Detection of Hepatitis E Virus (HEV) in Pork Sold in Saint-Louis, the North of Senegal
Abstract
:1. Introduction
2. Materials and Methods
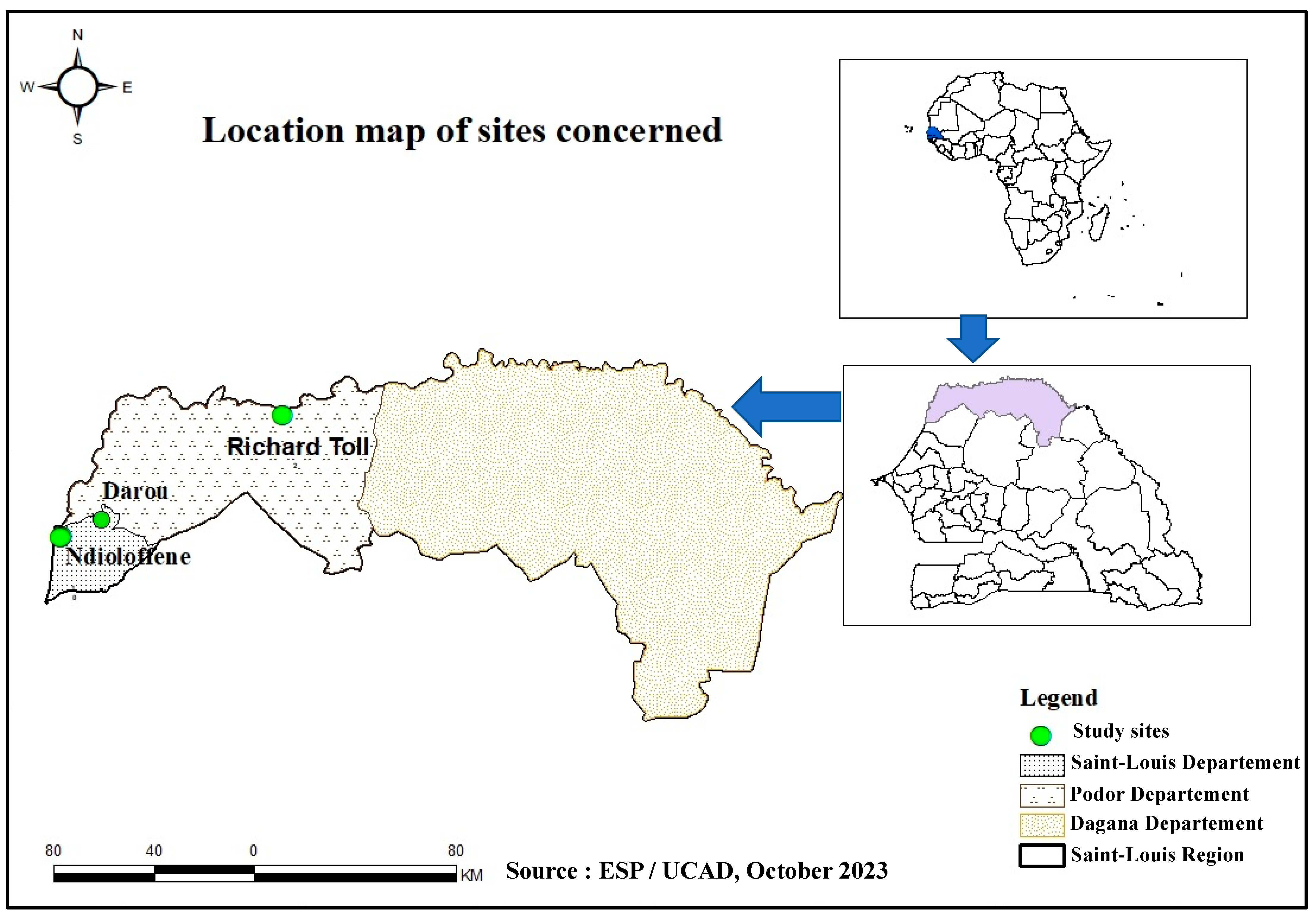
2.1. Sampling
2.2. Extraction of Total Nucleic Acid (TNA)
2.3. RT-PCR
2.4. Sequencing and Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Debing, Y.; Moradpour, D.; Neyts, J.; Gouttenoire, J. Update on hepatitis E virology: Implications for clinical practice. J. Hepatol. 2016, 65, 200–212. [Google Scholar] [CrossRef]
- Purdy, M.A.; Drexler, J.F.; Meng, X.-J.; Norder, H.; Okamoto, H.; Van der Poel, W.H.M.; Reuter, G.; de Souza, W.M.; Ulrich, R.G.; Smith, D.B. ICTV Virus Taxonomy Profile: Hepeviridae 2022. J. Gen. Virol. 2022, 103, 001778. [Google Scholar] [CrossRef]
- ICTV. Family: Hepeviridae. Available online: https://ictv.global/report/chapter/hepeviridae/hepeviridae (accessed on 2 January 2024).
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.-J.; Norder, H.; Okamoto, H.; van der Poel, W.H.M.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692–698. [Google Scholar] [CrossRef]
- Turlewicz-Podbielska, H.; Augustyniak, A.; Wojciechowski, J.; Pomorska-Mól, M. Hepatitis E Virus in Livestock—Update on Its Epidemiology and Risk of Infection to Humans. Animals 2023, 13, 3239. [Google Scholar] [CrossRef]
- Wang, J.; Li, N.; Zhang, H.; Li, F.; Fanning, S.; Jiang, T. Detection of Hepatitis E Virus in the Pig Livers and Retail Pork Samples Collected in Selected Cities in China. Foodborne Pathog. Dis. 2021, 18, 97–103. [Google Scholar] [CrossRef]
- 63 World Health Assembly. Viral Hepatitis: Report by the Secretariat. A63/15, 2010. Available online: https://iris.who.int/handle/10665/2383 (accessed on 8 November 2023).
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.-S.; Ijaz, S.; Izopet, J.; Dalton, H.R. Hepatitis E. Lancet 2012, 379, 2477–2488. [Google Scholar] [CrossRef]
- Purcell, R.H.; Emerson, S.U. Hepatitis E: An emerging awareness of an old disease. J. Hepatol. 2008, 48, 494–503. [Google Scholar] [CrossRef]
- Diouara, A.A.M.; Lo, S.; Nguer, C.M.; Senghor, A.; Diop Ndiaye, H.; Manga, N.M.; Danfakha, F.; Diallo, S.; Faye Dieme, M.E.; Thiam, O.; et al. Hepatitis E Virus Seroprevalence and Associated Risk Factors in Pregnant Women Attending Antenatal Consultations in Senegal. Viruses 2022, 14, 1742. [Google Scholar] [CrossRef]
- Aggarwal, R. Hepatitis E: Historical, contemporary and future perspectives. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. S1), 72–82. [Google Scholar] [CrossRef]
- Lhomme, S.; Abravanel, F.; Dubois, M.; Sandres-Saune, K.; Mansuy, J.-M.; Rostaing, L.; Kamar, N.; Izopet, J. Characterization of the Polyproline Region of the Hepatitis E Virus in Immunocompromised Patients. J. Virol. 2014, 88, 12017–12025. [Google Scholar] [CrossRef]
- WHO. The Global Prevalence of Hepatitis E Virus Infection and Susceptibility. Available online: https://www.who.int/publications-detail-redirect/WHO-IVB-10.14 (accessed on 8 November 2023).
- WHO. Hepatitis E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 11 August 2023).
- Crum-Cianflone, N.F.; Curry, J.; Drobeniuc, J.; Weintrob, A.; Landrum, M.; Ganesan, A.; Bradley, W.; Agan, B.K.; Kamili, S. Hepatitis E Virus Infection in HIV-infected Persons. Emerg. Infect. Dis. 2012, 18, 502–506. [Google Scholar] [CrossRef]
- Karna, R.; Hazam, R.K.; Borkakoti, J.; Kumar, A.; Kar, P. A 5-year Single-Center Experience of Hepatitis E Virus Infection during Pregnancy. J. Clin. Exp. Hepatol. 2020, 10, 135–138. [Google Scholar] [CrossRef]
- Santos-Silva, S.; Hemnani, M.; Lopez-Lopez, P.; Gonçalves, H.M.R.; Rivero-Juarez, A.; Van der Poel, W.H.M.; Nascimento, M.S.J.; Mesquita, J.R. A Systematic Review of Hepatitis E Virus Detection in Camels. Vet. Sci. 2023, 10, 323. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Kamili, S.; Khuroo, M.S. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J. Viral Hepat. 2009, 16, 519–523. [Google Scholar] [CrossRef]
- Pischke, S.; Hartl, J.; Pas, S.D.; Lohse, A.W.; Jacobs, B.C.; Van der Eijk, A.A. Hepatitis E virus: Infection beyond the liver? J. Hepatol. 2017, 66, 1082–1095. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Xia, J. Hepatitis E virus infection during pregnancy. Virol. J. 2020, 17, 73. [Google Scholar] [CrossRef]
- Sadio, B.D.; Faye, M.; Kaiser, M.; Diarra, M.; Balique, F.; Diagne, C.T.; Faye, O.; Diagne, M.M.; Fall, G.; Ndiaye, O.; et al. First hepatitis E outbreak in Southeastern Senegal. Sci. Rep. 2022, 12, 17878. [Google Scholar] [CrossRef]
- Takuissu, G.R.; Kenmoe, S.; Ndip, L.; Ebogo-Belobo, J.T.; Kengne-Ndé, C.; Mbaga, D.S.; Bowo-Ngandji, A.; Oyono, M.G.; Kenfack-Momo, R.; Tchatchouang, S.; et al. Hepatitis E Virus in Water Environments: A Systematic Review and Meta-analysis. Food Environ. Virol. 2022, 14, 223–235. [Google Scholar] [CrossRef]
- Izopet, J.; Tremeaux, P.; Marion, O.; Migueres, M.; Capelli, N.; Chapuy-Regaud, S.; Mansuy, J.-M.; Abravanel, F.; Kamar, N.; Lhomme, S. Hepatitis E virus infections in Europe. J. Clin. Virol. 2019, 120, 20–26. [Google Scholar] [CrossRef]
- Hakim, M.S.; Wang, W.; Bramer, W.M.; Geng, J.; Huang, F.; de Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The global burden of hepatitis E outbreaks: A systematic review. Liver Int. 2017, 37, 19–31. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Khuroo, M.S.; Khuroo, N.S. Transmission of Hepatitis E Virus in Developing Countries. Viruses 2016, 8, 253. [Google Scholar] [CrossRef]
- Singh, M.P.; Majumdar, M.; Goyal, K.; Lakshmi, P.V.M.; Bhatia, D.; Ratho, R.K. Investigation of suspected viral hepatitis outbreaks in North West India. Diagn. Microbiol. Infect. Dis. 2016, 84, 309–314. [Google Scholar] [CrossRef]
- Fatawou, M.A.; Chavely, M.G.; Henri, M.Y.M.; Daniel, K.N.; Claire, E.Z.M.; Richard, N. First Detection and Characterization of Hepatitis E Virus in Sewage Samples in Cameroon. Food Environ. Virol. 2023, 15, 255–261. [Google Scholar] [CrossRef]
- Nimgaonkar, I.; Ding, Q.; Schwartz, R.E.; Ploss, A. Hepatitis E virus: Advances and challenges. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 96–110. [Google Scholar] [CrossRef]
- Van der Poel, W.H. Food and environmental routes of Hepatitis E virus transmission. Curr. Opin. Virol. 2014, 4, 91–96. [Google Scholar] [CrossRef]
- Mirazo, S.; D`Albora, C.; Quintero Gil, D.; Cabrera, K.; Ramos, N.; Ordúz, S.; Arbiza, J. A case of incidental infection of Hepatitis E virus (HEV) genotype 1 in a domestic pig. Arch. Virol. 2018, 163, 3403–3407. [Google Scholar] [CrossRef]
- Pavio, N.; Kooh, P.; Cadavez, V.; Gonzales-Barron, U.; Thébault, A. Risk factors for sporadic hepatitis E infection: A systematic review and meta-analysis. Microb. Risk Anal. 2021, 17, 100129. [Google Scholar] [CrossRef]
- Bagulo, H.; Majekodunmi, A.O.; Welburn, S.C. Hepatitis E in Sub Saharan Africa—A significant emerging disease. One Health 2021, 11, 100186. [Google Scholar] [CrossRef]
- Shrestha, A.; Adhikari, A.; Bhattarai, M.; Rauniyar, R.; Debes, J.D.; Boonstra, A.; Lama, T.K.; Al Mahtab, M.; Butt, A.S.; Akbar, S.M.F.; et al. Prevalence and risk of hepatitis E virus infection in the HIV population of Nepal. Virol. J. 2017, 14, 228. [Google Scholar] [CrossRef]
- McCreary, C.; Martelli, F.; Grierson, S.; Ostanello, F.; Nevel, A.; Banks, M. Excretion of hepatitis E virus by pigs of different ages and its presence in slurry stores in the United Kingdom. Vet. Rec. 2008, 163, 261–265. [Google Scholar] [CrossRef]
- Carella, E.; Oberto, F.; Romano, A.; Peletto, S.; Vitale, N.; Costa, A.; Caruso, C.; Chiavacci, L.; Acutis, P.L.; Pite, L.; et al. Molecular and serological investigation of Hepatitis E virus in pigs slaughtered in Northwestern Italy. BMC Vet. Res. 2023, 19, 21. [Google Scholar] [CrossRef]
- Kokkinos, P.; Kozyra, I.; Lazic, S.; Bouwknegt, M.; Rutjes, S.; Willems, K.; Moloney, R.; de Roda Husman, A.M.; Kaupke, A.; Legaki, E.; et al. Harmonised investigation of the occurrence of human enteric viruses in the leafy green vegetable supply chain in three European countries. Food Environ. Virol. 2012, 4, 179–191. [Google Scholar] [CrossRef]
- Crossan, C.; Baker, P.J.; Craft, J.; Takeuchi, Y.; Dalton, H.R.; Scobie, L. Hepatitis E Virus Genotype 3 in Shellfish, United Kingdom. Emerg. Infect. Dis. 2012, 18, 2085–2087. [Google Scholar] [CrossRef]
- Meng, X.-J. Zoonotic and foodborne transmission of hepatitis E virus. Semin. Liver Dis. 2013, 33, 41–49. [Google Scholar] [CrossRef]
- Zahmanova, G.; Takova, K.; Tonova, V.; Koynarski, T.; Lukov, L.L.; Minkov, I.; Pishmisheva, M.; Kotsev, S.; Tsachev, I.; Baymakova, M.; et al. The Re-Emergence of Hepatitis E Virus in Europe and Vaccine Development. Viruses 2023, 15, 1558. [Google Scholar] [CrossRef]
- Doceul, V.; Bagdassarian, E.; Demange, A.; Pavio, N. Zoonotic Hepatitis E Virus: Classification, Animal Reservoirs and Transmission Routes. Viruses 2016, 8, 270. [Google Scholar] [CrossRef]
- Forero, J.E.; Gutiérrez-Vergara, C.; Parra Suescún, J.; Correa, G.; Rodríguez, B.; Gutiérrez, L.A.; Díaz, F.J.; López-Herrera, A. Phylogenetic analysis of Hepatitis E virus strains isolated from slaughter-age pigs in Colombia. Infect. Genet. Evol. 2017, 49, 138–145. [Google Scholar] [CrossRef]
- Thippornchai, N.; Leaungwutiwong, P.; Kosoltanapiwat, N.; Vuong, C.; Nguyen, K.; Okabayashi, T.; Lee, A. Survey of hepatitis E virus in pork products and pig stools in Nakhon Pathom Province, Thailand. Vet. Med. Sci. 2022, 8, 1975–1981. [Google Scholar] [CrossRef]
- Milojević, L.; Velebit, B.; Teodorović, V.; Kirbiš, A.; Petrović, T.; Karabasil, N.; Dimitrijević, M. Screening and Molecular Characterization of Hepatitis E Virus in Slaughter Pigs in Serbia. Food Environ. Virol. 2019, 11, 410–419. [Google Scholar] [CrossRef]
- Meng, X.J. Hepatitis E virus: Animal reservoirs and zoonotic risk. Vet. Microbiol. 2010, 140, 256–265. [Google Scholar] [CrossRef]
- Andraud, M.; Dumarest, M.; Cariolet, R.; Aylaj, B.; Barnaud, E.; Eono, F.; Pavio, N.; Rose, N. Direct contact and environmental contaminations are responsible for HEV transmission in pigs. Vet. Res. 2013, 44, 102. [Google Scholar] [CrossRef]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef]
- Montone, A.M.I.; De Sabato, L.; Suffredini, E.; Alise, M.; Zaccherini, A.; Volzone, P.; Di Maro, O.; Neola, B.; Capuano, F.; Di Bartolo, I. Occurrence of HEV-RNA in Italian Regional Pork and Wild Boar Food Products. Food Environ. Virol. 2019, 11, 420–426. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Lodder-Verschoor, F.; Van Der Poel, W.H.M.; Rutjes, S.A.; De Roda Husman, A.M. Hepatitis E Virus RNA in Commercial Porcine Livers in The Netherlands. J. Food Prot. 2007, 70, 2889–2895. [Google Scholar] [CrossRef]
- Feagins, A.R.; Opriessnig, T.; Guenette, D.K.; Halbur, P.G.; Meng, X.-J. Detection and characterization of infectious Hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J. Gen. Virol. 2007, 88, 912–917. [Google Scholar] [CrossRef]
- Feurer, C.; Le Roux, A.; Rossel, R.; Barnaud, E.; Dumarest, M.; Garry, P.; Pavio, N. High load of hepatitis E viral RNA in pork livers but absence in pork muscle at French slaughterhouses. Int. J. Food Microbiol. 2018, 264, 25–30. [Google Scholar] [CrossRef]
- Yazaki, Y.; Mizuo, H.; Takahashi, M.; Nishizawa, T.; Sasaki, N.; Gotanda, Y.; Okamoto, H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 2003, 84, 2351–2357. [Google Scholar] [CrossRef]
- Li, T.-C.; Chijiwa, K.; Sera, N.; Ishibashi, T.; Etoh, Y.; Shinohara, Y.; Kurata, Y.; Ishida, M.; Sakamoto, S.; Takeda, N.; et al. Hepatitis E Virus Transmission from Wild Boar Meat. Emerg. Infect. Dis. 2005, 11, 1958–1960. [Google Scholar] [CrossRef]
- Renou, C.; Afonso, A.-M.R.; Pavio, N. Foodborne Transmission of Hepatitis E Virus from Raw Pork Liver Sausage, France. Emerg. Infect. Dis. 2014, 20, 1945–1947. [Google Scholar] [CrossRef]
- Riveiro-Barciela, M.; Mínguez, B.; Gironés, R.; Rodriguez-Frías, F.; Quer, J.; Buti, M. Phylogenetic demonstration of hepatitis E infection transmitted by pork meat ingestion. J. Clin. Gastroenterol. 2015, 49, 165–168. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Frias, M.; Martinez-Peinado, A.; Risalde, M.A.; Rodriguez-Cano, D.; Camacho, A.; García-Bocanegra, I.; Cuenca-Lopez, F.; Gomez-Villamandos, J.C.; Rivero, A. Familial Hepatitis E Outbreak Linked to Wild Boar Meat Consumption. Zoonoses Public Health 2017, 64, 561–565. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, Y. Epidemiology of Hepatitis E. Adv. Exp. Med. Biol. 2016, 948, 39–59. [Google Scholar] [CrossRef]
- Kim, J.-H.; Nelson, K.E.; Panzner, U.; Kasture, Y.; Labrique, A.B.; Wierzba, T.F. A systematic review of the epidemiology of hepatitis E virus in Africa. BMC Infect. Dis. 2014, 14, 308. [Google Scholar] [CrossRef]
- Africa CDC. Hepatitis E Virus. Africa CDC. Available online: https://africacdc.org/disease/hepatitis-e-virus/ (accessed on 8 November 2023).
- Modiyinji, A.F.; Bigna, J.J.; Kenmoe, S.; Simo, F.B.N.; Amougou, M.A.; Ndangang, M.S.; Nola, M.; Njouom, R. Epidemiology of hepatitis E virus infection in animals in Africa: A systematic review and meta-analysis. BMC Vet. Res. 2021, 17, 50. [Google Scholar] [CrossRef]
- Salete de Paula, V.; Wiele, M.; Mbunkah, A.H.; Daniel, A.M.; Kingsley, M.T.; Schmidt-Chanasit, J. Hepatitis E Virus Genotype 3 Strains in Domestic Pigs, Cameroon. Emerg. Infect. Dis. 2013, 19, 686–688. [Google Scholar] [CrossRef]
- Kaba, M.; Colson, P.; Musongela, J.-P.; Tshilolo, L.; Davoust, B. Detection of hepatitis E virus of genotype 3 in a farm pig in Kinshasa (Democratic Republic of the Congo). Infect. Genet. Evol. 2010, 10, 154–157. [Google Scholar] [CrossRef]
- ISO 17604:2015; Microbiology of the Food Chain—Carcass Sampling for Microbiological Analysis. Available online: https://www.iso.org/obp/ui#iso:std:iso:17604:ed-2:v1:en (accessed on 11 December 2023).
- Diouara, A.A.M.; Ndiaye, H.D.; Guindo, I.; Bangoura, N.; Cissé, M.; Edmond, T.; Bougoudogo, F.; Mboup, S.; Peeters, M.; Ayouba, A.; et al. Antiretroviral treatment outcome in HIV-1-infected patients routinely followed up in capital cities and remote areas of Senegal, Mali and Guinea-Conakry. J. Int. AIDS Soc. 2014, 17, 19315. [Google Scholar] [CrossRef]
- Mizuo, H.; Suzuki, K.; Takikawa, Y.; Sugai, Y.; Tokita, H.; Akahane, Y.; Itoh, K.; Gotanda, Y.; Takahashi, M.; Nishizawa, T.; et al. Polyphyletic Strains of Hepatitis E Virus Are Responsible for Sporadic Cases of Acute Hepatitis in Japan. J. Clin. Microbiol. 2002, 40, 3209–3218. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Anisimova, M.; Gil, M.; Dufayard, J.-F.; Dessimoz, C.; Gascuel, O. Survey of Branch Support Methods Demonstrates Accuracy, Power, and Robustness of Fast Likelihood-based Approximation Schemes. Syst. Biol. 2011, 60, 685–699. [Google Scholar] [CrossRef]
- Rambaut. FigTree v. 1.4.4. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 11 December 2023).
- Pavio, N.; Merbah, T.; Thébault, A. Frequent Hepatitis E Virus Contamination in Food Containing Raw Pork Liver, France. Emerg. Infect. Dis. 2014, 20, 1925–1927. [Google Scholar] [CrossRef]
- Salines, M.; Andraud, M.; Rose, N. From the epidemiology of hepatitis E virus (HEV) within the swine reservoir to public health risk mitigation strategies: A comprehensive review. Vet. Res. 2017, 48, 31. [Google Scholar] [CrossRef]
- Boxman, I.L.A.; Jansen, C.C.C.; Hägele, G.; Zwartkruis-Nahuis, A.; Tijsma, A.S.L.; Vennema, H. Monitoring of pork liver and meat products on the Dutch market for the presence of HEV RNA. Int. J. Food Microbiol. 2019, 296, 58–64. [Google Scholar] [CrossRef]
- Di Bartolo, I.; Diez-Valcarce, M.; Vasickova, P.; Kralik, P.; Hernandez, M.; Angeloni, G.; Ostanello, F.; Bouwknegt, M.; Rodríguez-Lázaro, D.; Pavlik, I.; et al. Hepatitis E Virus in Pork Production Chain in Czech Republic, Italy, and Spain, 2010. Emerg. Infect. Dis. 2010, 18, 1282. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Rutjes, S.A.; Reusken, C.B.; Stockhofe-Zurwieden, N.; Frankena, K.; de Jong, M.C.; de Roda Husman, A.M.; van der Poel, W.H. The course of hepatitis E virus infection in pigs after contact-infection and intravenous inoculation. BMC Vet. Res. 2009, 5, 7. [Google Scholar] [CrossRef]
- Modiyinji, A.F.; Sanding, G.M.A.M.; Atsama, M.A.; Monamele, C.G.; Nola, M.; Njouom, R. Serological and molecular investigation of hepatitis E virus in pigs reservoirs from Cameroon reveals elevated seroprevalence and presence of genotype 3. PLoS ONE 2020, 15, e0229073. [Google Scholar] [CrossRef]
- El-Duah, P.; Dei, D.; Binger, T.; Sylverken, A.; Wollny, R.; Tasiame, W.; Oppong, S.; Adu-Sarkodie, Y.; Emikpe, B.; Folitse, R.; et al. Detection and genomic characterization of hepatitis E virus genotype 3 from pigs in Ghana, Africa. One Health Outlook 2020, 2, 10. [Google Scholar] [CrossRef]
- Traoré, K.A.; Ouoba, J.B.; Huot, N.; Rogée, S.; Dumarest, M.; Traoré, A.S.; Pavio, N.; Barro, N.; Roques, P. Hepatitis E Virus Exposure is Increased in Pork Butchers from Burkina Faso. Am. J. Trop. Med. Hyg. 2015, 93, 1356–1359. [Google Scholar] [CrossRef]
- Spahr, C.; Knauf-Witzens, T.; Vahlenkamp, T.; Ulrich, R.G.; Johne, R. Hepatitis E virus and related viruses in wild, domestic and zoo animals: A review. Zoonoses Public Health 2018, 65, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Primadharsini, P.P.; Nagashima, S.; Okamoto, H. Genetic Variability and Evolution of Hepatitis E Virus. Viruses 2019, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Lalèyê. La filière porcine au Sénégal: Commercialisation et consommation des viandes de porc et de phacochère dans les départements de Dakar, Fatick, Ziguinchor et Kolda. 2007. Available online: https://beep.ird.fr/greenstone/collect/eismv/index/assoc/TD07-2.dir/TD07-2.pdf (accessed on 11 December 2023).
- Ossebi, W.; Ayssiwede, S.; Nimbona, F.; Malou, R.; Djettin, A.; Diop, M.; Missohou, A. Analyse zootechnique et économique des systèmes d’élevage de porcs en Casamance (Sénégal). Rev. D’Élev. Méd. Vét. Pays Trop. 2019, 72, 13. [Google Scholar] [CrossRef]

| Sites | Sample Type | Sample Sizes | Prevalence of HEV (n) | 95% CI | p | |
|---|---|---|---|---|---|---|
| Pork Meat Samples | Pork Liver Samples | |||||
| Ndioloffène | 29 | 9 | 38 | 10.50% (n = 4) | [3.4–25.7%] | |
| Richard-Toll | 17 | 0 | 17 | 0% (n = 0) | [0–20.9%] | 0.1698 |
| Darou | 19 | 0 | 19 | 0% (n = 0) | [0–22.9%] | |
| TOTAL | 65 | 9 | 74 | 5.4% (n = 4) | [1.7–14%] | |
| Sample | Sample Sizes | Positive Samples | Prevalence of HEV | 95% CI | p |
|---|---|---|---|---|---|
| Pork meat | 65 | 2 | 3.1% | [0.53–11.6%] | 0.0699 |
| Pork liver | 9 | 2 | 22.2% | [3.9–59.8%] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tene, S.D.; Diouara, A.A.M.; Kane, A.; Sané, S.; Coundoul, S.; Thiam, F.; Nguer, C.M.; Diop, M.; Mbaye, M.N.; Mbengue, M.; et al. Detection of Hepatitis E Virus (HEV) in Pork Sold in Saint-Louis, the North of Senegal. Life 2024, 14, 512. https://doi.org/10.3390/life14040512
Tene SD, Diouara AAM, Kane A, Sané S, Coundoul S, Thiam F, Nguer CM, Diop M, Mbaye MN, Mbengue M, et al. Detection of Hepatitis E Virus (HEV) in Pork Sold in Saint-Louis, the North of Senegal. Life. 2024; 14(4):512. https://doi.org/10.3390/life14040512
Chicago/Turabian StyleTene, Sophie Deli, Abou Abdallah Malick Diouara, Alé Kane, Sarbanding Sané, Seynabou Coundoul, Fatou Thiam, Cheikh Momar Nguer, Mamadou Diop, Mame Ndew Mbaye, Malick Mbengue, and et al. 2024. "Detection of Hepatitis E Virus (HEV) in Pork Sold in Saint-Louis, the North of Senegal" Life 14, no. 4: 512. https://doi.org/10.3390/life14040512






