Prevalence of Helicobacter pylori Infection and Efficacy of Bismuth Quadruple and Levofloxacin Triple Eradication Therapies: A Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Design and Ethical Considerations
2.2. Participant Selection and Sample Collection
2.3. Treatment Strategies for H. pylori-Positive Patients
2.4. Monitoring and Follow-Up
3. Results
3.1. Baseline Characteristics
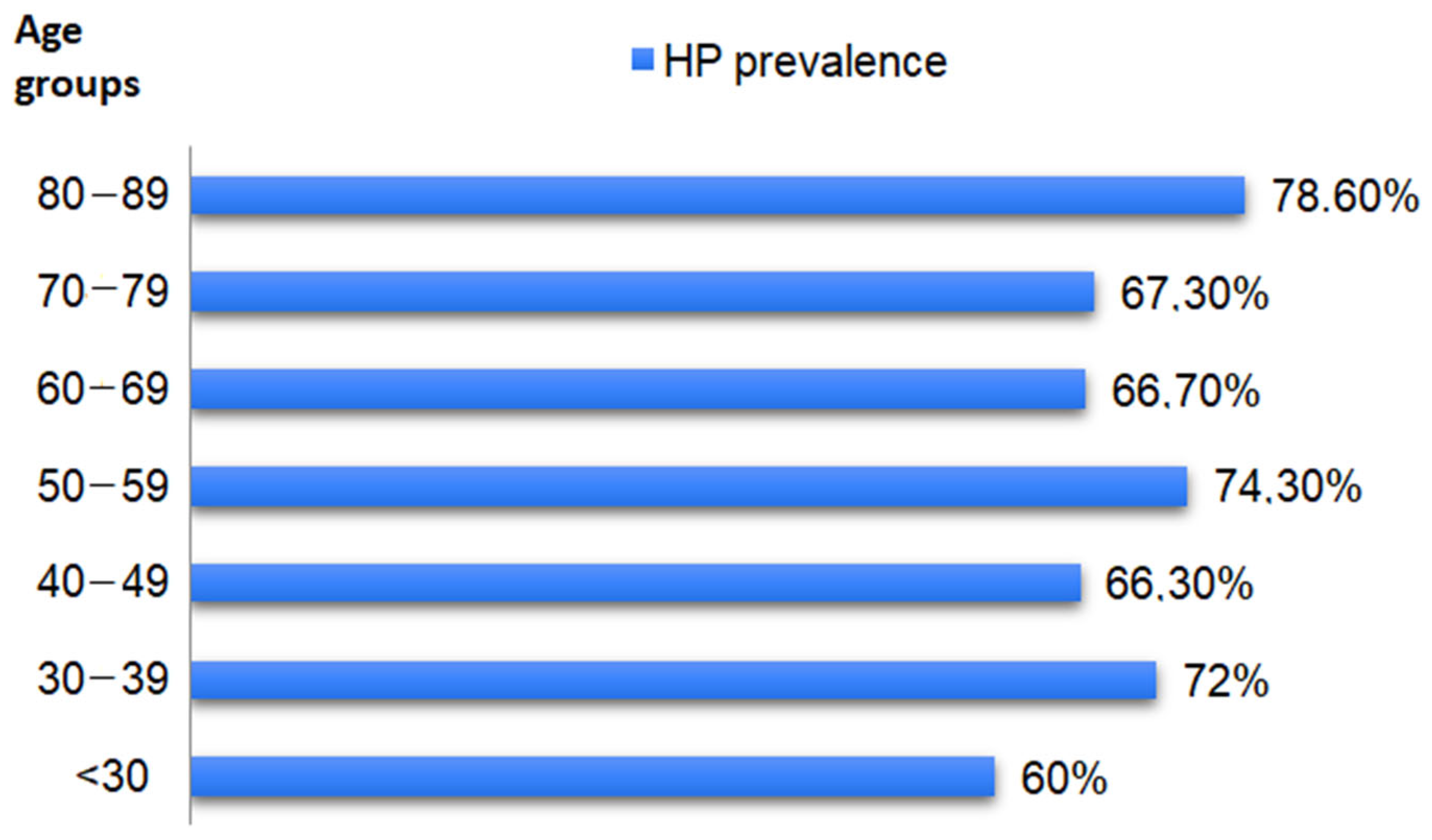
3.2. H. pylori Prevalence
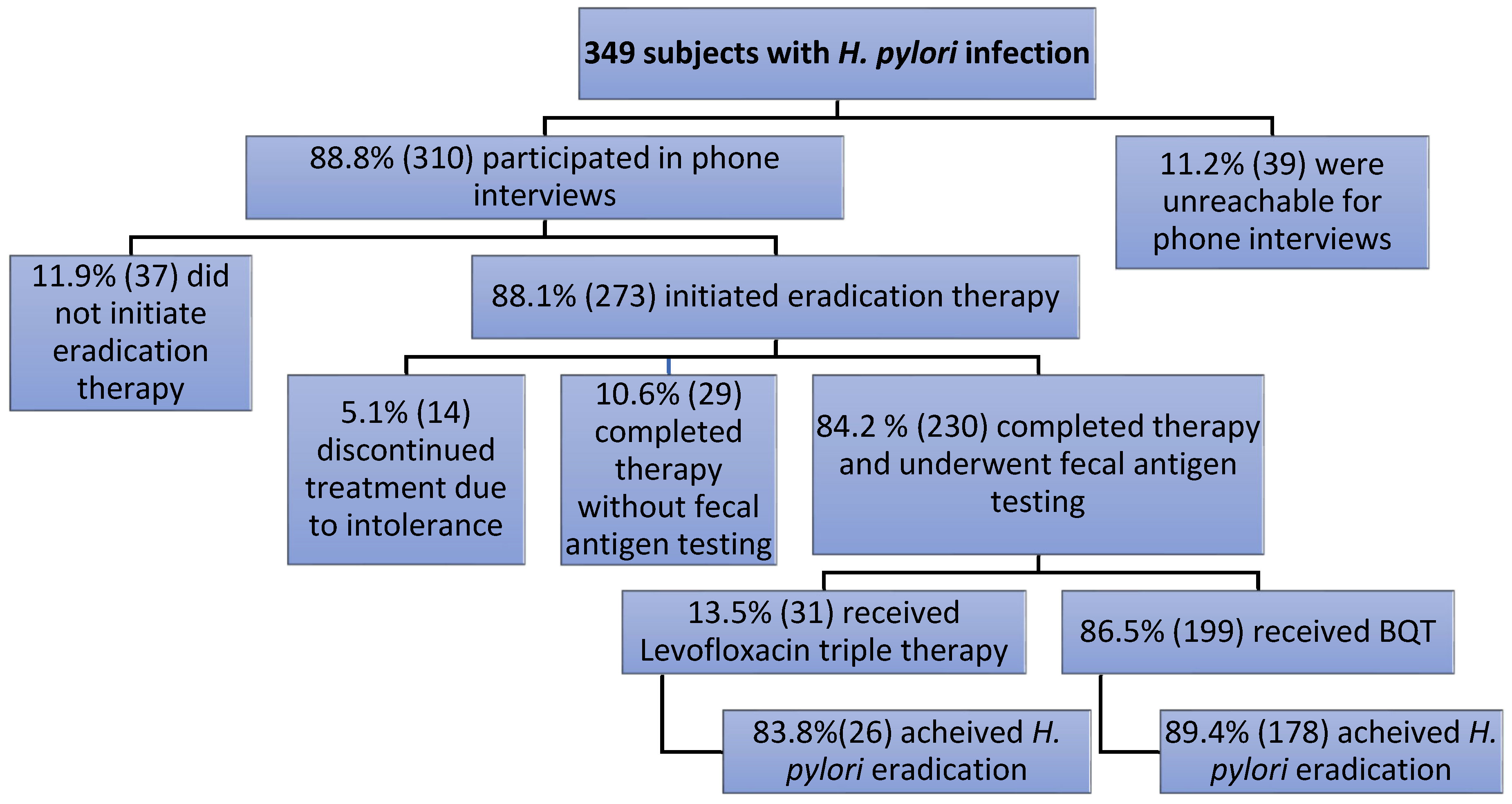
3.3. Follow-Up and Treatment in H. pylori-Positive Subjects
4. Discussion
4.1. Literature Findings
4.2. Study Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Shi, H.; Zhou, F.; Xie, L.; Lin, R. The Efficacy and Safety of Regimens for Helicobacter pylori Eradication Treatment in China A Systemic Review and Network Meta-Analysis. J. Clin. Gastroenterol. 2023, 58, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori Infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Jun, J.S.; Seo, J.H.; Youn, H.S.; Rhee, K.H. Changing Prevalence of Helicobacter pylori Infection in Children and Adolescents. Clin. Exp. Pediatr. 2021, 64, 21–25. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, L.H.; Zagari, R.M.; Bazzoli, F. Epidemiology of Helicobacter pylori Infection. Helicobacter 2014, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Malfertheiner, P.; Yu, H.T.; Kuo, C.L.; Chang, Y.Y.; Meng, F.T.; Wu, Y.X.; Hsiao, J.L.; Chen, M.J.; Lin, K.P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic Review with Meta-Analysis: The Worldwide Prevalence of Helicobacter pylori Infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global Prevalence of Helicobacter pylori Infection between 1980 and 2022: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Adeloye, D.; Luk, T.T.; Huang, L.; He, Y.; Xu, Y.; Ye, X.; Yi, Q.; Song, P.; Rudan, I. The Global Prevalence of and Factors Associated with Helicobacter pylori Infection in Children: A Systematic Review and Meta-Analysis. Lancet Child Adolesc. Health 2022, 6, 185–194. [Google Scholar] [CrossRef]
- Sugano, K.; Hiroi, S.; Yamaoka, Y. Prevalence of Helicobacter pylori Infection in Asia: Remembrance of Things Past? Gastroenterology 2018, 154, 257–258. [Google Scholar] [CrossRef]
- Popovici, E.L.; Moscalu, M.; Cojocaru, C.; Muzica, C.; Dimache, M.; Trifan, A.; Preventive Medicine-Laboratory. The Prevalence of Helicobacter pylori Infection in a Hospital from North-East of Romania. Med. Surg. J. 2020, 124, 461–465. [Google Scholar]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori Infection: The Maastricht VI/Florence Consensus Report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Tung, Y.C.; Tu, Y.K.; Yeh, H.Z.; Yang, J.C.; Hsu, P.I.; Kim, S.E.; Wu, M.F.; Liou, W.S.; Shiu, S.I. Efficacy of Second-Line Regimens for Helicobacter Pylori Eradication Treatment: A Systemic Review and Network Meta-Analysis. BMJ Open Gastroenterol. 2020, 7, e000472. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, M.; Annibale, B.; Capoccia, D.; Tari, R.; Lahner, E.; Osborn, J.; Leonetti, F.; Severi, C. The Eradication of Helicobacter Pylori Is Affected by Body Mass Index (BMI). Obes. Surg. 2008, 18, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lv, Y.; Yang, P.; Jiang, Y.; Qin, X.; Wang, X. Alcohol Increases Treatment Failure for Helicobacter pylori Eradication in Asian Populations. BMC Gastroenterol. 2023, 23, 365. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, P.; Qin, X.; Li, C.; Lv, Y.; Wang, X. Impact of Smoking on the Eradication of Helicobacter pylori. Helicobacter 2022, 27, e12860. [Google Scholar] [CrossRef] [PubMed]
- Azab, E.T.; Thabit, A.K.; McKee, S.; Al-Qiraiqiri, A. Levofloxacin versus Clarithromycin for Helicobacter Pylori Eradication: Are 14 Day Regimens Better than 10 Day Regimens? Gut. Pathog. 2022, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, X.; Chen, Q.; Zhang, W.; Lu, H. Inappropriate Treatment in Helicobacter pylori Eradication Failure: A Retrospective Study. Scand. J. Gastroenterol. 2018, 53, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P. Compliance, Adverse Events and Antibiotic Resistance in Helicobacter Pylori Treatment. Scand. J. Gastroenterol. 1993, 28, 34–37. [Google Scholar] [CrossRef]
- Shahbazi, S.; Vahdat Shariatpanahi, Z. Comparison between Daily Single-Dose Triple Therapy and Conventional Triple Therapy on Patient Compliance and Helicobacter pylori Eradication: A Randomized Controlled Trial. Indian J. Gastroenterol. 2018, 37, 550–554. [Google Scholar] [CrossRef]
- Huguet, J.M.; Ferrer-Barceló, L.; Suárez, P.; Barcelo-Cerda, S.; Sempere, J.; Saracino, I.M.; Fiorini, G.; Vaira, D.; Pérez-Aísa, Á.; Jonaitis, L.; et al. Role of Compliance in Helicobacter pylori Eradication Treatment: Results of the European Registry on H. Pylori Management. United Eur. Gastroenterol. J. 2024, 12, 691–704. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhan, B.; Xu, B.; Gao, H. Achieving Helicobacter pylori Eradication in the Primary Treatment Requires a Deep Integration of Personalization and Standardization. Helicobacter 2022, 27, 1. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Zurlo, C.; Fagoonee, S.; Rosso, C.; Armandi, A.; Caviglia, G.P.; Saracco, G.M.; Pellicano, R. A Retrospective Experience of Helicobacter pylori Histology in a Large Sample of Subjects in Northern Italy. Life 2021, 11, 650. [Google Scholar] [CrossRef]
- Acer, Ö.; Özüdoğru, O.; Yülek, Ö. Helicobacter pylori Prevalence in Patients Who Underwent Endoscopy in Our Region. Gevher Nesibe J. 2022, 7, 118–122. [Google Scholar] [CrossRef]
- Sepúlveda, M.; Maldonado, C.; Bravo, J.; Satizábal, N.; Gempeler, A.; Castro, A.; Escobar, J.; Herrera, J.; Rosso, F.; Rojas, N.; et al. Prevalence of Helicobacter pylori in Patients Undergoing Upper Digestive Tract Endoscopy at a Referral Hospital in Cali, Colombia, in 2020. Rev. Colomb. Gastroenterol. 2022, 37, 355–361. [Google Scholar] [CrossRef]
- Rokkas, T.; Gisbert, J.P.; Malfertheiner, P.; Niv, Y.; Gasbarrini, A.; Leja, M.; Megraud, F.; O’Morain, C.; Graham, D.Y. Comparative Effectiveness of Multiple Different First-Line Treatment Regimens for Helicobacter pylori Infection: A Network Meta-Analysis. Gastroenterology 2021, 161, 495–507.e4. [Google Scholar] [CrossRef] [PubMed]
- Costache, C.; Colosi, H.A.; Grad, S.; Paștiu, A.I.; Militaru, M.; Hădărean, A.P.; Țoc, D.A.; Neculicioiu, V.S.; Baciu, A.M.; Opris, R.V.; et al. Antibiotic Resistance in Helicobacter pylori Isolates from Northwestern and Central Romania Detected by Culture-Based and PCR-Based Methods. Antibiotics 2023, 12, 1672. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Borraccino, A.V.; Aloisio, A.; Russo, F.; Riezzo, G.; Galeano, G.; Pricci, M.; Girardi, B.; Celiberto, F.; Iannone, A.; et al. Concomitant and Bismuth Quadruple Therapy for Helicobacter pylori Eradication in Southern Italy: Preliminary Data from a Randomized Clinical Trial. Antibiotics 2024, 13, 348. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Bordin, D.; Tepes, B.; Pérez-Aisa, Á.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; et al. European Registry on Helicobacter pylori Management (Hp-EuReg): Patterns and Trends in First-Line Empirical Eradication Prescription and Outcomes of 5 Years and 21 533 Patients. Gut 2021, 70, 40–54. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Romano, M.; Gravina, A.G.; Solís-Muñoz, P.; Bermejo, F.; Molina-Infante, J.; Castro-Fernández, M.; Ortuño, J.; Lucendo, A.J.; Herranz, M.; et al. Helicobacter pylori Second-Line Rescue Therapy with Levofloxacin- and Bismuth-Containing Quadruple Therapy, after Failure of Standard Triple or Non-Bismuth Quadruple Treatments. Aliment. Pharmacol. Ther. 2015, 41, 768–775. [Google Scholar] [CrossRef]
- Romano, M.; Gravina, A.G.; Nardone, G.; Federico, A.; Dallio, M.; Martorano, M.; Mucherino, C.; Romiti, A.; Avallone, L.; Granata, L.; et al. Non-Bismuth and Bismuth Quadruple Therapies Based on Previous Clarithromycin Exposure Are as Effective and Safe in an Area of High Clarithromycin Resistance: A Real-Life Study. Helicobacter 2020, 25, e12694. [Google Scholar] [CrossRef] [PubMed]
- Castro-Fernández, M.; Romero-García, T.; Keco-Huerga, A.; Pabón-Jaén, M.; Lamas-Rojas, E.; Llorca-Fernández, R.; Grande-Santamaría, L.; Rojas-Feria, M. Compliance, Adverse Effects and Effectiveness of First Line Bismuth-Containing Quadruple Treatment (Pylera®) to Eradicate Helicobacter pylori Infection in 200 Patients. Rev. Esp. Enfermadades Dig. 2019, 111, 467–471. [Google Scholar] [CrossRef]
| Parameter | Subjects n = 507 |
|---|---|
| Mean age (years) | 57.15 ± 13.89 |
| Age subgroups | |
| <30 | 3.9% (20/507) |
| 30–39 | 8.5% (43/507) |
| 40–49 | 16.4% (83/507) |
| 50–59 | 20.7% (105/507) |
| 60–69 | 28.4% (144/507) |
| 70–79 | 19.3% (98/507) |
| 80–88 | 2.8 (14/507) |
| Gender | |
| Males | 191/507 (37.7%) |
| Females | 316/507 (62.3%) |
| Mean BMI (kg/m2) | 28.13 ± 5.59 |
| Place of residence | |
| Urban | 50.5% (256/507) |
| Rural | 49.5% (251/507) |
| Endoscopic findings | |
| Gastritis/Duodenitis | 82.6% (419/507)—342 poz |
| Hiatal hernia | 38.7% (196/507)—144 poz |
| Esophagitis | 10% (51/507)—39 poz |
| Gastric ulcer | 2.8% (14/507)—13 poz |
| Duodenal ulcer | 1% (5/507)—3 poz |
| Small polyps | 2.6% (13/507)—4 poz |
| Barrett’s esophagus | 2.8% (14/507)—4 poz |
| Other lesions | 4% (20/507)—2 poz |
| Normal endoscopy | 2% (10/507)—2 poz |
| No | Endoscopic Findings | Prevalence of HP | ||||
|---|---|---|---|---|---|---|
| I | Gastritis/Duodenitis | 81.6% (342/419) | ||||
| II | Hiatal hernia | 73.5% (144/196) | ||||
| III | Esophagitis | 76.5% (39/51) | ||||
| IV | Gastric ulcer | 92.9% (13/14) | ||||
| V | Duodenal ulcer | 60% (3/5) | ||||
| VI | Small polyps | 30.8% (4/13) | ||||
| VII | Barrett’s esophagus | 28.6% (4/14) | ||||
| VIII | Other lesions | 10% (2/20) | ||||
| IX | Normal endoscopy | 20% (2/10) | ||||
| p value | ||||||
| I/II | I/III | I/IV | I/V | I/VI | I/VII | I/VIII |
| 0.0360 | 0.5004 | 0.4663 | 0.5144 | <0.0001 | <0.0001 | <0.0001 |
| p value | ||||||
| I/IX | II/III | II/IV | II/V | II/VI | II/VII | II/VIII |
| <0.0001 | 0.7977 | 0.1948 | 0.8715 | 0.0030 | 0.0011 | <0.0001 |
| p value | ||||||
| II/IX | III/IV | III/V | III/VI | III/VII | III/VIII | III/IX |
| 0.0011 | 0.3259 | 0.7855 | 0.0051 | 0.0024 | <0.0001 | 0.0019 |
| p value | ||||||
| IV/V | IV/VI | IV/VII | IV/VIII | IV/IX | V/VI | V/VII |
| 0.3086 | 0.0033 | 0.0020 | <0.0001 | 0.0013 | 0.5495 | 0.4781 |
| p value | ||||||
| V/VIII | V/IX | VI/VII | VI/VIII | VI/IX | VII/VIII | VII/IX |
| 0.0608 | 0.3329 | 0.7668 | 0.2930 | 0.9158 | 0.3457 | 0.9987 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serena, P.; Popa, A.; Bende, R.; Miutescu, B.; Mare, R.; Borlea, A.; Aragona, G.; Groza, A.L.; Serena, L.; Popescu, A.; et al. Prevalence of Helicobacter pylori Infection and Efficacy of Bismuth Quadruple and Levofloxacin Triple Eradication Therapies: A Retrospective Analysis. Life 2024, 14, 885. https://doi.org/10.3390/life14070885
Serena P, Popa A, Bende R, Miutescu B, Mare R, Borlea A, Aragona G, Groza AL, Serena L, Popescu A, et al. Prevalence of Helicobacter pylori Infection and Efficacy of Bismuth Quadruple and Levofloxacin Triple Eradication Therapies: A Retrospective Analysis. Life. 2024; 14(7):885. https://doi.org/10.3390/life14070885
Chicago/Turabian StyleSerena, Patricia, Alexandru Popa, Renata Bende, Bogdan Miutescu, Ruxandra Mare, Andreea Borlea, Giovanni Aragona, Andrei Lucian Groza, Luca Serena, Alina Popescu, and et al. 2024. "Prevalence of Helicobacter pylori Infection and Efficacy of Bismuth Quadruple and Levofloxacin Triple Eradication Therapies: A Retrospective Analysis" Life 14, no. 7: 885. https://doi.org/10.3390/life14070885







