Emerging Transcatheter Therapies for Valvular Heart Disease: Focus on Mitral and Tricuspid Valve Procedures
Abstract
:1. Introduction
2. Transcatheter Interventions for Mitral Valve Disease
2.1. The Evolving Burden of Mitral Valve Disease
2.2. Transcatheter Mitral Valve Therapy
2.3. Leaflet Repair/Edge-to-Edge Technique
2.4. Indirect Annuloplasty Devices
2.5. Direct Annuloplasty Devices
2.6. Chordal Replacement
2.7. Transcatheter Mitral Valve Replacement (TMVR)
- The Intrepid TMVR system, manufactured by Medtronic in Minnesota, features a flexible dual-stent architecture that operates without the necessity for alternating alignment. The results of the Intrepid TMVR Early Feasibility Study [54] indicate that the advantages of this THV were sustained for up to 12 months, with low mortality, low need for re-operation, and almost outright removal of the MR. These findings demonstrate a favorable safety profile and long-lasting functionality of the valve.
- A completely recoverable and re-adjustable TMVR system is the Tendyne, made by Abbott Vascular in California. A 34 F sheath is utilized for its implantation through the apex. It features a porcine pericardial valve with three leaflets encased in two self-expanding stents from nitinol. The Real-World Tendyne European Experience Registry [55] outcomes indicate an elevated probability of technical success, long-lasting and thorough elimination of MR, notable therapeutic advantages, and a cardiovascular mortality rate of 17% within 12 months following the TMVR.
- The Tiara, developed by NeoVasc in Canada, is a device that includes bovine pericardial leaflets, and it is embedded within a self-expanding nitinol compound.
- The AltaValve, developed by 4C Medical in Minnesota, is a distinctive THV that is positioned in the LA. The device is composed of a nitinol body that is self-expanding with an elongated form and inside this, there is a bovine pericardial valve.
- The Cardiovalve, developed in Israel, is a self-expanding THV made from bovine pericardium. It consists of two segments, one for the LA and one for the LV. The product is offered in three different shapes, and it is provided using a 28 F sheath.
- The Cephea system, developed by Abbott Vascular, has a self-expanding structure with two plates. The inner compound contains a valve from bovine pericardium, and its layout enables the outer disk to adapt to different shapes while segregating the rest THV.
- The EVOQUE valve, developed by Edwards Lifesciences in California, is a self-expanding THV made of nitinol, with leaflets derived from bovine pericardium. The LV skeleton consists of nine hooks that connect to the leaflets of the MV and chords. The LA component of the device offers extra support by attaching to the annulus and it includes a skirt that prevents PVL. The initial trial conducted in the USA [56] has shown that the transseptal TMVR system is possible, with technical success in 92.9% of the patients, while non-cardiovascular death occurred in 7.1% of cases. The system resulted in improved MR and the overall clinical state of the patients.
- The HighLife system, developed by HighLife Medical in France, utilizes a sub-annular ring that is attached over the MV via a TF approach. This structure functions as a stabilizing point for the self-expanding THV, which incorporates three leaflets from bovine pericardium, and it can be implanted either via the apex of the heart or through the interatrial septum.
- The SAPIEN M3 system (Edwards Lifesciences, Irvine, CA, USA) consists of a nitinol structure that surrounds the MV and inside is attached a balloon-expandable THV through the interatrial septum. The valve resembles the SAPIEN 3 THV; nevertheless, it features an extra skirt to assist in sealing, avoiding any PVL. The ongoing ENCIRCLE trial (ClinicalTrials.gov identifier: NCT04153292) is designed to evaluate the safety and efficacy of the SAPIEN M3 THV in individuals with symptomatic, severe MR who are not suitable candidates for surgery or other percutaneous procedures.
2.8. Mitral Valve-in-Valve (MVIV), Valve-in-MAC (TMVR in-MAC), and Valve-in-Ring (MVIR)
2.9. Critical Appraisal of Transcatheter Mitral Valve Interventions
3. Transcatheter Interventions for Tricuspid Valve Disease
3.1. Transcatheter Edge-to-Edge Repair (TEER) of the Tricuspid Valve
3.2. Tricuspid Valve Replacement
3.3. Tricuspid Valve-in-Valve and Valve-in-Ring
3.4. Critical Appraisal of Transcatheter Tricuspid Valve Therapies
4. Transcatheter Pulmonary Valve Therapies
5. Special Considerations—Valve-in-Valve Procedures
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Bursi, F.; Faggiano, A.; Moscardelli, S.; Simeoli, P.S.; Guazzi, M.; Lorusso, R.; Carugo, S.; Faggiano, P. The Global Burden of Valvular Heart Disease: From Clinical Epidemiology to Management. J. Clin. Med. 2023, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.; Blanke, P.; Bax, J.J.; Leipsic, J. Multimodality imaging in valvular heart disease: How to use state-of-the-art technology in daily practice. Eur. Heart J. 2021, 42, 1912–1925. [Google Scholar] [CrossRef] [PubMed]
- Coisne, A.; Lancellotti, P.; Habib, G.; Garbi, M.; Dahl, J.S.; Barbanti, M.; Vannan, M.A.; Vassiliou, V.S.; Dudek, D.; Chioncel, O.; et al. ACC/AHA and ESC/EACTS Guidelines for the Management of Valvular Heart Diseases: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2023, 82, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Terre, J.A.; George, I. Over 15 years: The advancement of transcatheter aortic valve replacement. Ann. Cardiothorac. Surg. 2020, 9, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Apostolos, A.; Ktenopoulos, N.; Chlorogiannis, D.-D.; Katsaros, O.; Konstantinou, K.; Drakopoulou, M.; Tsalamandris, S.; Karanasos, A.; Synetos, A.; Latsios, G.; et al. Mortality Rates in Patients Undergoing Urgent Versus Elective Transcatheter Aortic Valve Replacement: A Meta-analysis. Angiology 2024, 33197241245733. [Google Scholar] [CrossRef] [PubMed]
- Katsaros, O.; Apostolos, A.; Ktenopoulos, N.; Koliastasis, L.; Kachrimanidis, I.; Drakopoulou, M.; Korovesis, T.; Karanasos, A.; Tsalamandris, S.; Latsios, G.; et al. Transcatheter Aortic Valve Implantation Access Sites: Same Goals, Distinct Aspects, Various Merits and Demerits. J. Cardiovasc. Dev. Dis. 2023, 11, 4. [Google Scholar] [CrossRef]
- Sagris, M.; Ktenopoulos, N.; Dimitriadis, K.; Papanikolaou, A.; Tzoumas, A.; Terentes-Printzios, D.; Synetos, A.; Soulaidopoulos, S.; Lichtenberg, M.; Korosoglou, G.; et al. Efficacy of intravascular lithotripsy (IVL) in coronary stenosis with severe calcification: A multicenter systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2024, 103, 710–721. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.S.; Redwood, S.R.; Allen, C.J.; Hurrell, H.; Chehab, O.; Rajani, R.; Prendergast, B.; Patterson, T. A 20-year journey in transcatheter aortic valve implantation: Evolution to current eminence. Front. Cardiovasc. Med. 2022, 9, 971762. [Google Scholar] [CrossRef]
- Karanasos, A.; Latsios, G.; Tsioufis, C.; Toutouzas, K. Trans-catheter aortic valve implantation: Passing on to adulthood. Hellenic J. Cardiol. 2021, 62, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Stathogiannis, K.; Synetos, A.; Latsios, G.; Karanasos, A.; Trantalis, G.; Toskas, P.; Drakopoulou, M.; Xanthopoulou, M.; Karmpalioti, M.; Simopoulou, C.; et al. Long-Term Outcomes and Valve Performance in Patients Undergoing Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 147, 80–87. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, G.; Apostolos, A.; Drakopoulou, M.; Simopoulou, C.; Karmpalioti, M.; Toskas, P.; Stathogiannis, K.; Xanthopoulou, M.; Ktenopoulos, N.; Latsios, G.; et al. Long-Term Outcomes of Aortic Stenosis Patients with Different Flow/Gradient Patterns Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2024, 13, 1200. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Vahl, T.P.; Thourani, V.H.; Makkar, R.R.; Hamid, N.; Khalique, O.K.; Daniels, D.; McCabe, J.M.; Satler, L.; Russo, M.; Cheng, W.; et al. Transcatheter aortic valve implantation in patients with high-risk symptomatic native aortic regurgitation (ALIGN-AR): A prospective, multicentre, single-arm study. Lancet 2024, 403, 1451–1459. [Google Scholar] [CrossRef]
- Dziadzko, V.; Clavel, M.-A.; Dziadzko, M.; Medina-Inojosa, J.R.; Michelena, H.; Maalouf, J.; Nkomo, V.; Thapa, P.; Enriquez-Sarano, M. Outcome and undertreatment of mitral regurgitation: A community cohort study. Lancet 2018, 391, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.-L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Nath, J.; Foster, E.; Heidenreich, P.A. Impact of tricuspid regurgitation on long-term survival. J. Am. Coll. Cardiol. 2004, 43, 405–409. [Google Scholar] [CrossRef]
- Arsalan, M.; Walther, T.; Smith, R.L., 2nd; Grayburn, P.A. Tricuspid regurgitation diagnosis and treatment. Eur. Heart J. 2017, 38, 634–638. [Google Scholar] [CrossRef]
- Ferencz, C.; Rubin, J.D.; Mccarter, R.J.; Brenner, J.I.; Neill, C.A.; Perry, L.W.; Hepner, S.I.; Downing, J.W. Congenital heart disease: Prevalence at livebirth. The Baltimore-Washington Infant Study. Am. J. Epidemiol. 1985, 121, 31–36. [Google Scholar] [CrossRef]
- Law, M.A.; Chatterjee, A. Transcatheter pulmonic valve implantation: Techniques, current roles, and future implications. World J. Cardiol. 2021, 13, 117–129. [Google Scholar] [CrossRef]
- Bonhoeffer, P.; Boudjemline, Y.; Saliba, Z.; Merckx, J.; Aggoun, Y.; Bonnet, D.; Acar, P.; Le Bidois, J.; Sidi, D.; Kachaner, J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000, 356, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.; Rhodes, J.F.; Fleming, G.A.; Kar, S.; Zahn, E.M.; Vincent, J.; Shirali, G.S.; Gorelick, J.; Fogel, M.A.; Fahey, J.T.; et al. 3-Year Outcomes of the Edwards SAPIEN Transcatheter Heart Valve for Conduit Failure in the Pulmonary Position From the COMPASSION Multicenter Clinical Trial. JACC Cardiovasc. Interv. 2018, 11, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Asgar, A.W.; Ducharme, A.; Pellerin, M.; Garceau, P.; Basmadjian, A.; Bouchard, D.; Bonan, R. The Evolution of Transcatheter Therapies for Mitral Valve Disease: From Mitral Valvuloplasty to Transcatheter Mitral Valve Replacement. Can. J. Cardiol. 2022, 38 (Suppl. 1), S54–S65. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Andell, P.; Li, X.; Martinsson, A.; Andersson, C.; Stagmo, M.; Zöller, B.; Sundquist, K.; Smith, J.G. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart 2017, 103, 1696–1703. [Google Scholar] [CrossRef]
- Harken, D.E.; Dexter, L.; Ellis, L.B.; Farrand, R.E.; Dickson, J.F., 3rd. The surgery of mitral stenosis: III. Finger-fracture valvuloplasty. Ann. Surg. 1951, 134, 722–742. [Google Scholar] [CrossRef]
- Inoue, K.; Owaki, T.; Nakamura, T.; Kitamura, F.; Miyamoto, N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J. Thorac. Cardiovasc. Surg. 1984, 87, 394–402. [Google Scholar] [CrossRef]
- I Stefanadis, C.; Stratos, C.G.; Lambrou, S.G.; Bahl, V.K.; Cokkinos, D.V.; A Voudris, V.; Foussas, S.G.; Tsioufis, C.P.; Toutouzas, P.K. Retrograde nontransseptal balloon mitral valvuloplasty: Immediate results and intermediate long-term outcome in 441 cases—A multicenter experience. J. Am. Coll. Cardiol. 1998, 32, 1009–1016. [Google Scholar] [CrossRef]
- Nobuyoshi, M.; Arita, T.; Shirai, S.-I.; Hamasaki, N.; Yokoi, H.; Iwabuchi, M.; Yasumoto, H.; Nosaka, H. Percutaneous balloon mitral valvuloplasty: A review. Circulation 2009, 119, e211–e219. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, G.T.; Weyman, A.E.; Abascal, V.M.; Block, P.C.; Palacios, I.F. Percutaneous balloon dilatation of the mitral valve: An analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br. Heart J. 1988, 60, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ishizu, K.; Kawaguchi, T.; Shirai, S.; Ando, K. Retrograde balloon aortic valvuloplasty with the newly invented Inoue balloon for aortic stenosis accompanied by severe heart failure: A case report. Clin. Case Rep. 2021, 9, 2011–2015. [Google Scholar] [CrossRef]
- Alfieri, O.; Maisano, F.; De Bonis, M.; Stefano, P.L.; Torracca, L.; Oppizzi, M.; La Canna, G. The double-orifice technique in mitral valve repair: A simple solution for complex problems. J. Thorac. Cardiovasc. Surg. 2001, 122, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Walther, C.; Fichtlscherer, S.; Holubec, T.; Vasa-Nicotera, M.; Arsalan, M.; Walther, T. New developments in transcatheter therapy of mitral valve disease. J. Thorac. Dis. 2020, 12, 1728–1739. [Google Scholar] [CrossRef]
- Kar, S.; von Bardeleben, R.S.; Rottbauer, W.; Mahoney, P.; Price, M.J.; Grasso, C.; Williams, M.; Lurz, P.; Ahmed, M.; Hausleiter, J.; et al. Contemporary Outcomes Following Transcatheter Edge-to-Edge Repair: 1-Year Results From the EXPAND Study. JACC Cardiovasc. Interv. 2023, 16, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Obadia, J.-F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Felbel, D.; Paukovitsch, M.; Förg, R.; Stephan, T.; Mayer, B.; Keßler, M.; Tadic, M.; Dahme, T.; Rottbauer, W.; Markovic, S.; et al. Comparison of transcatheter edge-to-edge and surgical repair in patients with functional mitral regurgitation using a meta-analytic approach. Front. Cardiovasc. Med. 2022, 9, 1063070. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.; Rubbio, A.P. The PASCAL transcatheter mitral valve repair system for the treatment of mitral regurgitation: Another piece to the puzzle of edge-to-edge technique. J. Thorac. Dis. 2017, 9, 4856–4859. [Google Scholar] [CrossRef]
- Praz, F.; Spargias, K.; Chrissoheris, M.; Büllesfeld, L.; Nickenig, G.; Deuschl, F.; Schueler, R.; Fam, N.P.; Moss, R.; Makar, M.; et al. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: A multicentre, prospective, observational, first-in-man study. Lancet 2017, 390, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Kar, S.; Spargias, K.; Kipperman, R.M.; O’Neill, W.W.; Ng, M.K.C.; Fam, N.P.; Walters, D.L.; Webb, J.G.; Smith, R.L.; et al. Transcatheter Valve Repair for Patients with Mitral Regurgitation: 30-Day Results of the CLASP Study. JACC Cardiovasc. Interv. 2019, 12, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Siminiak, T.; Wu, J.C.; Haude, M.; Hoppe, U.C.; Sadowski, J.; Lipiecki, J.; Fajadet, J.; Shah, A.M.; Feldman, T.; Kaye, D.M.; et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: Results of the TITAN Trial. Eur. J. Heart Fail. 2012, 14, 931–938. [Google Scholar] [CrossRef]
- Lipiecki, J.; Siminiak, T.; Sievert, H.; Müller-Ehmsen, J.; Degen, H.; Wu, J.C.; Schandrin, C.; Kalmucki, P.; Hofmann, I.; Reuter, D.; et al. Coronary sinus-based percutaneous annuloplasty as treatment for functional mitral regurgitation: The TITAN II trial. Open Heart 2016, 3, e000411. [Google Scholar] [CrossRef]
- Witte, K.K.; Lipiecki, J.; Siminiak, T.; Meredith, I.T.; Malkin, C.J.; Goldberg, S.L.; Stark, M.A.; von Bardeleben, R.S.; Cremer, P.C.; Jaber, W.A.; et al. The REDUCE FMR Trial: A Randomized Sham-Controlled Study of Percutaneous Mitral Annuloplasty in Functional Mitral Regurgitation. JACC Heart Fail. 2019, 7, 945–955. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Nickenig, G.; Latib, A.; Kuck, K.-H.; Baldus, S.; Schueler, R.; La Canna, G.; Agricola, E.; Kreidel, F.; Huntgeburth, M.; et al. Transcatheter mitral valve repair for functional mitral regurgitation using the Cardioband system: 1 year outcomes. Eur. Heart J. 2019, 40, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Schueler, R.; Dager, A.; Clark, P.M.; Abizaid, A.; Siminiak, T.; Buszman, P.; Demkow, M.; Ebner, A.; Asch, F.M.; et al. Treatment of Chronic Functional Mitral Valve Regurgitation With a Percutaneous Annuloplasty System. J. Am. Coll. Cardiol. 2016, 67, 2927–2936. [Google Scholar] [CrossRef]
- Baikoussis, N.G.; Koumallos, N.; Aggeli, K. Mitral valve repair with the use of the “Memo 3D ReChord” ring. J. Cardiothorac. Surg. 2023, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Seeburger, J.; Rinaldi, M.; Nielsen, S.L.; Salizzoni, S.; Lange, R.; Schoenburg, M.; Alfieri, O.; Borger, M.A.; Mohr, F.W.; Aidietis, A. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: The TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J. Am. Coll. Cardiol. 2014, 63, 914–919. [Google Scholar] [CrossRef]
- Gammie, J.S.; Bartus, K.; Gackowski, A.; D’Ambra, M.N.; Szymanski, P.; Bilewska, A.; Kusmierczyk, M.; Kapelak, B.; Rzucidlo-Resil, J.; Moat, N.; et al. Beating-Heart Mitral Valve Repair Using a Novel ePTFE Cordal Implantation Device: A Prospective Trial. J. Am. Coll. Cardiol. 2018, 71, 25–36. [Google Scholar] [CrossRef]
- del Val, D.; Ferreira-Neto, A.N.; Wintzer-Wehekind, J.; Dagenais, F.; Paradis, J.; Bernier, M.; O’Connor, K.; Beaudoin, J.; Freitas-Ferraz, A.B.; Rodés-Cabau, J. Early Experience With Transcatheter Mitral Valve Replacement: A Systematic Review. J. Am. Heart Assoc. 2019, 8, e013332. [Google Scholar] [CrossRef] [PubMed]
- Coisne, A.; Pontana, F.; Tchétché, D.; Richardson, M.; Longère, B.; Vahdat, O.; Berthoumieu, P.; Van Belle, E.; Rousse, N.; Lancellotti, P.; et al. Transcatheter mitral valve replacement: Factors associated with screening success and failure. EuroIntervention 2019, 15, e983–e989. [Google Scholar] [CrossRef] [PubMed]
- Hensey, M.; Brown, R.A.; Lal, S.; Sathananthan, J.; Ye, J.; Cheung, A.; Blanke, P.; Leipsic, J.; Moss, R.; Boone, R.; et al. Transcatheter Mitral Valve Replacement: An Update on Current Techniques, Technologies, and Future Directions. JACC Cardiovasc. Interv. 2021, 14, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Zahr, F.; Song, H.K.; Chadderdon, S.; Gada, H.; Mumtaz, M.; Byrne, T.; Kirshner, M.; Sharma, S.; Kodali, S.; George, I.; et al. 1-Year Outcomes Following Transfemoral Transseptal Transcatheter Mitral Valve Replacement: Intrepid TMVR Early Feasibility Study Results. JACC Cardiovasc. Interv. 2023, 16, 2868–2879. [Google Scholar] [CrossRef]
- Hell, M.M.; Wild, M.G.; Baldus, S.; Rudolph, T.; Treede, H.; Petronio, A.S.; Modine, T.; Andreas, M.; Coisne, A.; Duncan, A.; et al. Transapical Mitral Valve Replacement: 1-Year Results of the Real-World Tendyne European Experience Registry. JACC Cardiovasc. Interv. 2024, 17, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.; Hensey, M.; Fam, N.; Rodés-Cabau, J.; Daniels, D.; Smith, R.; Szeto, W.; Boone, R.; Ye, J.; Moss, R.; et al. Transcatheter Mitral Valve Replacement with the Transseptal EVOQUE System. JACC Cardiovasc. Interv. 2020, 13, 2418–2426. [Google Scholar] [CrossRef]
- Yousef, S.; Arnaoutakis, G.J.; Gada, H.; Smith, A.J.C.; Sanon, S.; Sultan, I. Transcatheter mitral valve therapies: State of the art. J. Card. Surg. 2022, 37, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.E.; Kliger, C.; Perk, G.; Maisano, F.; Cabalka, A.K.; Landzberg, M.; Rihal, C.; Kronzon, I. Transcatheter Therapies for the Treatment of Valvular and Paravalvular Regurgitation in Acquired and Congenital Valvular Heart Disease. J. Am. Coll. Cardiol. 2015, 66, 169–183. [Google Scholar] [CrossRef]
- Dvir, D.; Webb, J. Mitral valve-in-valve and valve-in-ring: Technical aspects and procedural outcomes. EuroIntervention 2016, 12, Y93–Y96. [Google Scholar] [CrossRef] [PubMed]
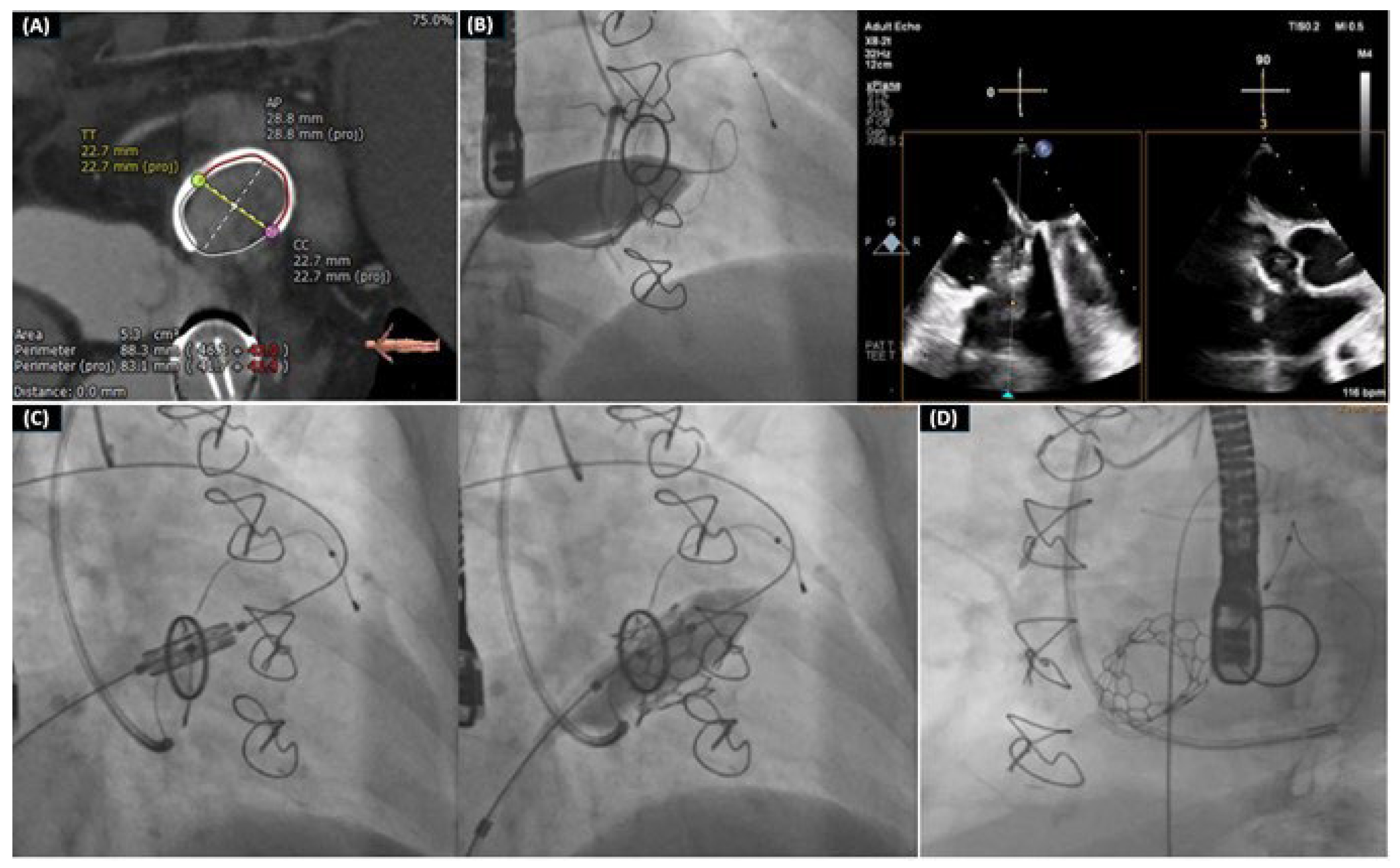
- Drakopoulou, M.; Latsios, G.; Synetos, A.; Benetos, G.; Soulaidopoulos, S.; Oikonomou, G.; Apostolos, A.; Aggeli, K.; Lozos, V.; Lymperiadis, D.; et al. Transcatheter mitral valve-in-valve replacement transeptally using a novel balloon-expandable device. J. Card. Surg. 2022, 37, 3376–3377. [Google Scholar] [CrossRef] [PubMed]
- Drakopoulou, M.; Oikonomou, G.; Latsios, G.; Synetos, A.; Benetos, G.; Simopoulou, C.; Apostolos, A.; Soulaidopoulos, S.; Aggeli, K.; Lozos, V.; et al. Takotsubo cardiomyopathy complicating transcatheter mitral valve-in-valve replacement. J. Geriatr. Cardiol. 2022, 19, 559–561. [Google Scholar] [PubMed]
- Romeo, J.D.; Bashline, M.J.; A Fowler, J.; E Kliner, D.; Toma, C.; Smith, A.C.; Sultan, I.; Sanon, S. Current Status of Transcatheter Tricuspid Valve Therapies. Heart Int. 2022, 16, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Hammerstingl, C.; Schueler, R.; Malasa, M.; Werner, N.; Nickenig, G. Transcatheter treatment of severe tricuspid regurgitation with the MitraClip system. Eur. Heart J. 2016, 37, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Muntane-Carol, G.; Alperi, A.; Faroux, L.; Bedard, E.; Philippon, F.; Rodes-Cabau, J. Transcatheter Tricuspid Valve Intervention: Coaptation Devices. Front. Cardiovasc. Med. 2020, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Hahn, R.T.; Alessandrini, H.; Latib, A.; Attinger-Toller, A.; Braun, D.; Brochet, E.; Connelly, K.A.; Denti, P.; Deuschl, F.; et al. The International Multicenter TriValve Registry: Which Patients Are Undergoing Transcatheter Tricuspid Repair? JACC Cardiovasc. Interv. 2017, 10, 1982–1990. [Google Scholar] [CrossRef]
- Mehr, M.; Taramasso, M.; Besler, C.; Ruf, T.; Connelly, K.A.; Weber, M.; Yzeiraj, E.; Schiavi, D.; Mangieri, A.; Vaskelyte, L.; et al. 1-Year Outcomes After Edge-to-Edge Valve Repair for Symptomatic Tricuspid Regurgitation: Results From the TriValve Registry. JACC Cardiovasc. Interv. 2019, 12, 1451–1461. [Google Scholar] [CrossRef]
- Lurz, P.; von Bardeleben, R.S.; Weber, M.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.-N.; Nabauer, M.; Tang, G.H.; et al. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2021, 77, 229–239. [Google Scholar] [CrossRef]
- Nickenig, G.; Weber, M.; Lurz, P.; von Bardeleben, R.S.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.-N.; Näbauer, M.; et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet 2019, 394, 2002–2011. [Google Scholar] [CrossRef]
- Sorajja, P.; Whisenant, B.; Hamid, N.; Naik, H.; Makkar, R.; Tadros, P.; Price, M.J.; Singh, G.; Fam, N.; Kar, S.; et al. Transcatheter Repair for Patients with Tricuspid Regurgitation. N. Engl. J. Med. 2023, 388, 1833–1842. [Google Scholar] [CrossRef]
- Aurich, M.; Volz, M.J.; Mereles, D.; Geis, N.A.; Frey, N.; Konstandin, M.H.; Raake, P.W. Initial Experience With the PASCAL Ace Implant System for Treatment of Severe Tricuspid Regurgitation. Circ. Cardiovasc. Interv. 2021, 14, e010770. [Google Scholar] [CrossRef]
- Fam, N.P.; Ho, E.C.; Zahrani, M.; Samargandy, S.; Connelly, K.A. Transcatheter Tricuspid Valve Repair With the PASCAL System. JACC Cardiovasc. Interv. 2018, 11, 407–408. [Google Scholar] [CrossRef]
- Fam, N.P.; Braun, D.; von Bardeleben, R.S.; Nabauer, M.; Ruf, T.; Connelly, K.A.; Ho, E.; Thiele, H.; Lurz, P.; Weber, M.; et al. Compassionate Use of the PASCAL Transcatheter Valve Repair System for Severe Tricuspid Regurgitation: A Multicenter, Observational, First-in-Human Experience. JACC Cardiovasc. Interv. 2019, 12, 2488–2495. [Google Scholar] [CrossRef]
- Kodali, S.; Hahn, R.T.; Eleid, M.F.; Kipperman, R.; Smith, R.; Lim, D.S.; Gray, W.A.; Narang, A.; Pislaru, S.V.; Koulogiannis, K.; et al. Feasibility Study of the Transcatheter Valve Repair System for Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2021, 77, 345–356. [Google Scholar] [CrossRef]
- Fam, N.P.; von Bardeleben, R.S.; Hensey, M.; Kodali, S.K.; Smith, R.L.; Hausleiter, J.; Ong, G.; Boone, R.; Ruf, T.; George, I.; et al. Transfemoral Transcatheter Tricuspid Valve Replacement With the EVOQUE System: A Multicenter, Observational, First-in-Human Experience. JACC Cardiovasc. Interv. 2021, 14, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Fam, N.P.; Ong, G.; Deva, D.P.; Peterson, M.D. Transfemoral Transcatheter Tricuspid Valve Replacement. JACC Cardiovasc. Interv. 2020, 13, e93–e94. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.; Hahn, R.T.; George, I.; Davidson, C.J.; Narang, A.; Zahr, F.; Chadderdon, S.; Smith, R.; Grayburn, P.A.; O’Neill, W.W.; et al. Transfemoral Tricuspid Valve Replacement in Patients With Tricuspid Regurgitation: TRISCEND Study 30-Day Results. JACC Cardiovasc. Interv. 2022, 15, 471–480. [Google Scholar] [CrossRef]
- Susheel Kodali, M. TRISCEND II: A Randomized Trial of Transcatheter Tricuspid Valve Replacement in Patients with Severe Tricuspid Regurgitation; TCT: San Francisco, CA, USA, 2023. [Google Scholar]
- Navia, J.L.; Kapadia, S.; Elgharably, H.; Harb, S.C.; Krishnaswamy, A.; Unai, S.; Mick, S.; Rodriguez, L.; Hammer, D.; Gillinov, A.M.; et al. First-in-Human Implantations of the NaviGate Bioprosthesis in a Severely Dilated Tricuspid Annulus and in a Failed Tricuspid Annuloplasty Ring. Circ. Cardiovasc. Interv. 2017, 10, e005840. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.L.; Ma, Y.; An, Z.; Cai, C.L.; Li, B.L.; Song, Z.G.; Han, L.; Wang, J.; Qiao, F.; Xu, Z.Y. First-in-Man Experience of Transcatheter Tricuspid Valve Replacement With LuX-Valve in High-Risk Tricuspid Regurgitation Patients. JACC Cardiovasc. Interv. 2020, 13, 1614–1616. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Turrión, S.; Briedis, K.; Estévez-Loureiro, R.; Sánchez-Recalde, A.; Cruz-González, I.; Pascual, I.; Mascherbauer, J.; Abdul-Jawad Altisent, O.; Nombela-Franco, L.; Pan, M.; et al. Bicaval TricValve Implantation in Patients With Severe Symptomatic Tricuspid Regurgitation: 1-Year Follow-Up Outcomes. JACC Cardiovasc. Interv. 2024, 17, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.-C.; Lim, S.-H.; Yi, G.; Hong, Y.S.; Lee, S.; Yoo, K.-J.; Kang, M.S.; Cho, B.K. Long-term clinical results of tricuspid valve replacement. Ann. Thorac. Surg. 2006, 1323, 81. [Google Scholar] [CrossRef]
- Guenther, T.; Noebauer, C.; Mazzitelli, D.; Busch, R.; Tassani-Prell, P.; Lange, R. Tricuspid valve surgery: A thirty-year assessment of early and late outcome. Eur. J. Cardiothorac. Surg. 2008, 34, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Qiao, W.-H.; Sun, F.-Q.; Ruan, X.-L.; Al Shirbini, M.; Hu, D.; Chen, S.; Dong, N.-G. Should a Mechanical or Biological Prosthesis Be Used for a Tricuspid Valve Replacement? A Meta-Analysis. J. Card. Surg. 2016, 31, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Sanon, S.; Cabalka, A.K.; Babaliaros, V.; Rihal, C.; Gafoor, S.; Webb, J.; Latib, A. Transcatheter Tricuspid Valve-in-Valve and Valve-in-Ring Implantation for Degenerated Surgical Prosthesis. JACC Cardiovasc. Interv. 2019, 12, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, V.; Donà, C.; Koschutnik, M.; Winter, M.-P.; Nitsche, C.; Kammerlander, A.A.; Bartko, P.E.; Hengstenberg, C.; Mascherbauer, J.; Goliasch, G. Transcatheter treatment by valve-in-valve and valve-in-ring implantation for prosthetic tricuspid valve dysfunction. Wien. Klin. Wochenschr. 2021, 133, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Van Garsse, L.A.; Bekke, R.M.T.; van Ommen, V.G. Percutaneous transcatheter valve-in-valve implantation in stenosed tricuspid valve bioprosthesis. Circulation 2011, 123, e219–e221. [Google Scholar] [CrossRef]
- Cullen, M.W.; Cabalka, A.K.; Alli, O.O.; Pislaru, S.V.; Sorajja, P.; Nkomo, V.T.; Malouf, J.F.; Cetta, F.; Hagler, D.J.; Rihal, C.S. Transvenous, antegrade Melody valve-in-valve implantation for bioprosthetic mitral and tricuspid valve dysfunction: A case series in children and adults. JACC Cardiovasc. Interv. 2013, 6, 598–605. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Cabalka, A.K.; Aboulhosn, J.A.; Eicken, A.; Boudjemline, Y.; Schubert, S.; Himbert, D.; Asnes, J.D.; Salizzoni, S.; Bocks, M.L.; et al. Transcatheter Tricuspid Valve-in-Valve Implantation for the Treatment of Dysfunctional Surgical Bioprosthetic Valves: An International, Multicenter Registry Study. Circulation 2016, 133, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Lurz, P.; Coats, L.; Khambadkone, S.; Nordmeyer, J.; Boudjemline, Y.; Schievano, S.; Muthurangu, V.; Lee, T.Y.; Parenzan, G.; Derrick, G.; et al. Percutaneous pulmonary valve implantation: Impact of evolving technology and learning curve on clinical outcome. Circulation 2008, 117, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, J.P.; Hellenbrand, W.E.; Zahn, E.M.; Jones, T.K.; Berman, D.P.; Vincent, J.A.; McElhinney, D.B. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation 2015, 131, 1960–1970. [Google Scholar] [CrossRef]
- Armstrong, A.K.; Balzer, D.T.; Cabalka, A.K.; Gray, R.G.; Javois, A.J.; Moore, J.W.; Rome, J.J.; Turner, D.R.; Zellers, T.M.; Kreutzer, J.; et al. One-year follow-up of the Melody transcatheter pulmonary valve multicenter post-approval study. JACC Cardiovasc. Interv. 2014, 7, 1254–1262. [Google Scholar] [CrossRef]
- Lehner, A.; Dashkalova, T.; Ulrich, S.; Rodriguez, S.F.; Mandilaras, G.; Jakob, A.; Dalla-Pozza, R.; Fischer, M.; Schneider, H.; Tarusinov, G.; et al. Intermediate outcomes of transcatheter pulmonary valve replacement with the Edwards Sapien 3 valve—German experience. Expert. Rev. Med. Devices 2019, 16, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.; Prachasilchai, P.; Promphan, W.; Rosenthal, E.; Sivakumar, K.; Kappanayil, M.; Sakidjan, I.; Walsh, K.P.; Kenny, D.; Thomson, J.; et al. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve: International experience. EuroIntervention 2019, 14, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Song, M.K.; Bae, E.J.; Park, E.-A.; Lee, W.; Lim, H.-G.; Kim, Y.J. Successful Feasibility Human Trial of a New Self-Expandable Percutaneous Pulmonary Valve (Pulsta Valve) Implantation Using Knitted Nitinol Wire Backbone and Trileaflet alpha-Gal-Free Porcine Pericardial Valve in the Native Right Ventricular Outflow Tract. Circ. Cardiovasc. Interv. 2018, 11, e006494. [Google Scholar] [CrossRef] [PubMed]

| Category | Device | Narrative | Access | Certification/Approval | |
|---|---|---|---|---|---|
| TEER |  | MitraClip (Abbot Vascular, Chicago, IL, USA) | “Edge-to-edge” technique by Alfieri et al. [34] | TF-TS | >80,000 implantations, CE and FDA approval |
 | PASCAL (Edwards Lifesciences, Irvine, CA, USA) | “Edge-to-edge” technique by Alfieri et al. [34] | TF-TS | CE approval | |
| Direct Annuloplasty |  | Cardioband (Edwards Lifesciences, Irvine, CA, USA) | Adjustable band to the posterior annulus | TF-TS | CE approval |
 | Mitralign (Mitralign, Inc., Tewksbury, MA, USA) | Enlargement of the posterior annulus with two pairs of pledges | TF-TS | CE approval | |
 | Memo 3D ReChord ring | Implantation of artificial neo-chordae | TA | CE approval | |
| IndirectAnnuloplasty |  | Carillon (Cardiac Dimensions, Lake Forest, CA, USA) | Nitinol anchors placed in the distal and proximal coronary sinus | TJ | CE approval |
| ChordalReplacement |  | NeoChord (NeoChord, St. Louis Park, MN, USA) | Implantation of artificial chords | TA | CE approval |
 | TSD-5 device (Harpoon Medical, Inc., Baltimore, MD, USA) | Implantation of artificial chords | TA | Ongoing trial for CE approval | |
| Device | Frame | Leaflets | Anchoring | Delivery | Recapturable | |
|---|---|---|---|---|---|---|
| Intrepid |  | Dual-stent self-expanding/nitinol | Bovine PD | Perimeter oversizing | TS | Yes |
| Tendyne |  | Dual-stent self-expanding/nitinol | Bovine PD | Apical pad | TA | Yes |
| Tiara |  | Self-expanding/nitinol | Bovine PD | D-shaped configuration with three anchors | TA | No |
| AltaValve |  | Spherical self-expanding/nitinol | Bovine PD | Supra-annular valveanchored in left atrium | TA/TS | Partially |
| Cardiovalve |  | Dual-frame self-expanding/nitinol | Bovine PD | MV leaflets/annulus | TS | Partially |
| Cephea |  | Dual-stent self-expanding/nitinol | NA | MV annulus: double disk | TS | Yes |
| EVOQUE | 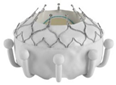 | Self-expanding/nitinol | Bovine PD | MV leaflets/annulus | TS | No |
| HighLife |  | Self-expanding/nitinol | Bovine PD | Sub-annular ring (valve-in-ring) | TS | No |
| SAPIEN M3 |  | Balloon-expandable cobalt–chromium | Bovine PD | Sub-annular nitinol dock | TS | Partially (dock) |
| Device | Frame | Leaflets | Anchoring | Delivery | |
|---|---|---|---|---|---|
| EVOQUE | 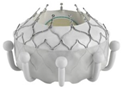 | Self-expanding (nickel–titanium) | Bovine PD | Intra-annular sealing skirt and anchors | TF |
| GATE |  | Self-expanding/nitinol | Xenogenic PD | Atrial winglets, ventricular graspers | TJ |
| INTREPID |  | Dual-stent system | Bovine PD | Recoverable before release | TF |
| LuX-Valve |  | Self-expanding/nitinol | Bovine PD | Leaflet fixation/septal anchoring | TA |
| TricValve |  | Self-expanding/nitinol | Pericardial tissue | NA | TF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ktenopoulos, N.; Katsaros, O.; Apostolos, A.; Drakopoulou, M.; Tsigkas, G.; Tsioufis, C.; Davlouros, P.; Toutouzas, K.; Karanasos, A. Emerging Transcatheter Therapies for Valvular Heart Disease: Focus on Mitral and Tricuspid Valve Procedures. Life 2024, 14, 842. https://doi.org/10.3390/life14070842
Ktenopoulos N, Katsaros O, Apostolos A, Drakopoulou M, Tsigkas G, Tsioufis C, Davlouros P, Toutouzas K, Karanasos A. Emerging Transcatheter Therapies for Valvular Heart Disease: Focus on Mitral and Tricuspid Valve Procedures. Life. 2024; 14(7):842. https://doi.org/10.3390/life14070842
Chicago/Turabian StyleKtenopoulos, Nikolaos, Odysseas Katsaros, Anastasios Apostolos, Maria Drakopoulou, Grigorios Tsigkas, Constantinos Tsioufis, Periklis Davlouros, Konstantinos Toutouzas, and Antonios Karanasos. 2024. "Emerging Transcatheter Therapies for Valvular Heart Disease: Focus on Mitral and Tricuspid Valve Procedures" Life 14, no. 7: 842. https://doi.org/10.3390/life14070842








