Acute and Preventive Treatment of COVID-19-Related Headache: A Series of 100 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Outcomes
2.2. Eligibility Criteria
2.3. Recruitment, Sampling, and Sample Size
2.4. Study Period
2.5. Intervention
2.6. Acute Medication
2.7. Preventive Medication
2.8. Ethics
2.9. Statistical Analysis
3. Results
3.1. COVID-19 Infection
3.2. COVID-19-Related Headache
3.3. Acute Medication
3.4. COVID-19 Infection
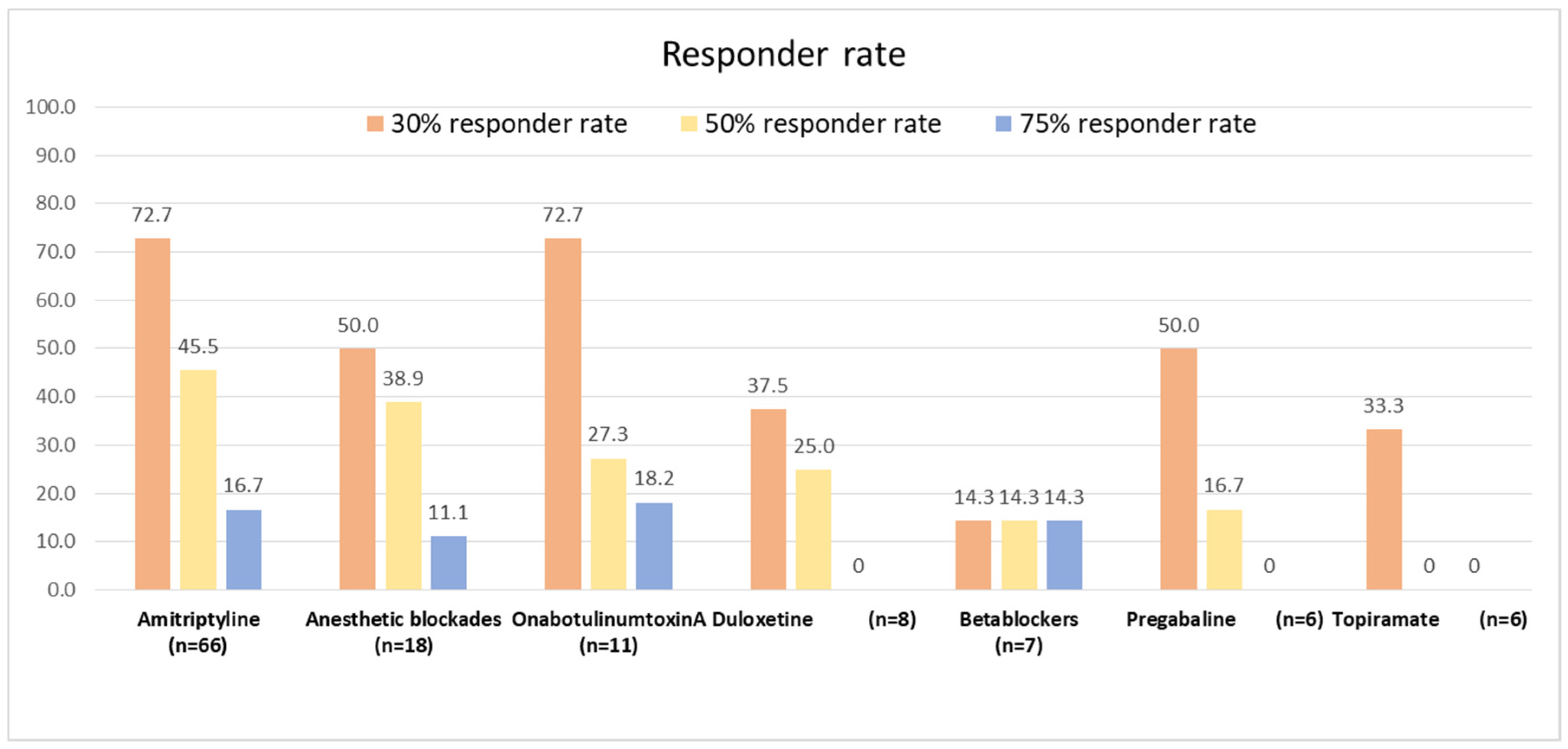
3.5. Responder Rate
3.6. Tolerability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Severity | Description |
|---|---|
| Mild illness | Patients with uncomplicated upper respiratory tract viral infection symptoms and non-specific symptoms such as fever, fatigue, cough (with or without sputum production), anorexia, malaise, muscle pain, sore throat, dyspnea, nasal congestion, diarrhea, nausea, or vomiting. |
| Pneumonia | Presence of pneumonia but no signs of severe pneumonia and no need for supplemental oxygen. CURB scale ≤ 1. |
| Severe pneumonia | Confirmed respiratory infection, plus one of the following: Respiratory rate > 30 breaths/min. Severe respiratory distress. SpO2 ≤ 93% on room air. |
| Acute respiratory distress syndrome (ARDS)2 | Onset: within 1 week of a known clinical insult or new or worsening respiratory symptoms. Chest imaging (radiograph, CT scan, or lung ultrasound): bilateral opacities, not fully explained by volume overload, lobar or lung collapse, or nodules. Origin of pulmonary infiltrates: respiratory failure not fully explained by cardiac failure or fluid overload. Need objective assessment (e.g., echocardiography) to exclude hydrostatic causes of infiltrates/edema if no risk factor present. Oxygenation impairment in adults: Mild ARDS: 200 mmHg < PaO2/FiO2a ≤ 300 mmHg (with PEEP or CPAP ≥ 5 cmH2O, or non-ventilated) Moderate ARDS: 100 mmHg < PaO2/FiO2 ≤ 200 mmHg (with PEEP ≥ 5 cmH2O, or non-ventilated) Severe ARDS: PaO2/FiO2 ≤ 100 mmHg (with PEEP ≥ 5 cmH2O, or non-ventilated) When PaO2 is not available, SpO2/FiO2 ≤ 315 implies ARDS (including in non-ventilated patients). |
References
- Fernández-de-Las-Peñas, C.; Navarro-Santana, M.; Gómez-Mayordomo, V.; Cuadrado, M.L.; García-Azorín, D.; Arendt-Nielsen, L.; Plaza-Manzano, G. Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: A meta-analysis of the current literature. Eur. J. Neurol. 2021, 28, 3820–3825. [Google Scholar] [CrossRef] [PubMed]
- García-Azorín, D.; Sierra, Á.; Trigo, J.; Alberdi, A.; Blanco, M.; Calcerrada, I.; Cornejo, A.; Cubero, M.; Gil, A.; García-Iglesias, C.; et al. Frequency and phenotype of headache in COVID-19: A study of 2194 patients. Sci. Rep. 2021, 11, 14674. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.; García-Azorín, D.; Planchuelo-Gómez, Á.; Martínez-Pías, E.; Talavera, B.; Hernández-Pérez, I.; Valle-Peñacoba, G.; Simón-Campo, P.; de Lera, M.; Chavarría-Miranda, A.; et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: A retrospective cohort study. J. Headache Pain 2020, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Azorin, D.; Layos-Romero, A.; Porta-Etessam, J.; Membrilla, J.A.; Caronna, E.; Gonzalez-Martinez, A.; Mencia, Á.S.; Segura, T.; Gonzalez-García, N.; Díaz-de-Terán, J.; et al. Post-COVID-19 persistent headache: A multicentric 9-months follow-up study of 905 patients. Cephalalgia 2022, 42, 804–809. [Google Scholar] [CrossRef]
- López, J.T.; García-Azorín, D.; Planchuelo-Gómez, Á.; García-Iglesias, C.; Dueñas-Gutiérrez, C.; Guerrero, Á.L. Phenotypic characterization of acute headache attributed to SARS-CoV-2: An ICHD-3 validation study on 106 hospitalized patients. Cephalalgia 2020, 40, 1432–1442. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Services and Systems, Communicable Diseases, Technical Advisory Group on SARS-CoV-2 Virus Evolution. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 17 May 2024).
- Sampaio Rocha-Filho, P.A.; Albuquerque, P.M.; Carvalho, L.C.L.S.; Dandara Pereira Gama, M.; Magalhães, J.E. Headache, anosmia, ageusia and other neurological symptoms in COVID-19: A cross-sectional study. J. Headache Pain 2022, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Camargo-Martínez, W.; Lozada-Martínez, I.; Escobar-Collazos, A.; Navarro-Coronado, A.; Moscote-Salazar, L.; Pacheco-Hernández, A.; Janjua, T.; Bosque-Varela, P. Post-COVID 19 neurological syndrome: Implications for sequelae’s treatment. J. Clin. Neurosci. 2021, 88, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Caronna, E.; Ballvé, A.; Llauradó, A.; Gallardo, V.J.; Ariton, D.M.; Lallana, S.; López Maza, S.; Olivé Gadea, M.; Quibus, L.; Restrepo, J.L.; et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia 2020, 40, 1410–1421. [Google Scholar] [CrossRef] [PubMed]
- Membrilla, J.A.; de Lorenzo, Í.; Sastre, M.; Díaz de Terán, J. Headache as a Cardinal Symptom of Coronavirus Disease 2019: A Cross-Sectional Study. Headache 2020, 60, 2176–2191. [Google Scholar] [CrossRef]
- Uygun, Ö.; Ertaş, M.; Ekizoğlu, E.; Bolay, H.; Özge, A.; Kocasoy Orhan, E.; Çağatay, A.A.; Baykan, B. Headache characteristics in COVID-19 pandemic-a survey study. J. Headache Pain 2020, 21, 121. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, A.; Fanjul, V.; Ramos, C.; Serrano Ballesteros, J.; Bustamante, M.; Villa Martí, A.; Álvarez, C.; García Del Álamo, Y.; Vivancos, J.; Gago-Veiga, A.B. Headache during SARS-CoV-2 infection as an early symptom associated with a more benign course of disease: A case-control study. Eur. J. Neurol. 2021, 28, 3426–3436. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Caronna, E.; Alpuente, A.; Torres-Ferrus, M.; Pozo-Rosich, P. Toward a better understanding of persistent headache after mild COVID-19: Three migraine-like yet distinct scenarios. Headache 2021, 61, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Dono, F.; Consoli, S.; Evangelista, G.; D’Apolito, M.; Russo, M.; Carrarini, C.; Calisi, D.; De Rosa, M.; Di Pietro, M.; De Angelis, M.V.; et al. New daily persistent headache after SARS-CoV-2 infection: A report of two cases. Neurol. Sci. 2021, 42, 3965–3968. [Google Scholar] [CrossRef]
- Tana, C.; Bentivegna, E.; Cho, S.J.; Harriott, A.M.; García-Azorín, D.; Labastida-Ramirez, A.; Ornello, R.; Raffaelli, B.; Beltrán, E.R.; Ruscheweyh, R.; et al. Long COVID headache. J. Headache Pain 2022, 23, 93. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Tassorelli, C.; Diener, H.C.; Dodick, D.W.; Silberstein, S.D.; Lipton, R.B.; Ashina, M.; Becker, W.J.; Ferrari, M.D.; Goadsby, P.J.; Pozo-Rosich, P.; et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia 2018, 38, 815–832. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Tassorelli, C.; Dodick, D.W.; Silberstein, S.D.; Lipton, R.B.; Ashina, M.; Becker, W.J.; Ferrari, M.D.; Goadsby, P.J.; Pozo-Rosich, P.; et al. Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: Fourth edition. Cephalalgia. 2019, 39, 687–710. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martínez, A.; Guerrero Peral, A.L.; Arias, S.; Silva, L.; Sierra-Mencía, A.; Gago-Veiga, A.B.; García-Azorín, D. Amitriptyline for post-COVID-19 headache. Effectiveness, tolerability, and response predictors. J. Neurol. 2022, 269, 5702–5709. [Google Scholar] [CrossRef]
- Stovner, L.J.; Hagen, K.; Linde, M.; Steiner, T.J. The global prevalence of headache: An update, with analysis of the influences of methodological factors on prevalence estimates. J. Headache Pain 2022, 23, 34. [Google Scholar] [CrossRef]
- Matías-Guiu, J.; Porta-Etessam, J.; Mateos, V.; Díaz-Insa, S.; Lopez-Gil, A.; Fernández, C.; Scientific Committee of the PALM Program. One-year prevalence of migraine in Spain: A nationwide population-based survey. Cephalalgia 2011, 31, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Arca, K.N.; Smith, J.H.; Chiang, C.C.; Starling, A.J.; Robertson, C.E.; Halker Singh, R.B.; Schwedt, T.J.; Kissoon, N.R.; Garza, I.; Rozen, T.D.; et al. COVID-19 and Headache Medicine: A Narrative Review of Non-Steroidal Anti-Inflammatory Drug (NSAID) and Corticosteroid Use. Headache 2020, 60, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.; Bosco-Levy, P.; Thurin, N.; Blin, P.; Droz-Perroteau, C. NSAIDs and COVID-19: A Systematic Review and Meta-analysis. Drug Saf. 2021, 44, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, L.; Evers, S.; Linde, M.; Mitsikostas, D.D.; Sandrini, G.; Schoenen, J. EFNS guideline on the treatment of tension-type headache—Report of an EFNS task force. Eur. J. Neurol. 2010, 17, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Burch, R. Preventive Migraine Treatment. Continuum 2021, 27, 613–632. [Google Scholar] [CrossRef]
- Everitt, H.; Baldwin, D.S.; Stuart, B.; Lipinska, G.; Mayers, A.; Malizia, A.L.; Manson, C.C.; Wilson, S. Antidepressants for insomnia in adults. Cochrane Database Syst. Rev. 2018, CD010753. [Google Scholar] [CrossRef] [PubMed]
- van den Driest, J.J.; Bierma-Zeinstra, S.M.A.; Bindels, P.J.E.; Schiphof, D. Amitriptyline for musculoskeletal complaints: A systematic review. Fam. Pract. 2017, 34, 138–146. [Google Scholar] [CrossRef]
- Moore, R.A.; Derry, S.; Aldington, D.; Cole, P.; Wiffen, P.J. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015, CD008242. [Google Scholar] [CrossRef]
- Guaiana, G.; Barbui, C.; Hotopf, M. Amitriptyline for depression. Cochrane Database Syst. Rev. 2007, CD004186. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Martini Ferreira, A.; Ribeiro, R.T.; Zukerman, E.; Cipolla-Neto, J.; Peres, M.F. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1127–1132. [Google Scholar] [CrossRef]
- Dodick, D.W.; Freitag, F.; Banks, J.; Saper, J.; Xiang, J.; Rupnow, M.; Biondi, D.; Greenberg, S.J.; Hulihan, J.; CAPSS-277 Investigator Group. Topiramate versus amitriptyline in migraine prevention: A 26-week, multicenter, randomized, double-blind, double-dummy, parallel-group noninferiority trial in adult migraineurs. Clin. Ther. 2009, 31, 542–559. [Google Scholar] [CrossRef]
- Jackson, J.L.; Cogbill, E.; Santana-Davila, R.; Eldredge, C.; Collier, W.; Gradall, A.; Sehgal, N.; Kuester, J. A Comparative Effectiveness Meta-Analysis of Drugs for the Prophylaxis of Migraine Headache. PLoS ONE 2015, 10, e0130733. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Shah, N.D. Post-infectious new daily persistent headache may respond to intravenous methylprednisolone. J. Headache Pain 2010, 11, 59–66. [Google Scholar] [CrossRef]
- Karadaş, Ö.; Gül, H.L.; Öztürk, B.; Sonkaya, A.R.; Özön, A.Ö.; Shafiyev, J.; Sir, E. Greater occipital nerve block efficacy in COVID 19 associated headache: A preliminary study. Acta Neurobiol. Exp. 2021, 81, 386–392. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Vostanis, P.; Bell, C.A. Counselling and psychotherapy post-COVID-19. Couns. Psychother. Res. 2020, 20, 389–393. [Google Scholar] [CrossRef]
- Beghi, E.; Giussani, G.; Westenberg, E.; Allegri, R.; Garcia-Azorin, D.; Guekht, A.; Frontera, J.; Kivipelto, M.; Mangialasche, F.; Mukaetova-Ladinska, E.B.; et al. Acute and post-acute neurological manifestations of COVID-19: Present findings, critical appraisal, and future directions. J. Neurol. 2022, 269, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef]
- Crivelli, L.; Palmer, K.; Calandri, I.; Guekht, A.; Beghi, E.; Carroll, W.; Frontera, J.; García-Azorín, D.; Westenberg, E.; Winkler, A.S.; et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimer’s Dement. 2022, 18, 1047–1066. [Google Scholar] [CrossRef]
- Cacciatore, M.; Raggi, A.; Pilotto, A.; Cristillo, V.; Guastafierro, E.; Toppo, C.; Magnani, F.G.; Sattin, D.; Mariniello, A.; Silvaggi, F.; et al. Neurological and Mental Health Symptoms Associated with Post-COVID-19 Disability in a Sample of Patients Discharged from a COVID-19 Ward: A Secondary Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4242. [Google Scholar] [CrossRef]
- Torrente, A.; Alonge, P.; Di Stefano, V.; Baschi, R.; Ornello, R.; Correnti, E.; Lupica, A.; Camarda, C.; Farinella, G.; Raieli, V.; et al. New-onset headache following COVID-19: An Italian multicentre case series. J. Neurol. Sci. 2023, 446, 120591. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Nessi, F.S.; Ascanio, L.C.; Pineda-Arapé, A.G.; Omaña-Ávila, Ó.D.; Mendoza-Millán, D.L.; Romero, S.R.; Almao-Rivero, A.B.; Camejo-Ávila, N.A.; Gebran-Chedid, K.J.; Rodriguez-Saavedra, C.M.; et al. New daily persistent headache after SARS-CoV-2 infection in Latin America: A cross-sectional study. BMC Infect Dis. 2023, 23, 877. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia: An official clinical practice guideline of the American Thoracic Society and Infectious Disease Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Taylor Thompson, B.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [PubMed]


| Variable | Proportion (n = 100) |
|---|---|
| Headache location | |
| Strictly holocranial | 63% |
| Strictly hemicranial | 15% |
| Both holocranial and hemicranial | 22% |
| Headache topography | |
| Frontal | 48% |
| Occipital | 36% |
| Temporal | 27% |
| Periocular | 21% |
| Parietal | 15% |
| Vertex | 9% |
| Cervical | 5% |
| Facial | 4% |
| Quality of pain | |
| Pressing | 75% |
| Throbbing | 27% |
| Stabbing | 27% |
| Associated symptoms | |
| Photophobia | 58% |
| Phonophobia | 47% |
| Osmophobia | 10% |
| Cranial autonomic symptoms | 7% |
| Nausea | 36% |
| Vomiting | 7% |
| Worsening by physical activity | 46% |
| Drug n, % | Not Tolerated | No Response (0–30%) | Partial Response (31–50%) | Standard Response (50–75%) | Optimal Response (>75%) |
|---|---|---|---|---|---|
| Amitriptyline (n = 66) | 2 (3.0%) | 16 (24.2%) | 18 (27.3%) | 19 (28.8%) | 11 (16.7%) |
| Anesthetic blockade (n = 18) | 1 (5.6%) | 8 (44.4%) | 2 (11.1%) | 5 (27.8%) | 2 (11.1%) |
| OnabotulinumtoxinA (n = 11) | 0 (0%) | 3 (27.3%) | 5 (45.4%) | 1 (9.1%) | 2 (18.2%) |
| Duloxetine (n = 8) | 1 (12.5%) | 4 (50%) | 1 (12.5%) | 2 (25%) | 0 (0%) |
| Betablockers (n = 7) | 1 (14.3%) | 5 (71.4%) | 0 (0%) | 0 (05) | 1 (14.3%) |
| Pregabaline (n = 6) | 0 (0%) | 3 (50%) | 2 (33.3%) | 1 (16.7%) | 0 (0%) |
| Topiramate (n = 6) | 1 (16.7%) | 3 (50%) | 2 (33.3%) | 0 (0%) | 0 (0%) |
| Mirtazapine (n = 5) | 0 (0%) | 2 (40%) | 1 (20%) | 1 (20%) | 1 (20%) |
| Flunarizine (n = 5) | 1 (20%) | 1 (20%) | 2 (40%) | 0 (0%) | 1 (20%) |
| Steroids (n = 4) | 1 (25%) | 1 (25%) | 1 (25%) | 1 (25%) | 0 (0%) |
| Venlafaxine (n = 4) | 1 (25%) | 2 (50%) | 1 (25%) | 0 (0%) | 0 (0%) |
| Gabapentine (n = 1) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) |
| Zonisamide (n = 1) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) |
| Candesartan (n = 1) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Azorín, D.; García-Ruiz, C.; Sierra-Mencía, Á.; González-Osorio, Y.; Recio-García, A.; González-Celestino, A.; García-Iglesias, C.; Planchuelo-Gómez, Á.; Íñiguez, A.E.; Guerrero-Peral, Á.L. Acute and Preventive Treatment of COVID-19-Related Headache: A Series of 100 Patients. Life 2024, 14, 910. https://doi.org/10.3390/life14070910
García-Azorín D, García-Ruiz C, Sierra-Mencía Á, González-Osorio Y, Recio-García A, González-Celestino A, García-Iglesias C, Planchuelo-Gómez Á, Íñiguez AE, Guerrero-Peral ÁL. Acute and Preventive Treatment of COVID-19-Related Headache: A Series of 100 Patients. Life. 2024; 14(7):910. https://doi.org/10.3390/life14070910
Chicago/Turabian StyleGarcía-Azorín, David, Claudia García-Ruiz, Álvaro Sierra-Mencía, Yésica González-Osorio, Andrea Recio-García, Ana González-Celestino, Cristina García-Iglesias, Álvaro Planchuelo-Gómez, Ana Echavarría Íñiguez, and Ángel L. Guerrero-Peral. 2024. "Acute and Preventive Treatment of COVID-19-Related Headache: A Series of 100 Patients" Life 14, no. 7: 910. https://doi.org/10.3390/life14070910
APA StyleGarcía-Azorín, D., García-Ruiz, C., Sierra-Mencía, Á., González-Osorio, Y., Recio-García, A., González-Celestino, A., García-Iglesias, C., Planchuelo-Gómez, Á., Íñiguez, A. E., & Guerrero-Peral, Á. L. (2024). Acute and Preventive Treatment of COVID-19-Related Headache: A Series of 100 Patients. Life, 14(7), 910. https://doi.org/10.3390/life14070910







