Duloxetine-Induced Antidiuresis in Rats with Lithium-Induced Nephrogenic Diabetes Insipidus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Immunoblot Analysis
2.3. Immunohistochemistry
2.4. Quantitative Polymerase Chain Reaction (qPCR)
2.5. Cyclic Adenosine Monophosphate (cAMP) and Prostaglandin E2 (PGE2) Measurement from Rat Kidneys
2.6. Statistics
3. Results
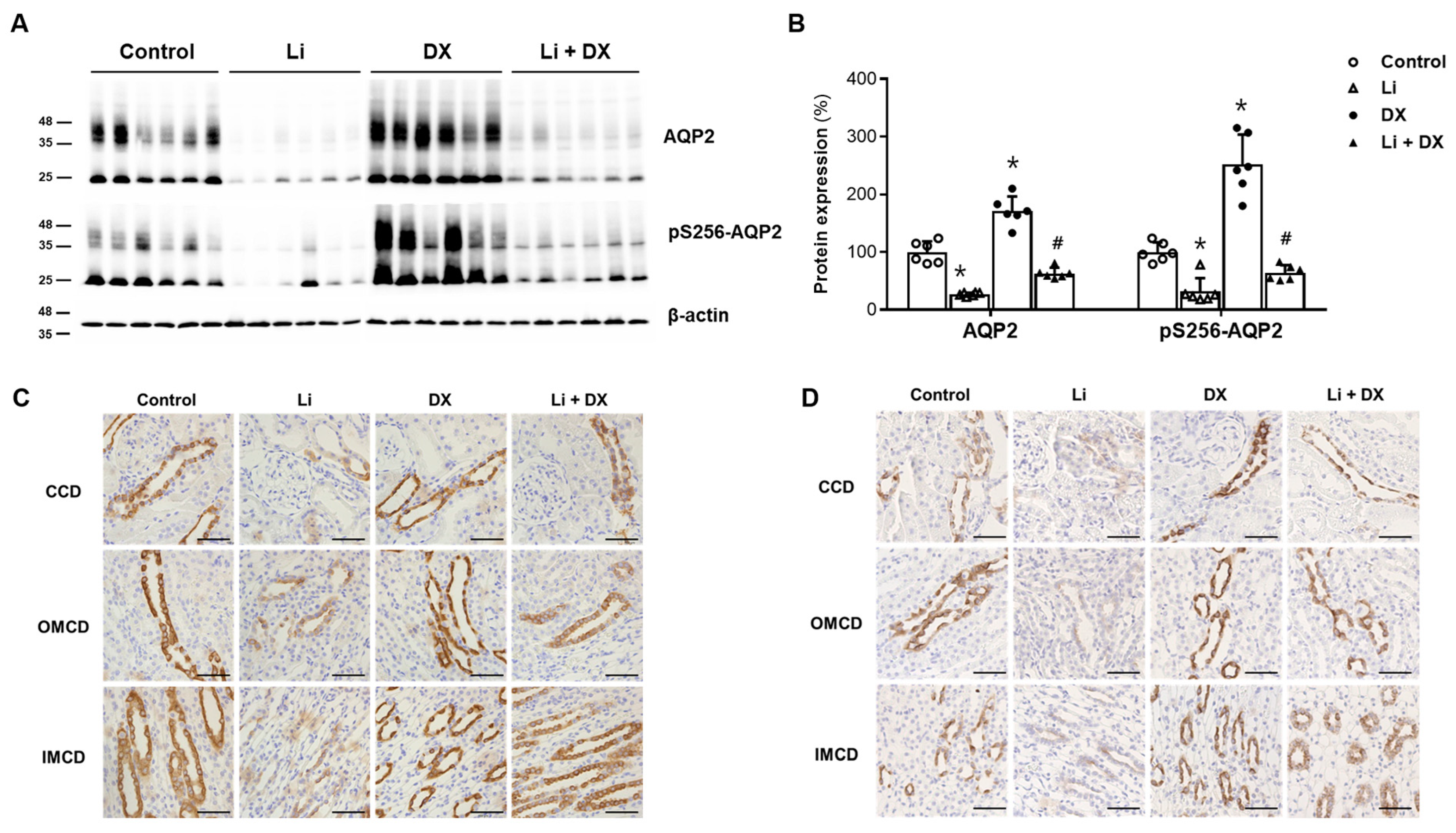
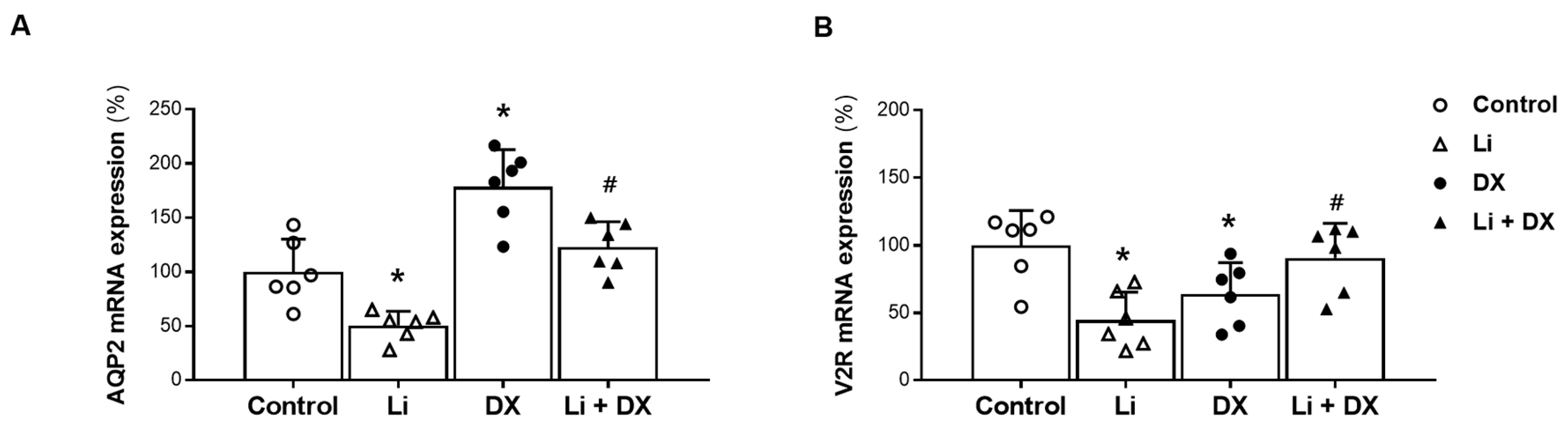
3.1. Animal Experiment 1: Effects of Duloxetine Treatment in Li-NDI
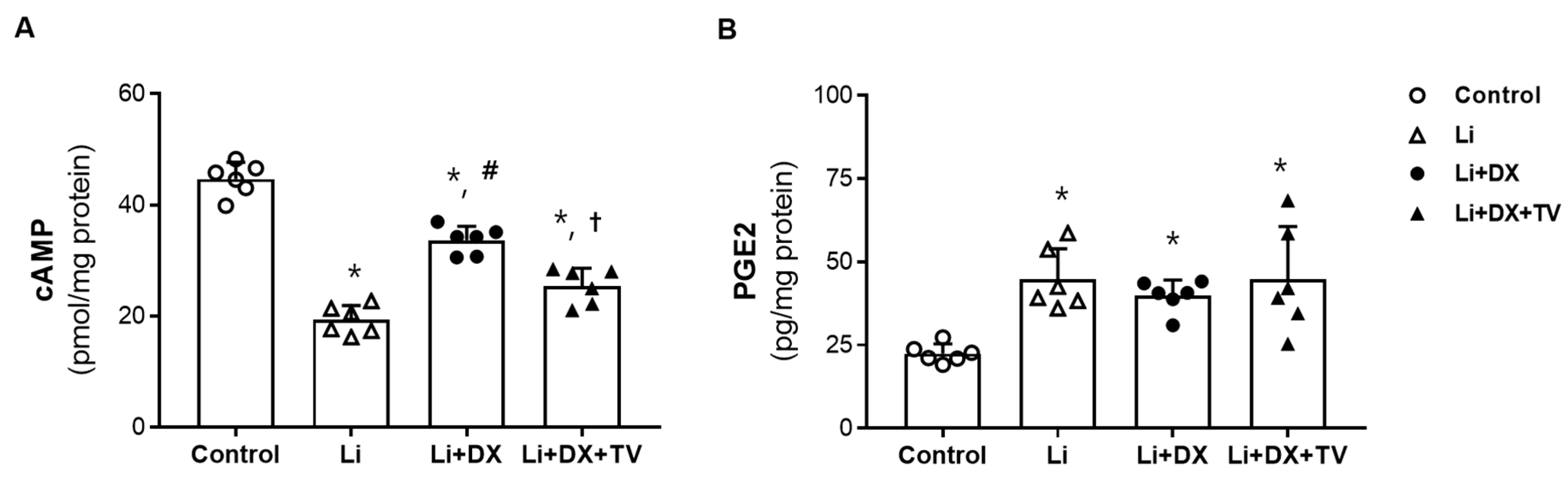
3.2. Animal Experiment 2: Reversal of Duloxetine Effects by Tolvaptan Co-Treatment in Li-NDI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seifert, J.; Letmaier, M.; Greiner, T.; Schneider, M.; Deest, M.; Eberlein, C.K.; Bleich, S.; Grohmann, R.; Toto, S. Psychotropic drug-induced hyponatremia: Results from a drug surveillance program-an update. J. Neural. Transm. 2021, 128, 1249–1264. [Google Scholar] [CrossRef]
- Moyses, Z.P.; Nakandakari, F.K.; Magaldi, A.J. Fluoxetine effect on kidney water reabsorption. Nephrol. Dial. Transplant. 2008, 23, 1173–1178. [Google Scholar] [CrossRef]
- Kim, S.; Jo, C.H.; Kim, G.-H. Psychotropic drugs upregulate aquaporin-2 via vasopressin-2 receptor/cAMP/protein kinase A signaling in inner medullary collecting duct cells. Am. J. Physiol.-Ren. Physiol. 2021, 320, F963–F971. [Google Scholar] [CrossRef]
- Shelton, R.C. Serotonin and norepinephrine reuptake inhibitors. In Antidepressants. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 250, pp. 145–180. [Google Scholar] [CrossRef]
- Amoako, A.O.; Brown, C.; Riley, T. Syndrome of inappropriate antidiuretic hormone secretion: A story of duloxetine-induced hyponatraemia. BMJ Case Rep. 2015, 2015, bcr2014208037. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Matsuki, K.; Kondou, S.; Furukawa, S.; Onji, M. Duloxetine-induced syndrome of inappropriate secretion of antidiuretic hormone in a super-elderly patient. Intern. Med. 2022, 61, 1099–1103. [Google Scholar] [CrossRef]
- Yoshida, K.; Aburakawa, Y.; Suzuki, Y.; Kuroda, K.; Kimura, T. Acute hyponatremia resulting from duloxetine-induced syndrome of inappropriate antidiuretic hormone secretion. Intern. Med. 2019, 58, 1939–1942. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Yasui-Furukori, N.; Oda, Y.; Yang, M.; Suzuki, Y.; Shinozaki, M.; Shimizu, T.; Shimoda, K. Asymptomatic syndrome of inappropriate secretion of antidiuretic hormone (SIADH) following duloxetine treatment for pain with depression: Two case reports. Neuropsychopharmacol. Rep. 2022, 42, 387–390. [Google Scholar] [CrossRef]
- Kim, G.-H. Pathophysiology of drug-induced hyponatremia. J. Clin. Med. 2022, 11, 5810. [Google Scholar] [CrossRef] [PubMed]
- Liamis, G.; Megapanou, E.; Elisaf, M.; Milionis, H. Hyponatremia-inducing drugs. Front. Horm. Res. 2019, 52, 167–177. [Google Scholar] [CrossRef]
- Sands, J.M.; Klein, J.D. Physiological insights into novel therapies for nephrogenic diabetes insipidus. Am. J. Physiol.-Ren. Physiol. 2016, 311, F1149–F1152. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research—Accessdata.Fda.Gov. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/021427_s000_Cymbalta_Pharmr_P2.pdf (accessed on 30 July 2024).
- Miyazaki, T.; Yamamura, Y.; Onogawa, T.; Nakamura, S.; Kinoshita, S.; Nakayama, S.; Fujiki, H.; Mori, T. Therapeutic effects of tolvaptan, a potent, selective nonpeptide vasopressin V2 receptor antagonist, in rats with acute and chronic severe hyponatremia. Endocrinology 2005, 146, 3037–3043. [Google Scholar] [CrossRef] [PubMed]
- El-Shabrawy, M.; Mishriki, A.; Attia, H.; Emad Aboulhoda, B.; Emam, M.; Wanas, H. Protective effect of tolvaptan against cyclophosphamide-induced nephrotoxicity in rat models. Pharmacol. Res. Perspect. 2020, 8, e00659. [Google Scholar] [CrossRef]
- Tanabe, N.; Takami, T.; Fujisawa, K.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. Effectiveness of tolvaptan monotherapy and low-dose furosemide/tolvaptan combination therapy for hepatoprotection and diuresis in a rat cirrhotic model. J. Clin. Biochem. Nutr. 2017, 61, 53–59. [Google Scholar] [CrossRef]
- Klein, J.D.; Khanna, I.; Pillarisetti, R.; Hagan, R.A.; LaRocque, L.M.; Rodriguez, E.L.; Sands, J.M. An AMPK activator as a therapeutic option for congenital nephrogenic diabetes insipidus. JCI Insight 2021, 6, e146419. [Google Scholar] [CrossRef]
- Han, S.W.; Yi, J.H.; Kang, K.P.; Kim, H.Y.; Kim, S.W.; Choi, H.Y.; Ha, S.K.; Kim, G.-H.; Kim, Y.W.; Jeong, K.H.; et al. Safety and efficacy of tolvaptan in Korean patients with hyponatremia caused by the syndrome of inappropriate antidiuretic hormone. J. Korean Med. Sci. 2018, 33, e112. [Google Scholar] [CrossRef]
- Muta, T.; Takasugi, M.; Kuroiwa, A. Chlorpropamide alters AVP-receptor binding of rat renal tubular membranes. Eur. J. Pharmacol. 1989, 159, 191–194. [Google Scholar] [CrossRef]
- Hensen, J.; Haenelt, M.; Gross, P. Water retention after oral chlorpropamide is associated with an increase in renal papillary arginine vasopressin receptors. Eur. J. Endocrinol. 1995, 132, 459–464. [Google Scholar] [CrossRef]
- Kim, S.; Choi, H.J.; Jo, C.H.; Park, J.S.; Kwon, T.H.; Kim, G.-H. Cyclophosphamide-induced vasopressin-independent activation of aquaporin-2 in the rat kidney. Am. J. Physiol.-Ren. Physiol. 2015, 309, F474–F483. [Google Scholar] [CrossRef] [PubMed]
- Glavaš, M.; Gitlin-Domagalska, A.; Dębowski, D.; Ptaszyńska, N.; Łęgowska, A.; Rolka, K. Vasopressin and its analogues: From natural hormones to multitasking peptides. Int. J. Mol. Sci. 2022, 23, 3068. [Google Scholar] [CrossRef] [PubMed]
- Olesen, E.T.; Fenton, R.A. Is there a role for PGE2 in urinary concentration? J. Am. Soc. Nephrol. 2013, 24, 169–178. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Y.; Zheng, F.; Guan, Y.; Zhang, X. Prostaglandin E2 in the regulation of water transport in renal collecting ducts. Int. J. Mol. Sci. 2017, 18, 2539. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Hashimoto, K.; Ota, Z. Involvement of prostaglandin E2, cAMP, and vasopressin in lithium-induced polyuria. Am. J. Physiol. 1988, 254, R863–R869. [Google Scholar] [CrossRef]
- Walker, R.J.; Weggery, S.; Bedford, J.J.; McDonald, F.J.; Ellis, G.; Leader, J.P. Lithium-induced reduction in urinary concentrating ability and urinary aquaporin 2 (AQP2) excretion in healthy volunteers. Kidney Int. 2005, 67, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Olesen, E.T.; Rützler, M.R.; Moeller, H.B.; Praetorius, H.A.; Fenton, R.A. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc. Natl. Acad. Sci. USA 2011, 108, 12949–12954. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward (F) and Reverse (R) Primer Sequences | PCR Product (bp) | GenBank Accession No. |
|---|---|---|---|
| AQP2 | F 5′-CATGTCTCCTTCCTTCGAGCTG-3′ R 5′-CCCCACGGATTTCTACTGGAGT-3′ | 125 | NM_012909.2 |
| V2R | F 5′-GCTCTTCATCTTTGCTCAGCGT-3′ R 5′-TCCAGGTGACATAGGCACGAA-3′ | 110 | NM_019136 |
| Parameter | Control (n = 6) | Li (n = 6) | DX (n = 6) | Li + DX (n = 6) |
|---|---|---|---|---|
| Body weight (g) | 316 ± 13 | 261 ± 6 * | 299 ± 7 * | 266 ± 13 |
| Urine output (mL/day/100 g BW) | 4.9 ± 0.8 | 56.5 ± 7.8 * | 3.8 ± 0.4 * | 28.7 ± 6.4 # |
| Urine osmolality (mOsm/kg H2O) | 1918 ± 170 | 227 ± 38 * | 2181 ± 148 * | 336 ± 95 # |
| Urine sodium (mmol/day/100 g BW) | 0.89 ± 0.14 | 1.37 ± 0.20 * | 0.84 ± 0.12 | 0.90 ± 0.20 # |
| Urine creatinine (mg/day/100 g BW) | 3.38 ± 0.80 | 4.64 ± 0.90 * | 3.13 ± 0.36 | 3.03 ± 0.63 # |
| Plasma osmolality (mOsm/kg H2O) | 307 ± 10 | 314 ± 9 | 308 ± 6 | 308 ± 3 |
| Plasma sodium (mmol/L) | 141 ± 3 | 143 ± 2 | 143 ± 3 | 144 ± 2 |
| Plasma creatinine (mg/dL) | 0.31 ± 0.03 | 0.35 ± 0.05 | 0.34 ± 0.05 | 0.33 ± 0.03 |
| Parameter | Control (n = 6) | Li (n = 6) | Li + DX (n = 6) | Li + DX + TV (n = 6) |
|---|---|---|---|---|
| Body weight (g) | 344 ± 13 | 288 ± 20 * | 300 ± 10 | 283 ± 3 † |
| Urine output (mL/day/100 g BW) | 6.7 ± 0.8 | 67.6 ± 5.7 * | 17.5 ± 5.5 # | 64.7 ± 5.3 † |
| Urine osmolality (mOsm/kg H2O) | 1610 ± 165 | 188 ± 21 * | 542 ± 247 # | 179 ± 19 † |
| Urine sodium (mmol/day/100 g BW) | 0.78 ± 0.06 | 1.14 ± 0.13 * | 0.57 ± 0.09 # | 0.93 ± 0.08 † |
| Urine creatinine (mg/day/100 g BW) | 3.31 ± 0.30 | 4.07 ± 0.46 * | 2.62 ± 0.46 # | 3.28 ± 0.35 † |
| Plasma osmolality (mOsm/kg H2O) | 321 ± 7 | 315 ± 6 | 321 ± 9 | 317 ± 5 |
| Plasma sodium (mmol/L) | 144 ± 2 | 142 ± 1 | 142 ± 7 | 143 ± 2 |
| Plasma creatinine (mg/dL) | 0.39 ± 0.07 | 0.50 ± 0.09 | 0.51 ± 0.13 | 0.45 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Jo, C.H.; Kim, G.-H. Duloxetine-Induced Antidiuresis in Rats with Lithium-Induced Nephrogenic Diabetes Insipidus. Life 2024, 14, 1012. https://doi.org/10.3390/life14081012
Kim S, Jo CH, Kim G-H. Duloxetine-Induced Antidiuresis in Rats with Lithium-Induced Nephrogenic Diabetes Insipidus. Life. 2024; 14(8):1012. https://doi.org/10.3390/life14081012
Chicago/Turabian StyleKim, Sua, Chor Ho Jo, and Gheun-Ho Kim. 2024. "Duloxetine-Induced Antidiuresis in Rats with Lithium-Induced Nephrogenic Diabetes Insipidus" Life 14, no. 8: 1012. https://doi.org/10.3390/life14081012
APA StyleKim, S., Jo, C. H., & Kim, G.-H. (2024). Duloxetine-Induced Antidiuresis in Rats with Lithium-Induced Nephrogenic Diabetes Insipidus. Life, 14(8), 1012. https://doi.org/10.3390/life14081012







