Genetic and Physiological Insights into Salt Resistance in Rice through Analysis of Germination, Seedling Traits, and QTL Identification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Salinity Resistance Assessment at the Germination Stage
2.3. Salinity Resistance Assessment at the Seedling Stage
2.4. Genetic and QTL Mapping Analysis
2.5. Principal Component Analysis
2.6. Quantification of Physiological Parameters
2.7. Quantification of ROS Enzyme Activity in Leaves
2.8. Statistical Analysis
3. Results
3.1. Phenotypic Analysis of Rice Germination and Seedling Traits Under Salt Stress
3.2. PCA of Rice Germination and Seedling Traits under Salt Stress
3.3. QTL Analysis for Salt Resistance-Related Traits during Seed Germination and Seedling Stages
3.4. QTL Analysis of PC Composite Scores for Salt Resistance-Related Traits during Seed Germination and Seedling Stages
3.5. Correlation Analysis of qESC9 with Salt Resistance Composite Score and Identification of Extreme Phenotype Lines
3.6. Analysis of Salt Resistance Physiological Indices in Extreme Phenotype Lines
4. Discussion
4.1. qESC9 Plays a Crucial Role in Salinity Resistance in Rice
4.2. Implications for Breeding Programs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dar, M.H.; Bano, D.A.; Waza, S.A.; Zaidi, N.W.; Majid, A.; Shikari, A.B.; Ahangar, M.A.; Hossain, M.; Kumar, A.; Singh, U.S. Abiotic stress tolerance-progress and pathways of sustainable rice production. Sustainability 2021, 13, 2078. [Google Scholar] [CrossRef]
- Singh, R.K.; Kota, S.; Flowers, T.J. Salt tolerance in rice: Seedling and reproductive stage QTL mapping come of age. Theor. Appl. Genet. 2021, 134, 3495–3533. [Google Scholar] [CrossRef]
- Ganie, S.A.; Molla, K.A.; Henry, R.J.; Bhat, K.; Mondal, T.K. Advances in understanding salt tolerance in rice. Theor. Appl. Genet. 2019, 132, 851–870. [Google Scholar] [CrossRef]
- Qin, H.; Li, Y.; Huang, R. Advances and challenges in the breeding of salt-tolerant rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef]
- Rasheed, A.; Li, H.; Nawaz, M.; Mahmood, A.; Hassan, M.U.; Shah, A.N.; Hussain, F.; Azmat, S.; Gillani, S.F.A.; Majeed, Y. Molecular tools, potential frontiers for enhancing salinity tolerance in rice: A critical review and future prospective. Front. Plant Sci. 2022, 13, 966749. [Google Scholar] [CrossRef] [PubMed]
- Lokeshkumar, B.; Krishnamurthy, S.; Rathor, S.; Warriach, A.S.; Vinaykumar, N.; Dushyanthakumar, B.; Sharma, P.C. Morphophysiological Diversity and Haplotype Analysis of Saltol QTL Region in Diverse Rice Landraces for Salinity Tolerance. Rice Sci. 2023, 30, 306–320. [Google Scholar] [CrossRef]
- Maniruzzaman, S.; Rahman, M.A.; Hasan, M.; Rasul, M.G.; Molla, A.H.; Khatun, H.; Iftekharuddaula, K.; Kabir, M.S.; Akter, S. Molecular Mapping to Discover Reliable Salinity-Resilient QTLs from the Novel Landrace Akundi in Two Bi-Parental Populations Using SNP-Based Genome-Wide Analysis in Rice. Int. J. Mol. Sci. 2023, 24, 11141. [Google Scholar] [CrossRef]
- Haque, M.A.; Rafii, M.Y.; Yusoff, M.M.; Ali, N.S.; Yusuff, O.; Datta, D.R.; Anisuzzaman, M.; Ikbal, M.F. Advanced breeding strategies and future perspectives of salinity tolerance in rice. Agronomy 2021, 11, 1631. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Chen, H.; Wang, F.; Li, Z.; Zuo, J.; Fan, M.; Luo, B.; Feng, P.; Wang, J. Mapping combined with principal component analysis identifies excellent lines with increased rice quality. Sci. Rep. 2022, 12, 5969. [Google Scholar] [CrossRef]
- Lenz, M.; Müller, F.-J.; Zenke, M.; Schuppert, A. Principal components analysis and the reported low intrinsic dimensionality of gene expression microarray data. Sci. Rep. 2016, 6, 25696. [Google Scholar] [CrossRef]
- Yao, F.; Coquery, J.; Lê Cao, K.-A. Independent principal component analysis for biologically meaningful dimension reduction of large biological data sets. BMC Bioinform. 2012, 13, 24. [Google Scholar] [CrossRef]
- Chen, K.M.; Wong, A.K.; Troyanskaya, O.G.; Zhou, J. A sequence-based global map of regulatory activity for deciphering human genetics. Nat. Genet. 2022, 54, 940–949. [Google Scholar] [CrossRef]
- Yano, K.; Morinaka, Y.; Wang, F.; Huang, P.; Takehara, S.; Hirai, T.; Ito, A.; Koketsu, E.; Kawamura, M.; Kotake, K. GWAS with principal component analysis identifies a gene comprehensively controlling rice architecture. Proc. Natl. Acad. Sci. USA 2019, 116, 21262–21267. [Google Scholar] [CrossRef]
- Yang, J.; Yang, G.; Yang, M.; Su, L.; Xia, A.; Li, D.; Huang, C.; Zhou, D.; Liu, Y.; Wang, H. Quantitative trait locus analysis of seed germination and early seedling growth in rice. Front. Plant Sci. 2019, 10, 1582. [Google Scholar] [CrossRef]
- Cock, J.; Yoshida, S.; Forno, D.A. Laboratory Manual for Physiological Studies of Rice; The International Rice Research Institute: Los Baños, Philippines, 1976. [Google Scholar]
- Allen, S.; Dobrenz, A.; Schonhorst, M.; Stoner, J. Heritability of NaCl tolerance in germinating alfalfa seeds 1. Agron. J. 1985, 77, 99–101. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Y.; Tan, J.; Hu, S.; Yu, J.; Xue, Q. A genome-wide microsatellite polymorphism database for the indica and japonica rice. DNA Res. 2007, 14, 37–45. [Google Scholar] [CrossRef]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- McCouch, S.R.; CGSNL (Committee on Gene Symbolization, Nomenclature and Linkage, Rice Genetics Cooperative). Gene nomenclature system for rice. Rice 2008, 1, 72–84. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, S.; Fu, Y.; Su, Z.; Wang, X.; Sun, C. Identification of a drought tolerant introgression line derived from Dongxiang common wild rice (O. rufipogon Griff.). Plant Mol. Biol. 2006, 62, 247–259. [Google Scholar] [CrossRef]
- Ferguson, I.B.; Watkins, C.B.; Harman, J.E. Inhibition by calcium of senescence of detached cucumber cotyledons: Effect on ethylene and hydroperoxide production. Plant Physiol. 1983, 71, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, H.; Zhu, L.; Feng, P.; Fan, M.; Wang, J. WSL214 negatively regulates ROS accumulation and pathogen defense response in rice. Plant Cell Rep. 2023, 42, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Asch, J.; Johnson, K.; Mondal, S.; Asch, F. Comprehensive assessment of extraction methods for plant tissue samples for determining sodium and potassium via flame photometer and chloride via automated flow analysis. J. Plant Nutr. Soil. Sci. 2022, 185, 308–316. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Jaffar, M.A.; Song, A.; Faheem, M.; Chen, S.; Jiang, J.; Liu, C.; Fan, Q.; Chen, F. Involvement of CmWRKY10 in drought tolerance of chrysanthemum through the ABA-signaling pathway. Int. J. Mol. Sci. 2016, 17, 693. [Google Scholar] [CrossRef] [PubMed]
- Moukette, B.; Pieme, C.A.; Nya Biapa, P.C.; Njimou, J.R.; Ama Moor, V.J.; Stoller, M.; Bravi, M.; Ngogang, J.Y. Phenolic content of Hypodaphnis zenkeri and its antioxidant effects against Fenton reactions mediated oxidative Injuries on Liver Homogenate. Antioxidants 2014, 3, 866–889. [Google Scholar] [CrossRef]
- Ara, N.; Nakkanong, K.; Lv, W.; Yang, J.; Hu, Z.; Zhang, M. Antioxidant enzymatic activities and gene expression associated with heat tolerance in the stems and roots of two cucurbit species (“Cucurbita maxima” and “Cucurbita moschata”) and their interspecific inbred line “Maxchata”. Int. J. Mol. Sci. 2013, 14, 24008–24028. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Gautam, R.K.; Singh, R.K.; Krishnamurthy, S.L.; Ali, S.; Sakthivel, K.; Iquebal, M.A.; Rai, A.; Kumar, D. Harmonizing technological advances in phenomics and genomics for enhanced salt tolerance in rice from a practical perspective. Rice 2019, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nayyeripasand, L.; Garoosi, G.A.; Ahmadikhah, A. Genome-wide association study (GWAS) to identify salt-tolerance QTLs carrying novel candidate genes in rice during early vegetative stage. Rice 2021, 14, 1–21. [Google Scholar] [CrossRef]
- Theerawitaya, C.; Samphumphuang, T.; Tisarum, R.; Siangliw, M.; Cha-Um, S.; Takabe, T.; Toojinda, T. Expression level of Na+ homeostasis-related genes and salt-tolerant abilities in backcross introgression lines of rice crop under salt stress at reproductive stage. Protoplasma 2020, 257, 1595–1606. [Google Scholar] [CrossRef]
- Chen, T.; Shabala, S.; Niu, Y.; Chen, Z.H.; Shabala, L.; Meinke, H.; Venkataraman, G.; Pareek, A.; Xu, J.; Zhou, M. Molecular mechanisms of salinity tolerance in rice. Crop J. 2021, 9, 506–520. [Google Scholar] [CrossRef]
- Ren, R.; Li, D.; Zhen, C.; Chen, D.; Chen, X. Specific roles of Os4BGlu10, Os6BGlu24, and Os9BGlu33 in seed germination, root elongation, and drought tolerance in rice. Planta 2019, 249, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, Y.; Liu, Y.; Tan, Y.; Huang, J.; Shu, Q. Mutation of inositol 1, 3, 4-trisphosphate 5/6-kinase6 impairs plant growth and phytic acid synthesis in rice. Plants 2019, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Xiong, S.; Li, M.; Tian, L.; Qing Qu, L. OsFes1C, a potential nucleotide exchange factor for OsBiP1, is involved in the ER and salt stress responses. Plant Physiol. 2021, 187, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Gutha, L.R.; Reddy, A.R. Rice DREB1B promoter shows distinct stress-specific responses, and the overexpression of cDNA in tobacco confers improved abiotic and biotic stress tolerance. Plant Mol. Biol. 2008, 68, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L.; encode transcription activators that function in drought-, high-salt-and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, M.; Cao, L.; Dang, Z.; Ruan, N.; Wang, Y.; Huang, Y.; Wu, J.; Zhang, M.; Xu, Z.; et al. OsUGE3-mediated cell wall polysaccharides accumulation improves biomass production, mechanical strength, and salt tolerance. Plant Cell Environ. 2022, 45, 2492–2507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, F.; Lin, Q.; Sheng, P.; Wu, Z.; Jin, X.; Chen, W.; Li, S.; Luo, S.; Duan, E.; et al. A regulatory loop establishes the link between the circadian clock and abscisic acid signaling in rice. Plant Physiol. 2023, 191, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, S.; Kim, T.H.; Lee, J.H.; Park, J.; Lee, J.; Lee, J.Y.; Cho, L.H.; Choi, J.Y.; Lee, W.; et al. Natural variations at the Stay-Green gene promoter control lifespan and yield in rice cultivars. Nat. Commun. 2022, 11, 2819. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Chakrabarti, S.; Sarkar, A.; Singh, A.; Grover, A. Heat shock factor gene family in rice: Genomic organization and transcript expression profiling in response to high temperature, low temperature and oxidative stresses. Plant Physiol. Biochem. 2009, 47, 785–795. [Google Scholar] [CrossRef]
- Ganapati, R.K.; Naveed, S.A.; Zafar, S.; Wang, W.; Xu, J. Saline-alkali tolerance in rice: Physiological response, molecular mechanism, and QTL identification and application to breeding. Rice Sci. 2022, 29, 412–434. [Google Scholar] [CrossRef]
- Muthuramalingam, P.; Jeyasri, R.; Rakkammal, K.; Satish, L.; Shamili, S.; Karthikeyan, A.; Valliammai, A.; Priya, A.; Selvaraj, A.; Gowri, P.; et al. Multi-Omics and integrative approach towards understanding salinity tolerance in rice: A review. Biology 2022, 11, 1022. [Google Scholar] [CrossRef] [PubMed]

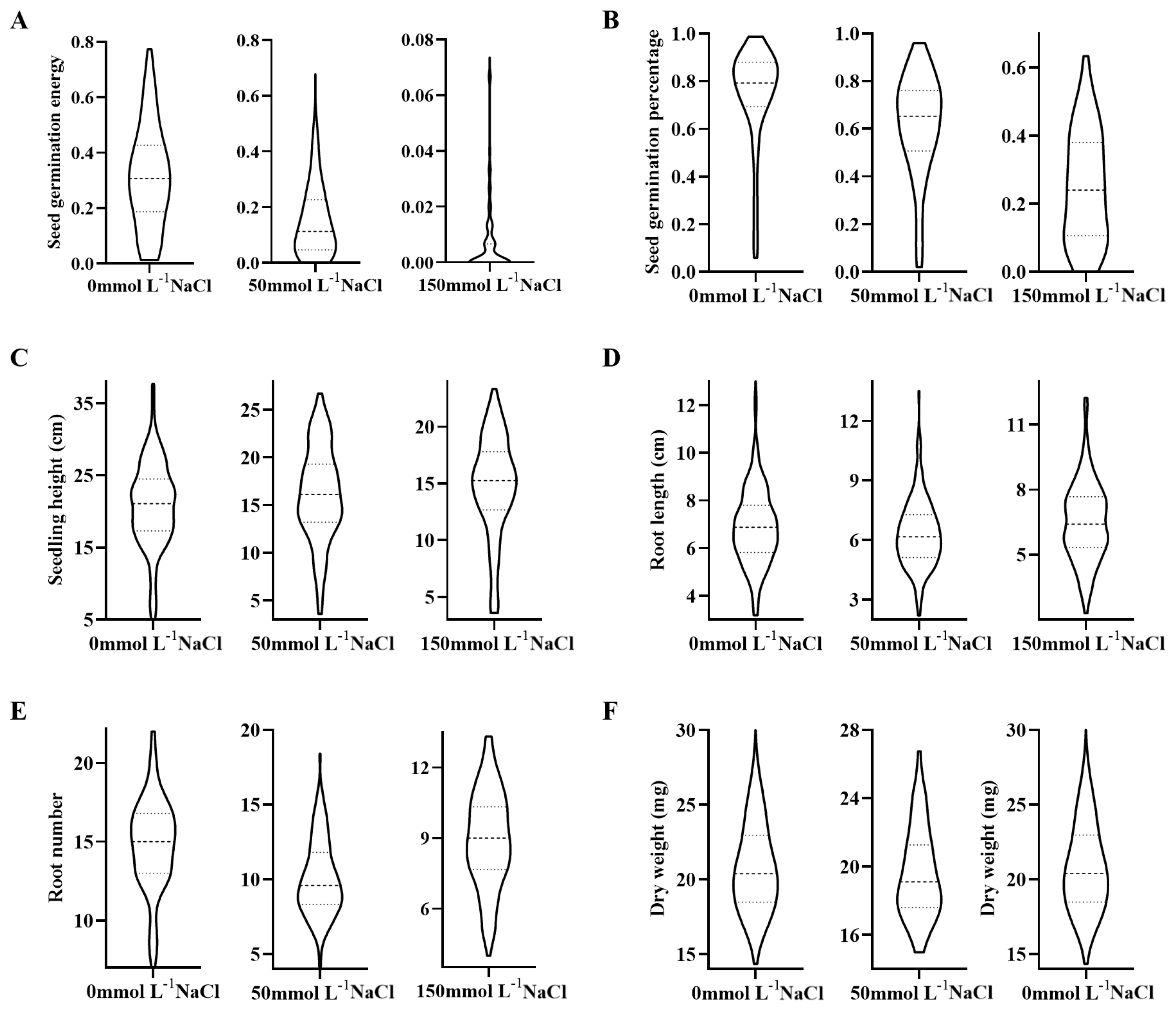

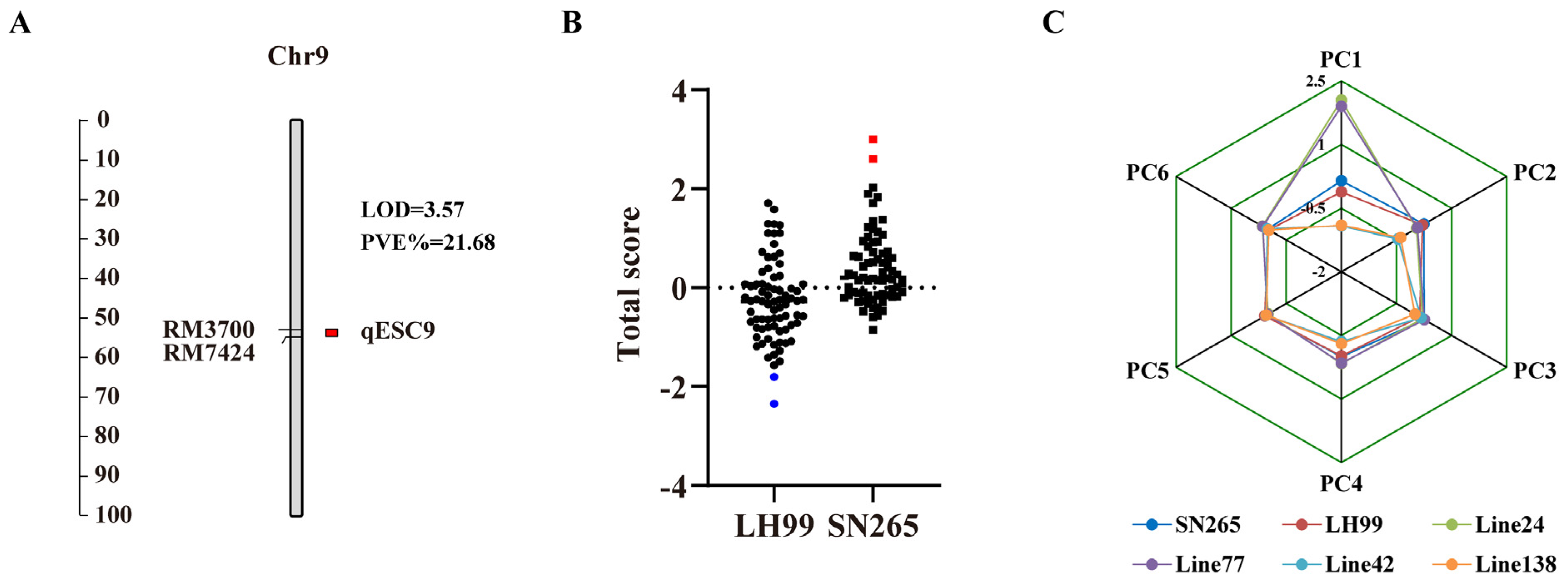
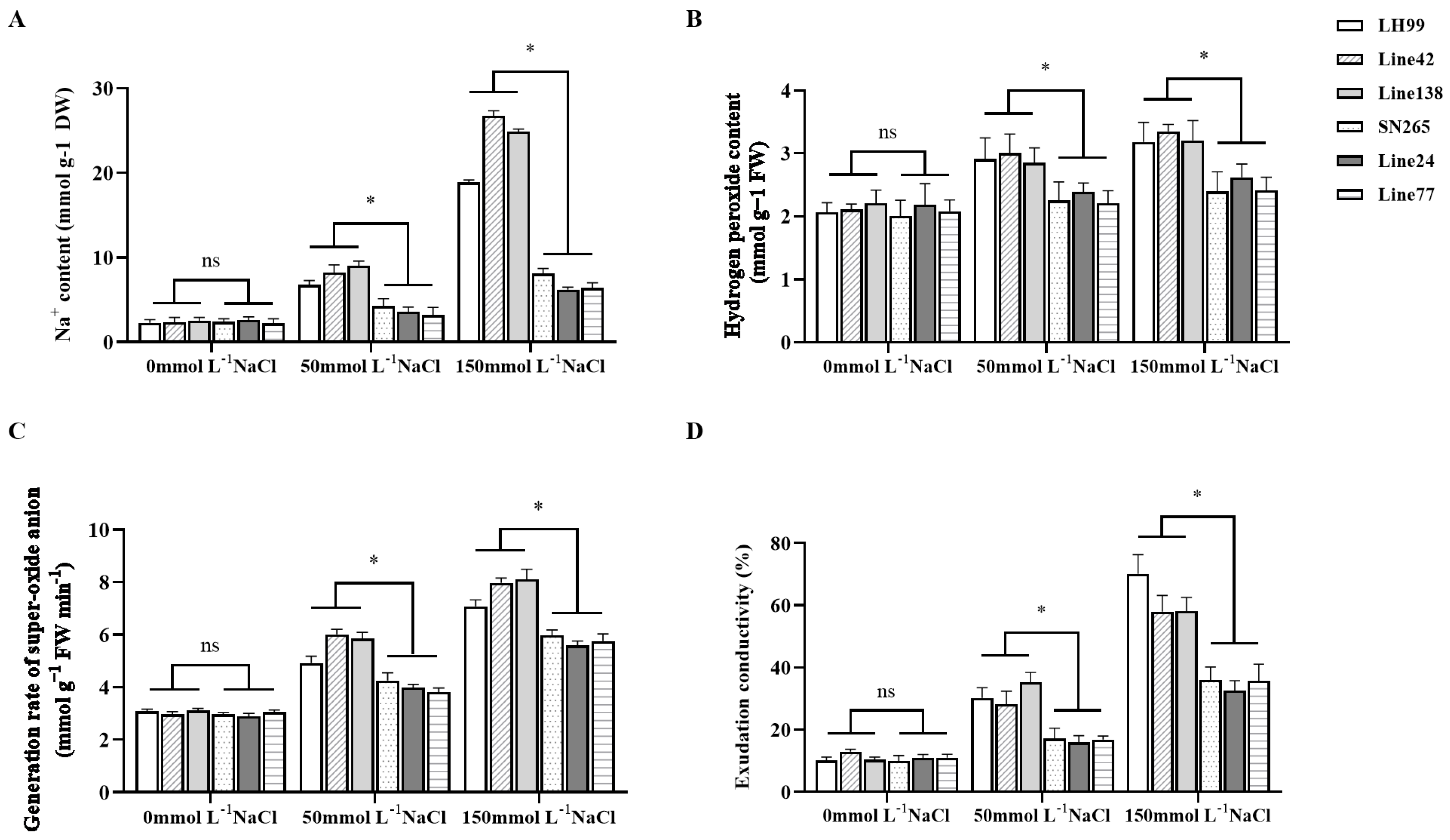
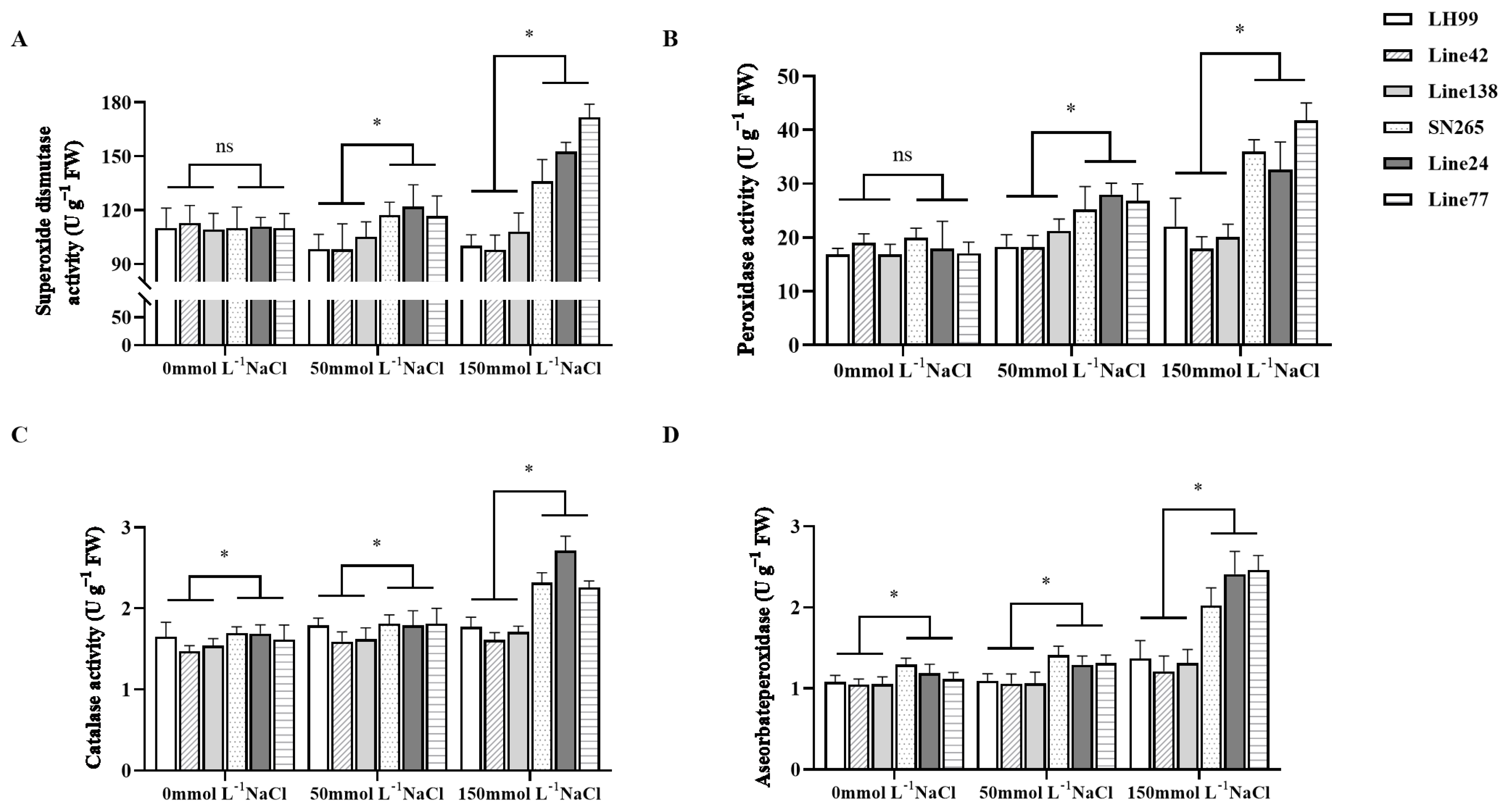

| Trait | Abridge | Conditions (mmol L−1 NaCl) | Abridge |
|---|---|---|---|
| Seed germination energy | SGE | 0 | C-SGE |
| 50 | L-SGE | ||
| 150 | H-SGE | ||
| Seed germination percentage | SGP | 0 | C-SGP |
| 50 | L-SGP | ||
| 150 | H-SGP | ||
| Seedling height | SSH | 0 | C-SSH |
| 50 | L-SSH | ||
| 150 | H-SSH | ||
| Root length | SRL | 0 | C-SRL |
| 50 | L-SRL | ||
| 150 | H-SRL | ||
| Root number | SRN | 0 | C-SRN |
| 50 | L-SRN | ||
| 150 | H-SRN | ||
| Dry weight | SDW | 0 | C-SDW |
| 50 | L-SDW | ||
| 150 | H-SDW |
| Traits | Salt Stress Intensity | Chr. | QTL | Location Interval | LOD Value | Add. | Var. (%) | Source of Potentiating Allele |
|---|---|---|---|---|---|---|---|---|
| Seed germination energy | 1 | 5 | qSGE5 | RM519-RM413 | 2.78 | 0.04 | 8.08 | SN265 |
| 1 | 7 | qSGE7 | PSM142-RI05304 | 3.31 | 0.05 | 8.54 | SN265 | |
| Seed germination percentage | 2 | 7 | qSGP7 | RI05304-RM11 | 3.77 | 0.07 | 10.16 | SN265 |
| Seedling height | 3 | 7 | qSSH7 | RM1186-RM6835 | 3.30 | −1.54 | 8.60 | LH99 |
| 3 | 9 | qSSH9-1 | RM3700-RM7424 | 2.52 | 1.56 | 8.75 | SN265 | |
| 4 | 9 | qSSH9-2 | RM7424B-STS18 | 3.16 | 1.72 | 9.45 | SN265 | |
| Root number | 4 | 6 | qSRN6 | RI02763- RI04969 | 2.64 | 0.65 | 8.02 | SN265 |
| Dry weight | 4 | 6 | qSDW6 | RI02729-RM454 | 2.57 | −1.86 | 7.10 | LH99 |
| 4 | 9 | qSDW9 | RM3700-RM7424 | 2.92 | 2.01 | 8.59 | SN265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Wang, Q.; Wang, X.; Yuan, B.; Wang, G.; Wang, F.; Wang, J. Genetic and Physiological Insights into Salt Resistance in Rice through Analysis of Germination, Seedling Traits, and QTL Identification. Life 2024, 14, 1030. https://doi.org/10.3390/life14081030
Yuan J, Wang Q, Wang X, Yuan B, Wang G, Wang F, Wang J. Genetic and Physiological Insights into Salt Resistance in Rice through Analysis of Germination, Seedling Traits, and QTL Identification. Life. 2024; 14(8):1030. https://doi.org/10.3390/life14081030
Chicago/Turabian StyleYuan, Jie, Qi Wang, Xueying Wang, Bo Yuan, Guojiao Wang, Fengbin Wang, and Jiayu Wang. 2024. "Genetic and Physiological Insights into Salt Resistance in Rice through Analysis of Germination, Seedling Traits, and QTL Identification" Life 14, no. 8: 1030. https://doi.org/10.3390/life14081030




