Can Gentisic Acid Serve as a High-Performance Antioxidant with Lower Toxicity for a Promising New Topical Application?
Abstract
:1. Introduction
2. Materials and Methods
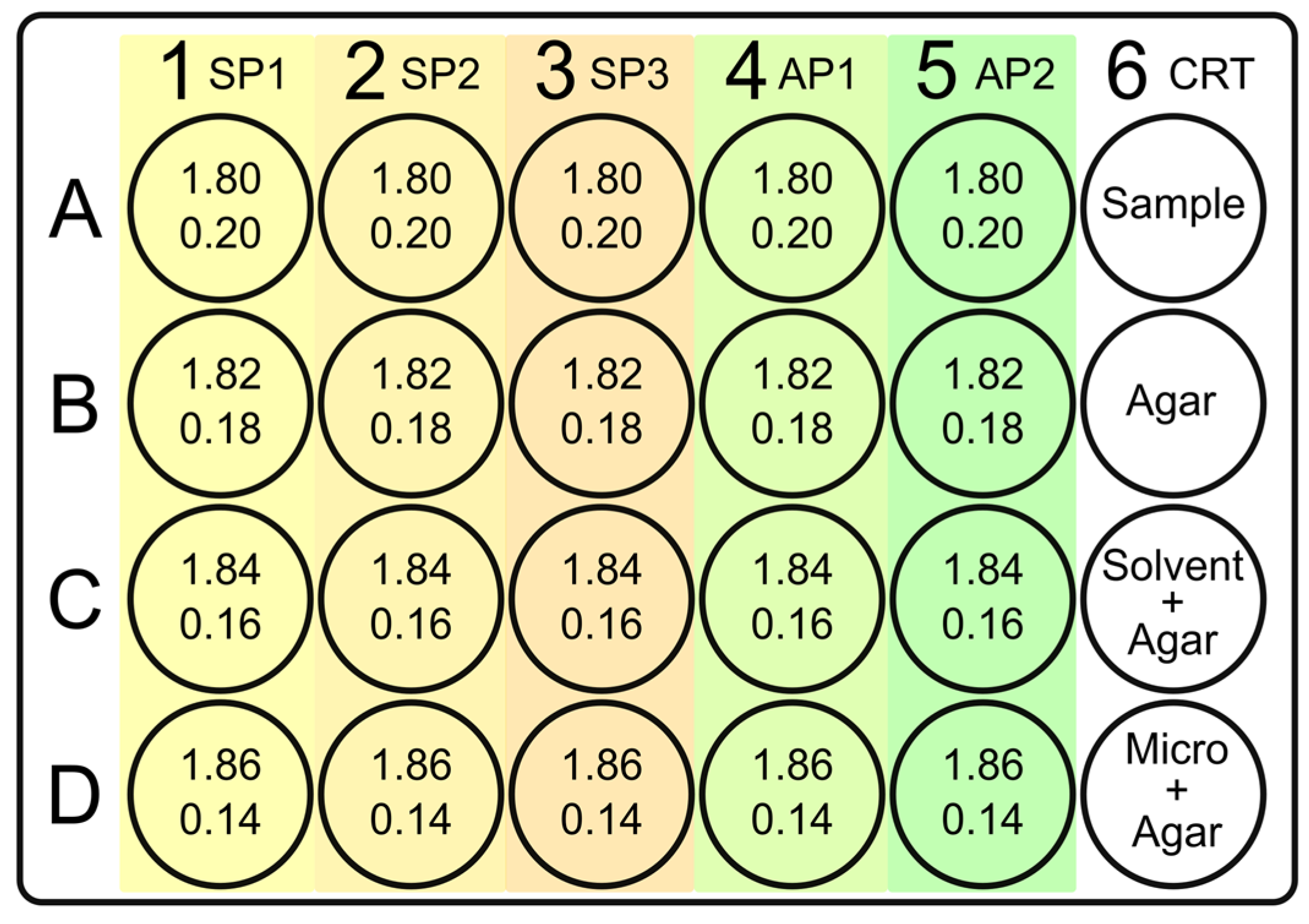
2.1. The Kinetic Method for Antioxidant Potential
2.1.1. DPPH Calibration Curve
2.1.2. DPPH Radical Scavenging Kinetic Curve
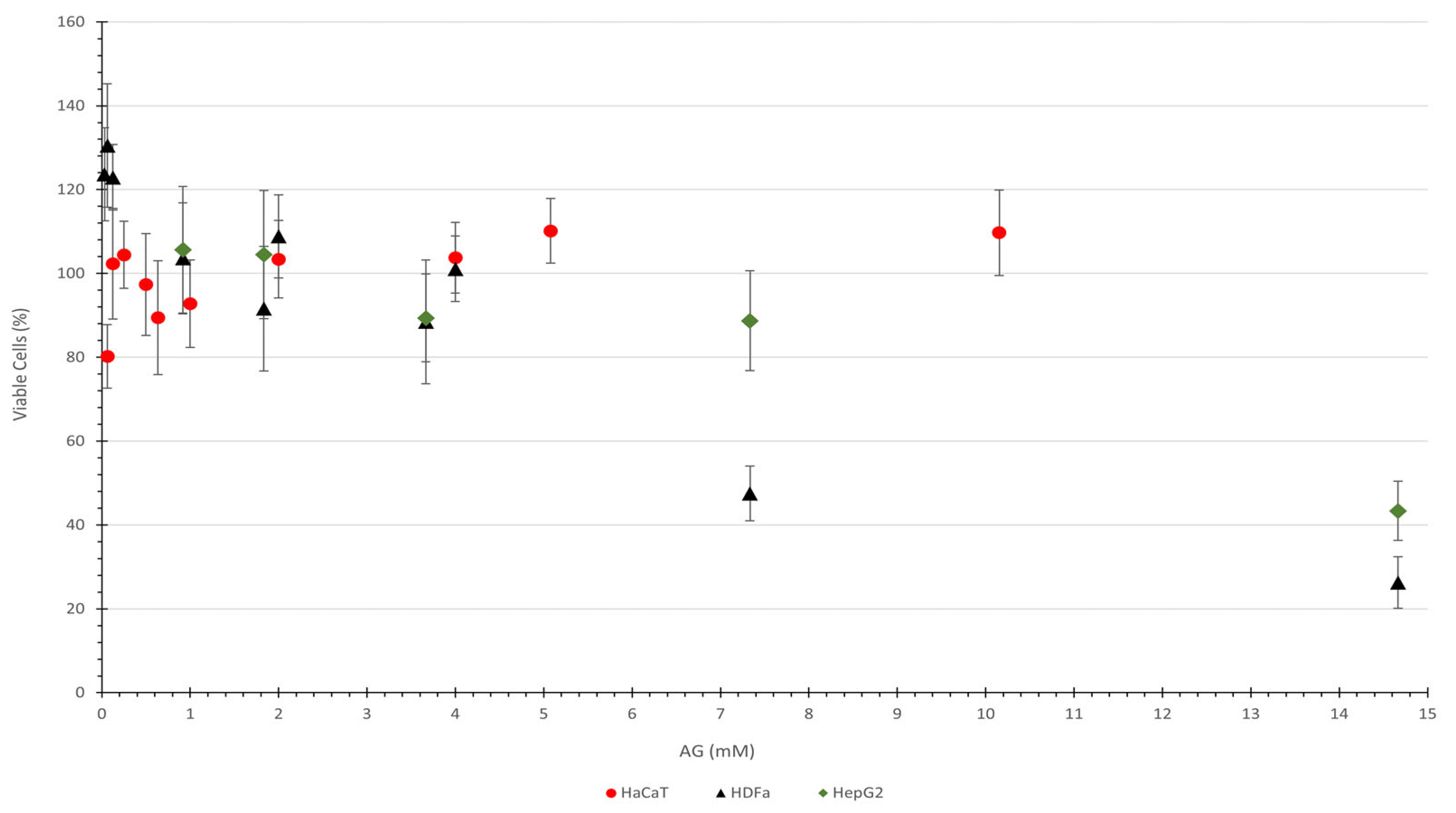
2.2. In Vitro Cytotoxic Potential through Neutral Red Uptake (NRU)
2.3. Determination of Antimicrobial Activity
2.4. Data Analysis
3. Results
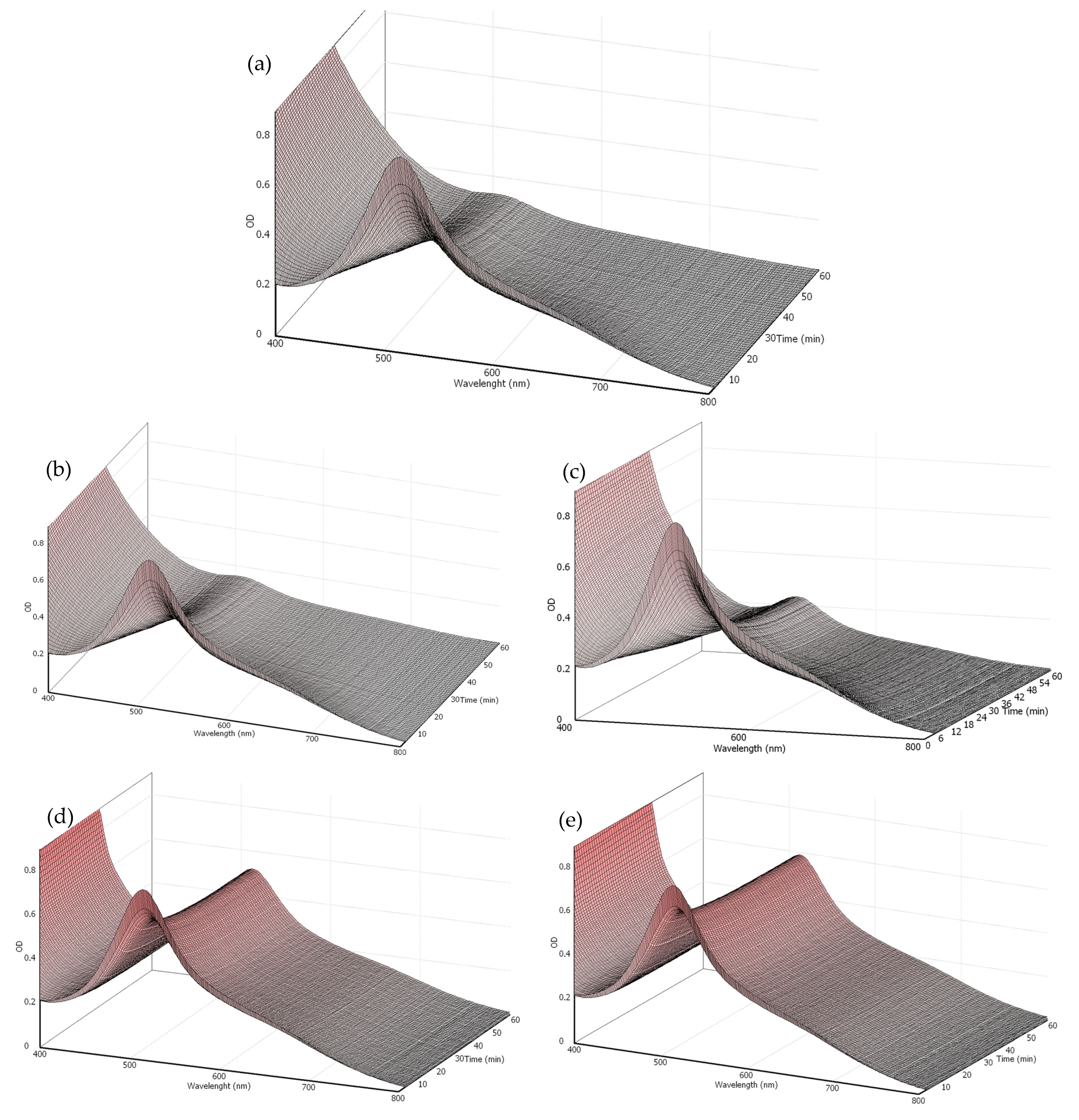
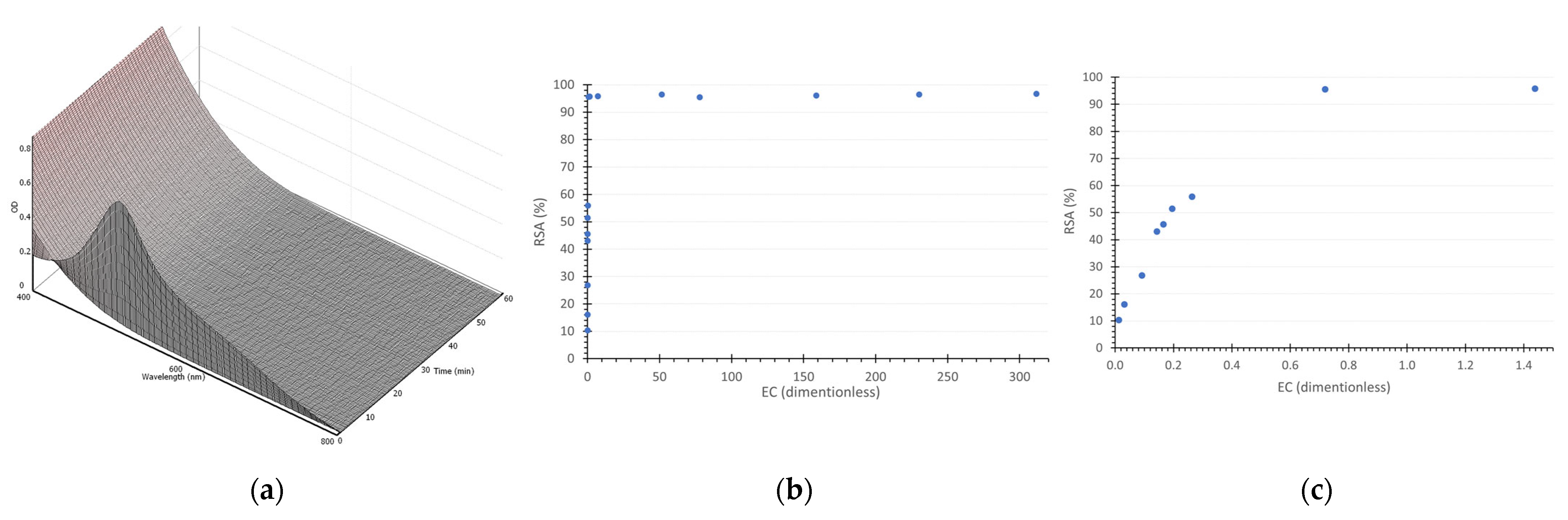
3.1. Antioxidant Potential
3.2. Evaluation of Cytotoxic Potential
3.3. Assessment of Antimicrobial Potential
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nutricati, E.; De Pascali, M.; Negro, C.; Bianco, P.A.; Quaglino, F.; Passera, A.; Pierro, R.; Marcone, C.; Panattoni, A.; Sabella, E.; et al. Signaling Cross-Talk between Salicylic and Gentisic Acid in the “Candidatus Phytoplasma Solani” Interaction with Sangiovese Vines. Plants 2023, 12, 2695. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A Review on Gentisic Acid as a Plant Derived Phenolic Acid and Metabolite of Aspirin: Comprehensive Pharmacology, Toxicology, and Some Pharmaceutical Aspects. Phytother. Res. 2020, 34, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Vega-Ruiz, Y.C.; Hayano-Kanashiro, C.; Gámez-Meza, N.; Medina-Juárez, L.A. Determination of Chemical Constituents and Antioxidant Activities of Leaves and Stems from Jatropha Cinerea (Ortega) Müll. Arg and Jatropha Cordata (Ortega) Müll. Arg. Plants 2021, 10, 212. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Dabić Zagorac, D.Č.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.J.; Natić, M.M. Identification and Quantification of Phenolic Compounds in Berry Skin, Pulp, and Seeds in 13 Grapevine Varieties Grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A Major Update of the Phenol-Explorer Database to Incorporate Data on the Effects of Food Processing on Polyphenol Content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Skroza, D.; Šimat, V.; Vrdoljak, L.; Jolić, N.; Skelin, A.; Čagalj, M.; Frleta, R.; Generalić Mekinić, I. Investigation of Antioxidant Synergisms and Antagonisms among Phenolic Acids in the Model Matrices Using FRAP and ORAC Methods. Antioxidants 2022, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Gangabhagirathi, R.; Venu, S.; Adhikari, S.; Mukherjee, T. Antioxidant Activity and Free Radical Scavenging Reactions of Gentisic Acid: In-Vitro and Pulse Radiolysis Studies. Free Radic. Res. 2012, 46, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mardani-Ghahfarokhi, A.; Farhoosh, R. Antioxidant Activity and Mechanism of Inhibitory Action of Gentisic and α-Resorcylic Acids. Sci. Rep. 2020, 10, 19487. [Google Scholar] [CrossRef]
- Borges, R.S.; Castle, S.L. The Antioxidant Properties of Salicylate Derivatives: A Possible New Mechanism of Anti-Inflammatory Activity. Bioorg. Med. Chem. Lett. 2015, 25, 4808–4811. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, Q.; Zeng, F.; Cai, M.; Ding, D. The Protective Effect of Gentisic Acid on Rheumatoid Arthritis via the RAF/ERK Signaling Pathway. J. Orthop. Surg. Res. 2022, 17, 109. [Google Scholar] [CrossRef]
- Kabra, M.P.; Bhandari, S.S.; Sharma, A.; Gupta, R.B. Evaluation of Anti-Parkinson’s Activity of Gentisic Acid in Different Animal Models. J. Acute Dis. 2014, 3, 141–144. [Google Scholar] [CrossRef]
- Noei Razliqi, R.; Ahangarpour, A.; Mard, S.A.; Khorsandi, L. Gentisic Acid Protects against Diabetic Nephropathy in Nicotinamide-Streptozotocin Administered Male Mice by Attenuating Oxidative Stress and Inflammation: The Role of MiR-200a/Keap1/Nrf2 Pathway, Renin-Angiotensin System (RAS) and NF-KB. Chem. Biol. Interact. 2023, 380, 110507. [Google Scholar] [CrossRef] [PubMed]
- Razliqi, R.N.; Ahangarpour, A.; Mard, S.A.; Khorsandi, L. Gentisic Acid Ameliorates Type 2 Diabetes Induced by Nicotinamide-Streptozotocin in Male Mice by Attenuating Pancreatic Oxidative Stress and Inflammation through Modulation of Nrf2 and NF-KB Pathways. Life Sci. 2023, 325, 121770. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Kee, H.J.; Ryu, Y.; Choi, S.Y.; Kim, G.R.; Kim, H.-S.; Kee, S.-J.; Jeong, M.H. Gentisic Acid Prevents the Transition from Pressure Overload-Induced Cardiac Hypertrophy to Heart Failure. Sci. Rep. 2019, 9, 3018. [Google Scholar] [CrossRef] [PubMed]
- Altinoz, M.A.; Elmaci, I.; Cengiz, S.; Emekli-Alturfan, E.; Ozpinar, A. From Epidemiology to Treatment: Aspirin’s Prevention of Brain and Breast-Cancer and Cardioprotection May Associate with Its Metabolite Gentisic Acid. Chem. Biol. Interact. 2018, 291, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.M.; Nabors, L.B.; Thompson, Z.J.; Rozmeski, C.M.; Anic, G.A.; Olson, J.J.; LaRocca, R.V.; Chowdhary, S.A.; Forsyth, P.A.; Thompson, R.C. Analgesic Use and the Risk of Primary Adult Brain Tumor. Eur. J. Epidemiol. 2016, 31, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Sivak-Sears, N.R.; Schwartzbaum, J.A.; Miike, R.; Moghadassi, M.; Wrensch, M. Case-Control Study of Use of Nonsteroidal Antiinflammatory Drugs and Glioblastoma Multiforme. Am. J. Epidemiol. 2004, 159, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Shi, L.; Zeng, J.; Wen, Z. Aspirin as a Potential Modality for the Chemoprevention of Breast Cancer: A Dose-Response Meta-Analysis of Cohort Studies from 857,831 Participants. Oncotarget 2017, 8, 40389–40401. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Kornelius, E.; Chiou, J.-Y.; Lai, Y.-R.; Lo, S.-C.; Peng, C.-H.; Huang, C.-N. Low-Dose Aspirin Reduces Breast Cancer Risk in Women with Diabetes: A Nationwide Retrospective Cohort Study in Taiwan. J. Women’s Health 2017, 26, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Smith-Warner, S.A.; Collins, L.C.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Use of Aspirin, Other Nonsteroidal Anti-Inflammatory Drugs, and Acetaminophen and Postmenopausal Breast Cancer Incidence. J. Clin. Oncol. 2012, 30, 3468–3477. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Khan, N.; Sultana, S. Study on Prevention of Two-Stage Skin Carcinogenesis by Hibiscus Rosa Sinensis Extract and the Role of Its Chemical Constituent, Gentisic Acid, in the Inhibition of Tumour Promotion Response and Oxidative Stress in Mice. Eur. J. Cancer Prev. 2004, 13, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J.; Shin, Y.-K.; Kim, K.-Y. Gentisic Acid Stimulates Keratinocyte Proliferation through ERK1/2 Phosphorylation. Int. J. Med. Sci. 2020, 17, 626–631. [Google Scholar] [CrossRef] [PubMed]
- PubChem Compound Summary for CID 3469, 2,5-Dihydroxybenzoic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2_5-Dihydroxybenzoic-acid (accessed on 9 July 2024).
- Corrêa, M.A. Cosmetologia/Ciência e Técnica; Livraria e Editora Medfarma: São Paulo, Brazil, 2012; ISBN 9788589248099. [Google Scholar]
- Dorado, J.G.; Fraile, P.A. Anatomía y Fisiología de La Piel. Pediatr. Integral 2021, 24, 156.e1–156.e13. [Google Scholar]
- Venus, M.; Waterman, J.; Mcnab, I. Basic Physiology of the Skin. Surgery 2010, 29, 471–474. [Google Scholar]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and Maintenance of Skin Barrier Function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, X.; Cheng, G. Human Skin Bacterial Microbiota Homeostasis: A Delicate Balance between Health and Disease. mLife 2023, 2, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Kim, H.S. Interaction between the Microbiota and the Skin Barrier in Aging Skin: A Comprehensive Review. Front. Physiol. 2024, 15, 1322205. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial Community Variation in Human Body Habitats across Space and Time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; McFall-Ngai, M.; Relman, D.A. An Ecological and Evolutionary Perspective on Human–Microbe Mutualism and Disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Galvan, A.; Pellicciari, C.; Calderan, L. Recreating Human Skin In Vitro: Should the Microbiota Be Taken into Account? Int. J. Mol. Sci. 2024, 25, 1165. [Google Scholar] [CrossRef] [PubMed]
- Scholz, C.F.P.; Kilian, M. The Natural History of Cutaneous Propionibacteria, and Reclassification of Selected Species within the Genus Propionibacterium to the Proposed Novel Genera Acidipropionibacterium Gen. Nov., Cutibacterium Gen. Nov. and Pseudopropionibacterium Gen. Nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Kalinowska, M.; Gołębiewska, E.; Świderski, G.; Męczyńska-Wielgosz, S.; Lewandowska, H.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Świsłocka, R.; Samsonowicz, M.; et al. Plant-Derived and Dietary Hydroxybenzoic Acids—A Comprehensive Study of Structural, Anti-/pro-Oxidant, Lipophilic, Antimicrobial, and Cytotoxic Activity in MDA-MB-231 and MCF-7 Cell Lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef] [PubMed]
- JASP Team JASP. 2023. Available online: https://jasp-stats.org/download/ (accessed on 15 February 2023).
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH. Free Radical Method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and Pitfalls in Measuring Antioxidant Efficacy: A Critical Evaluation of ABTS, DPPH, and ORAC Assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-Evaluation of the 2,2-Diphenyl-1-Picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.; Baghdadi, A.; Tayebi-Meigooni, A. Alpha-Mangostin-Rich Extracts from Mangosteen Pericarp: Optimization of Green Extraction Protocol and Evaluation of Biological Activity. Molecules 2018, 23, 1852. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Bukhori, M.F.M.; Rahmat, M.H.; Rahmat, A. Assessment and Comparison of Phytochemical Constituents and Biological Activities of Bitter Bean (Parkia Speciosa Hassk.) Collected from Different Locations in Malaysia. Chem. Cent. J. 2018, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, J.; Furman, C.; Bernier, J.-L.; Duriez, P.; Teissier, E.; Cotelle, N. Antioxidant Properties of Di-Tert-Butylhydroxylated Flavonoids. Free Radic. Biol. Med. 2000, 29, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Santos, S.N.; Castanha, R.F.; Haber, L.L.; Marques, M.O.M.; Scramin, S.; Melo, I.S. Determinação Quantitativa Da Atividade Antioxidante de Extratos Brutos de Microrganismos Pelo Método de Captura de Radical Livre DPPH. Comun. Técnico Embrapa Meio Ambiente 2011, 1, 1–5. [Google Scholar]
- Da Silveira, A.C.; Kassuia, Y.S.; Domahovski, R.C.; Lazzarotto, M. Método de DPPH Adaptado: Uma Ferramenta Para Analisar Atividade Antioxidante de Polpa de Frutos da Erva-Mate de Forma Rápida e Reprodutível. Comun. Técnico Embrapa Meio Ambiente 2018, 1. Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1101294/ (accessed on 15 February 2023).
- Pérez-González, A.; Galano, A.; Alvarez-Idaboy, J.R. Dihydroxybenzoic Acids as Free Radical Scavengers: Mechanisms, Kinetics, and Trends in Activity. New J. Chem. 2014, 38, 2639. [Google Scholar] [CrossRef]
- Do Carmo, M.A.V.; Fidelis, M.; Pressete, C.G.; Marques, M.J.; Castro-Gamero, A.M.; Myoda, T.; Granato, D.; Azevedo, L. Hydroalcoholic Myrciaria Dubia (Camu-Camu) Seed Extracts Prevent Chromosome Damage and Act as Antioxidant and Cytotoxic Agents. Food Res. Int. 2019, 125, 108551. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen Fragmentation Promotes Oxidative Stress and Elevates Matrix Metalloproteinase-1 in Fibroblasts in Aged Human Skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Saleh, E.A.M.; Al-Dolaimy, F.; Almajidi, Y.Q.; Baymakov, S.; Kader, M.M.A.; Ullah, M.I.; Abbas, A.H.R.; Khlewee, I.H.; Bisht, Y.S.; Alsaalamy, A.H. Oxidative Stress Affects the Beginning of the Growth of Cancer Cells through a Variety of Routes. Pathol. Res. Pract. 2023, 249, 154664. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Ertürk Bergdahl, G.; Saleh, K.; Magnúsdóttir, H.; Stødkilde, K.; Andersen, C.B.F.; Lundqvist, K.; Jensen, A.; Brüggemann, H.; Lood, R. Common Skin Bacteria Protect Their Host from Oxidative Stress through Secreted Antioxidant RoxP. Sci. Rep. 2019, 9, 3596. [Google Scholar] [CrossRef]
- Mestrallet, G.; Rouas-Freiss, N.; LeMaoult, J.; Fortunel, N.O.; Martin, M.T. Skin Immunity and Tolerance: Focus on Epidermal Keratinocytes Expressing HLA-G. Front. Immunol. 2021, 12, 772516. [Google Scholar] [CrossRef] [PubMed]
- Vandal, J.; Abou-Zaid, M.M.; Ferroni, G.; Leduc, L.G. Antimicrobial Activity of Natural Products from the Flora of Northern Ontario, Canada. Pharm. Biol. 2015, 53, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Feldeková, E.; Kosová, M.; Berčíková, M.; Dragoun, M.; Klojdová, I.; Hrádková, I.; Šmidrkal, J. Antimicrobial Properties of Phenolic Acid Alkyl Esters. Czech J. Food Sci. 2022, 40, 438–444. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Truglio, M.; De Maio, F.; Lucantoni, F.; Cardinali, G.; Pontone, M.; Bernardi, T.; Sanguinetti, M.; Capitanio, B.; et al. Skin Dysbiosis and Cutibacterium Acnes Biofilm in Inflammatory Acne Lesions of Adolescents. Sci. Rep. 2022, 12, 21104. [Google Scholar] [CrossRef] [PubMed]
- Corvec, S.; Dagnelie, M.-A.; Khammari, A.; Dréno, B. Taxonomy and Phylogeny of Cutibacterium (Formerly Propionibacterium) Acnes in Inflammatory Skin Diseases. Ann. Dermatol. Venereol. 2019, 146, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Song, H.; Jin, J.S.; Lee, W.J.; Kim, J. Genomic and Phenotypic Characterization of Cutibacterium Acnes Bacteriophages Isolated from Acne Patients. Antibiotics 2022, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium Acnes and Acne Vulgaris: New Insights from the Integration of Population Genetic, Multi-Omic, Biochemical and Host-Microbe Studies. Microorganisms 2019, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Mias, C.; Mengeaud, V.; Bessou-Touya, S.; Duplan, H. Recent Advances in Understanding Inflammatory Acne: Deciphering the Relationship between Cutibacterium Acnes and Th17 Inflammatory Pathway. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Rozas, M.; Hart de Ruijter, A.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From Dysbiosis to Healthy Skin: Major Contributions of Cutibacterium Acnes to Skin Homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef] [PubMed]
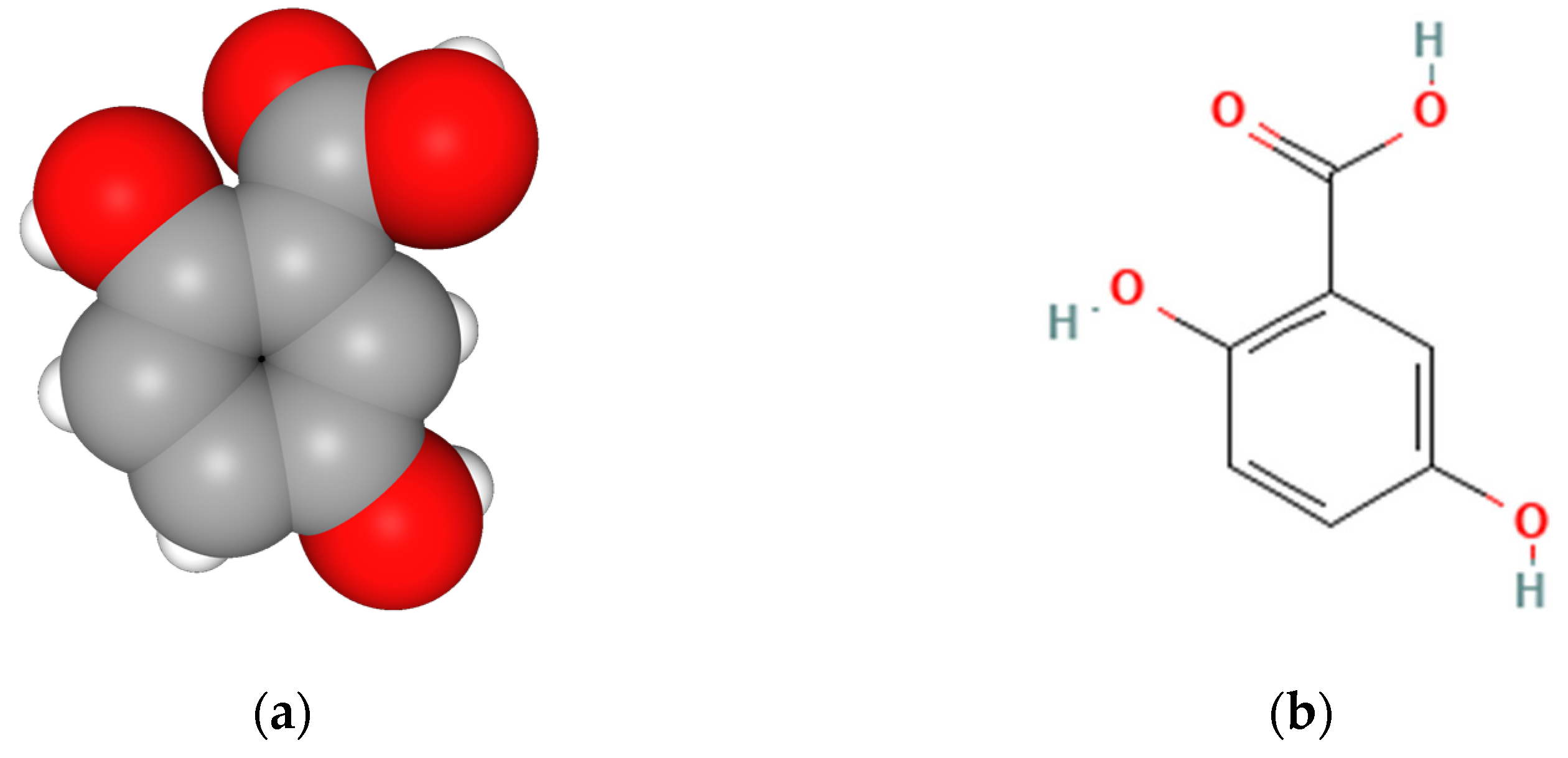

| Samples/ Microorganisms | MIC | |||
|---|---|---|---|---|
| Staphylococcus aureus | Escherichia coli | Candida albicans | Cutibacterium acnes | |
| GA * | 4.15 ± 0.09 | 4.00 ± 0.69 | 3.00 ± 0.26 | 3.60 ± 0.00 |
| Ampicillin ** | <3.5 ± 0.00 | 14.00 ± 0.00 | - | >0.5 ± 0.00 |
| Fluconazole ** | - | - | 8.00 ± 0.00 | - |
| Experiment control | Passed | Passed | Passed | Passed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, L.B.; Lepore, E.D.; Medeiros, C.C.B.; Sorrechia, R.; Pietro, R.C.L.R.; Corrêa, M.A. Can Gentisic Acid Serve as a High-Performance Antioxidant with Lower Toxicity for a Promising New Topical Application? Life 2024, 14, 1022. https://doi.org/10.3390/life14081022
Cunha LB, Lepore ED, Medeiros CCB, Sorrechia R, Pietro RCLR, Corrêa MA. Can Gentisic Acid Serve as a High-Performance Antioxidant with Lower Toxicity for a Promising New Topical Application? Life. 2024; 14(8):1022. https://doi.org/10.3390/life14081022
Chicago/Turabian StyleCunha, Luciano B., Eloah D. Lepore, Camila Cristina B. Medeiros, Rodrigo Sorrechia, Rosemeire C. L. R. Pietro, and Marcos A. Corrêa. 2024. "Can Gentisic Acid Serve as a High-Performance Antioxidant with Lower Toxicity for a Promising New Topical Application?" Life 14, no. 8: 1022. https://doi.org/10.3390/life14081022
APA StyleCunha, L. B., Lepore, E. D., Medeiros, C. C. B., Sorrechia, R., Pietro, R. C. L. R., & Corrêa, M. A. (2024). Can Gentisic Acid Serve as a High-Performance Antioxidant with Lower Toxicity for a Promising New Topical Application? Life, 14(8), 1022. https://doi.org/10.3390/life14081022





