Genetic Differences between Male and Female Pattern Hair Loss in a Korean Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Genotyping
2.3. Imputation and Quality Control
2.4. Literature Survey
2.5. Statistics and Software
3. Results
3.1. Population Characteristics
3.2. Recapitulation Study
3.3. GWAS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, W.S.; Lee, H.J. Characteristics of Androgenetic Alopecia in Asian. Ann. Dermatol. 2012, 24, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.C.C.; Sinclair, R.D. Prevalence of Male and Female Pattern Hair Loss in Maryborough. J. Investig. Dermatol. Symp. Proc. 2005, 10, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, M.J.; Sadoughifar, R.; Schwartz, R.A.; Lotti, T.M.; Janniger, C.K. Female Pattern Hair Loss: A Comprehensive Review. Dermatol. Ther. 2020, 33, e14055. [Google Scholar] [CrossRef] [PubMed]
- Maguire, H.C.; Kligman, A.M. Common baldness in women. Geriatrics 1963, 18, 329–333. [Google Scholar]
- Ludwig, E. Androgenetic alopecia in women. Arch. Klin. Exp. Dermatol. 1964, 219, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.A. Diagnostic and Predictive Value of Horizontal Sections of Scalp Biopsy Specimens in Male Pattern Androgenetic Alopecia. J. Am. Acad. Dermatol. 1993, 28, 755–763. [Google Scholar] [CrossRef]
- Whiting, D.A. Scalp biopsy as a diagnostic and prognostic tool in androgenetic alopecia. Dermatol. Ther. 1998, 8, 24–33. [Google Scholar]
- Whiting, D.A.; Waldstreicher, J.; Sanchez, M.; Kaufman, K.D. Measuring Reversal of Hair Miniaturization in Androgenetic Alopecia by Follicular Counts in Horizontal Sections of Serial Scalp Biopsies: Results of Finasteride 1 Mg Treatment of Men and Postmenopausal Women. J. Investig. Dermatol. Symp. Proc. 1999, 4, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.B. Patterned loss of hair in man; types and incidence. Ann. N. Y. Acad. Sci. 1951, 53, 708–728. [Google Scholar] [CrossRef]
- Norwood, O.T. Male pattern baldness: Classification and incidence. South. Med. J. 1975, 68, 1359–1365. [Google Scholar] [CrossRef]
- Ramos, P.M.; Miot, H.A. Female Pattern Hair Loss: A Clinical and Pathophysiological Review. An. Bras. Dermatol. 2015, 90, 529–543. [Google Scholar] [CrossRef]
- Olsen, E.A. Female Pattern Hair Loss. In Hair Growth and Disorders; Blume-Peytavi, U., Tosti, A., Trüeb, R.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 171–186. [Google Scholar] [CrossRef]
- Herskovitz, I.; Tosti, A. Female Pattern Hair Loss. Int. J. Endocrinol. Metab. 2013, 11, e9860. [Google Scholar] [CrossRef] [PubMed]
- Karrer-Voegeli, S.; Rey, F.; Reymond, M.J.; Meuwly, J.Y.; Gaillard, R.C.; Gomez, F. Androgen Dependence of Hirsutism, Acne, and Alopecia in Women: Retrospective Analysis of 228 Patients Investigated for Hyperandrogenism. Medicine 2009, 88, 32–45. [Google Scholar] [CrossRef]
- Herskovitz, I.; de Sousa, I.C.V.; Tosti, A. Vellus Hairs in the Frontal Scalp in Early Female Pattern Hair Loss. Int. J. Trichology 2013, 5, 118–120. [Google Scholar] [CrossRef]
- Futterweit, W.; Dunaif, A.; Yeh, H.C.; Kingsley, P. The Prevalence of Hyperandrogenism in 109 Consecutive Female Patients with Diffuse Alopecia. J. Am. Acad. Dermatol. 1988, 19, 831–836. [Google Scholar] [CrossRef]
- Olsen, E.A. Androgenetic alopecia. In Disorders of Hair Growth: Diagnosis and Treatment; Olsen, E.A., Ed.; McGraw-Hill: New York, NY, USA, 1994; pp. 257–283. [Google Scholar]
- Olsen, E.A. The Midline Part: An Important Physical Clue to the Clinical Diagnosis of Androgenetic Alopecia in Women. J. Am. Acad. Dermatol. 1999, 40, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Messenger, A.G.; Shapiro, J.; Bergfeld, W.F.; Hordinsky, M.K.; Roberts, J.L.; Stough, D.; Washenik, K.; Whiting, D.A. Evaluation and Treatment of Male and Female Pattern Hair Loss. J. Am. Acad. Dermatol. 2005, 52, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Chen, J.Y.F.; Hsu, W.L.; Yu, S.; Chen, W.C.; Chiu, S.H.; Yang, H.R.; Lin, S.Y.; Wu, C.Y. Female Pattern Hair Loss: An Overview with Focus on the Genetics. Genes 2023, 14, 1326. [Google Scholar] [CrossRef]
- MacArthur, J.; Bowler, E.; Cerezo, M.; Gil, L.; Hall, P.; Hastings, E.; Junkins, H.; McMahon, A.; Milano, A.; Morales, J.; et al. The New NHGRI-EBI Catalog of Published Genome-Wide Association Studies (GWAS Catalog). Nucleic Acids Res. 2017, 45, D896–D901. [Google Scholar] [CrossRef]
- Boughton, A.P.; Welch, R.P.; Flickinger, M.; VandeHaar, P.; Taliun, D.; Abecasis, G.R.; Boehnke, M. LocusZoom.Js: Interactive and Embeddable Visualization of Genetic Association Study Results. Bioinformatics 2021, 37, 3017–3018. [Google Scholar] [CrossRef]
- GTEx Consortium. The Genotype-Tissue Expression (GTEx) Project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J.; Südhof, T.C. Snares and Munc18 in Synaptic Vesicle Fusion. Nat. Rev. Neurosci. 2002, 3, 641–653. [Google Scholar] [CrossRef]
- Plooster, M.; Rossi, G.; Farrell, M.S.; McAfee, J.C.; Bell, J.L.; Ye, M.; Diering, G.H.; Won, H.; Gupton, S.L.; Brennwald, P. Schizophrenia-Linked Protein tSNARE1 Regulates Endosomal Trafficking in Cortical Neurons. J. Neurosci. 2021, 41, 9466–9481. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, P.; Wang, D.; Glessner, J.; Hadley, D.; Gur, R.E.; Cohen, N.; Li, Q.; Hakonarson, H. GWAS Meta Analysis Identifies TSNARE1 as a Novel Schizophrenia/Bipolar Susceptibility Locus. Sci. Rep. 2013, 3, 3075. [Google Scholar] [CrossRef] [PubMed]
- Goda, Y. SNAREs and Regulated Vesicle Exocytosis. Proc. Natl. Acad. Sci. USA 1997, 94, 769–772. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J.; Buddenkotte, J.; Buhl, T.; Steinhoff, M. Role of SNAREs in Atopic Dermatitis-Related Cytokine Secretion and Skin-Nerve Communication. J. Investig. Dermatol. 2019, 139, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Kadono, N.; Hagiwara, N.; Tagawa, T.; Maekubo, K.; Hirai, Y. Extracellularly Extruded Syntaxin-4 Is a Potent Cornification Regulator of Epidermal Keratinocytes. Mol. Med. 2015, 21, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Kadono, N.; Miyazaki, T.; Okugawa, Y.; Nakajima, K.; Hirai, Y. The Impact of Extracellular Syntaxin4 on HaCaT Keratinocyte Behavior. Biochem. Biophys. Res. Commun. 2012, 417, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Ungermann, C.; Langosch, D. Functions of SNAREs in Intracellular Membrane Fusion and Lipid Bilayer Mixing. J. Cell Sci. 2005, 118, 3819–3828. [Google Scholar] [CrossRef]
- Liu, D.; Xu, Q.; Meng, X.; Liu, X.; Liu, J. Status of Research on the Development and Regeneration of Hair Follicles. Int. J. Med. Sci. 2024, 21, 80–94. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W. The Molecular Mechanism of Natural Products Activating Wnt/β-Catenin Signaling Pathway for Improving Hair Loss. Life 2022, 12, 1856. [Google Scholar] [CrossRef] [PubMed]
- van Genderen, C.; Okamura, R.M.; Fariñas, I.; Quo, R.G.; Parslow, T.G.; Bruhn, L.; Grosschedl, R. Development of Several Organs That Require Inductive Epithelial-Mesenchymal Interactions Is Impaired in LEF-1-Deficient Mice. Genes Dev. 1994, 8, 2691–2703. [Google Scholar] [CrossRef]
- Reddy, S.T.; Andl, T.; Lu, M.M.; Morrisey, E.E.; Millar, S.E. Expression of Frizzled Genes in Developing and Postnatal Hair Follicles. J. Investig. Dermatol. 2004, 123, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Tan, Y.; Li, M.; Dey, S.K.; Das, S.K. Canonical Wnt Signaling Is Critical to Estrogen-Mediated Uterine Growth. Mol. Endocrinol. 2004, 18, 3035–3049. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilpa, S.; Rattanasirisin, N.; Panchaprateep, R.; Orprayoon, N.; Phutrakul, P.; Suwan, A.; Jaisamrarn, U. Prevalence of Female Pattern Hair Loss in Postmenopausal Women: A Cross-Sectional Study. Menopause 2022, 29, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Famenini, S.; Slaught, C.; Duan, L.; Goh, C. Demographics of Women with Female Pattern Hair Loss and the Effectiveness of Spironolactone Therapy. J. Am. Acad. Dermatol. 2015, 73, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Hartwig, J.H.; Italiano, J.E. The Biogenesis of Platelets from Megakaryocyte Proplatelets. J. Clin. Investig. 2005, 115, 3348–3354. [Google Scholar] [CrossRef] [PubMed]
- Arita, K.; Akiyama, M.; Tsuji, Y.; McMillan, J.R.; Eady, R.a.J.; Shimizu, H. Gap Junction Development in the Human Fetal Hair Follicle and Bulge Region. Br. J. Dermatol. 2004, 150, 429–434. [Google Scholar] [CrossRef]
- Churko, J.M.; Chan, J.; Shao, Q.; Laird, D.W. The G60S Connexin43 Mutant Regulates Hair Growth and Hair Fiber Morphology in a Mouse Model of Human Oculodentodigital Dysplasia. J. Investig. Dermatol. 2011, 131, 2197–2204. [Google Scholar] [CrossRef]
- Flores, A.F.; Varela-Vazquez, A.; Mayan, M.D.; Fonseca, E. Expression of Connexin 43 in the Human Hair Follicle: Emphasis on the Connexin 43 Protein Levels in the Bulge and through the Keratinization Process. J. Cutan. Pathol. 2018, 45, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Emori, C.; Kobayashi, M.; Maruyama, N.; Fujii, W.; Naito, K.; Sugiura, K. Cooperative Effects of Oocytes and Estrogen on the Forkhead Box L2 Expression in Mural Granulosa Cells in Mice. Sci. Rep. 2022, 12, 20158. [Google Scholar] [CrossRef] [PubMed]
- Winterhager, E.; Kidder, G.M. Gap Junction Connexins in Female Reproductive Organs: Implications for Women’s Reproductive Health. Hum. Reprod. Update 2015, 21, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Moore, R.K.; Wang, X.; Sharma, S.; Miyoshi, T.; Shimasaki, S. Essential Role of the Oocyte in Estrogen Amplification of Follicle-Stimulating Hormone Signaling in Granulosa Cells. Endocrinology 2005, 146, 3362–3367. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.E. Molecular Mechanisms Regulating Hair Follicle Development. J. Investig. Dermatol. 2002, 118, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional Hair Follicle Regeneration: An Updated Review. Signal Transduct. Target. Ther. 2021, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; He, J.; Wang, J.; Chen, X.; Yang, R. Regulation of Signaling Pathways in Hair Follicle Stem Cells. Burns Trauma. 2022, 10, tkac022. [Google Scholar] [CrossRef]
- Yesudian, P.D. Hair Abnormalities in Genetic Disorders of Junctions. Int. J. Trichology 2009, 1, 15–17. [Google Scholar] [CrossRef]
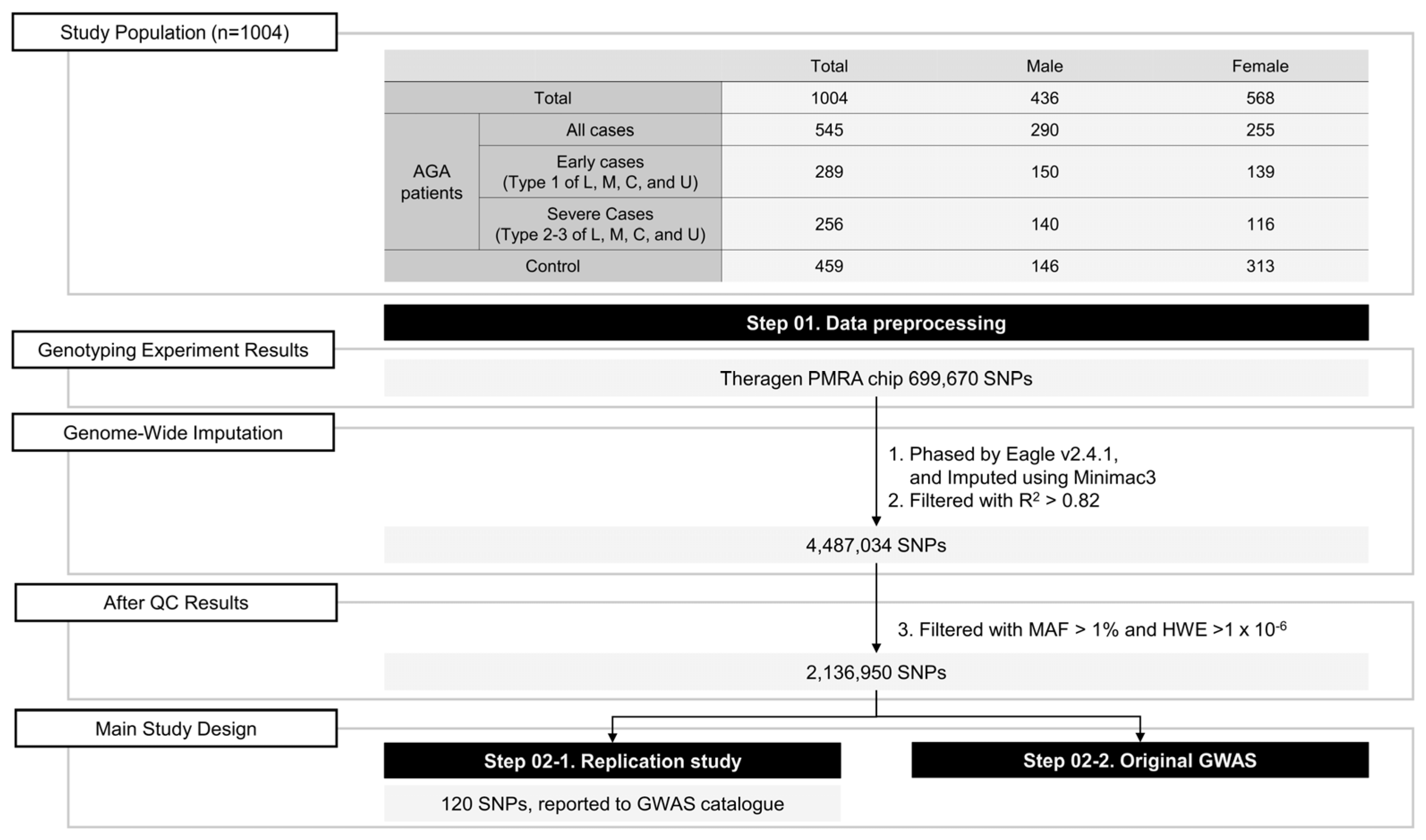

| All Subjects (n = 1004) | Males (n = 436, 43.4%) | Females (n = 568, 56.6%) | |||||
|---|---|---|---|---|---|---|---|
| n (% of All) | Age (Mean ± s.d) | n (% of Males) | Age (Mean ± s.d) | n (% of Females) | Age (Mean ± s.d) | ||
| AGA cases | All cases | 545 (54.3) | 46.7 ± 14.6 | 290 (66.5) | 45.4 ± 14.9 | 255 (44.9) | 48.2 ± 14.1 |
| Early cases | 289 (28.8) | 42.4 ± 13.8 | 150 (34.4) | 39.6 ± 14.0 | 139 (24.5) | 45.5 ± 14.0 | |
| Severe cases | 256 (25.5) | 51.5 ± 14.0 | 140 (32.1) | 51.5 ± 14.0 | 116 (20.4) | 51.5 ± 14.0 | |
| Control | 459 (45.7) | 53.2 ± 7.1 | 146(33.5) | 52.9 ± 7.1 | 313 (55.1) | 53.3 ± 7.2 | |
| SNP | Mapped Gene | Function | A1 | Case Type | Present Study | Previous Study | Meta-Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Subjects | Male | Female | OR | p | OR | p | Q | I | ||||||||
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |||||||||||
| rs9282858 | SRD5A2 | Missense | T | All | 0.28 (0.14–0.55) | 2.3 × 10−4 | 0.47 (0.20–1.10) | 8.3 × 10−2 | 0.10 (0.02–0.45) | 2.5 × 10−3 | 0.60 | 9.0 × 10−18 | 0.60 | 2.0 × 10−17 | 0.58 | 0 |
| Early | 0.31 (0.12–0.82) | 1.8 × 10−2 | 0.62 (0.19–2.09) | 4.4 × 10−1 | 0.10 (0.01–0.82) | 3.1 × 10−2 | ||||||||||
| Severe | 0.31 (0.13–0.71) | 6.0 × 10−3 | 0.47 (0.17–1.28) | 1.4 × 10−1 | 0.11 (0.01–0.84) | 3.3 × 10−2 | ||||||||||
| rs3827760 | EDAR | Missense | A | All | 0.62 (0.47–0.81) | 6.3 × 10−4 | 0.73 (0.48–1.10) | 1.4 × 10−1 | 0.54 (0.37–0.79) | 1.4 × 10−3 | 0.66 | 1.0 × 10−14 | 0.67 | 3.4 × 10−16 | 0.66 | 0 |
| Early | 0.46 (0.31–0.68) | 9.0 × 10−5 | 0.54 (0.30–1.00) | 4.8 × 10−2 | 0.41 (0.24–0.69) | 7.1 × 10−4 | ||||||||||
| Severe | 0.81 (0.59–1.12) | 2.0 × 10−1 | 0.92 (0.58–1.43) | 7.0 × 10−1 | 0.71 (0.45–1.14) | 1.6 × 10−1 | ||||||||||
| rs201563 | PAX1 (20p11.22) | 3′ Downstream | T | All | 1.37 (1.11–1.70) | 3.2 × 10−3 | 1.49 (1.06–2.09) | 2.1 × 10−2 | 1.30 (0.99–1.71) | 5.6 × 10−2 | 1.55 | 3.0 × 10−81 | 1.55 | 7.4 × 10−82 | 0.83 | 0 |
| Early | 1.27 (0.97–1.66) | 8.3 × 10−2 | 1.49 (0.97–2.30) | 6.9 × 10−2 | 1.15 (0.81–1.62) | 4.4 × 10−1 | ||||||||||
| Severe | 1.50 (1.17–1.93) | 1.3 × 10−3 | 1.59 (1.10–2.31) | 1.5 × 10−2 | 1.44 (1.03–2.01) | 3.2 × 10−2 | ||||||||||
| rs2073963 | HDAC9 | Intron | G | All | 1.24 (1.02–1.51) | 3.2 × 10−2 | 0.94 (0.69–1.27) | 6.8 × 10−1 | 1.50 (1.16–1.94) | 1.9 × 10−3 | 1.29 | 1.0 × 10−12 | 1.27 | 3.6 × 10−11 | 0.05 | 74.66 |
| Early | 1.20 (0.94–1.54) | 1.4 × 10−1 | 0.94 (0.64–1.39) | 7.7 × 10−1 | 1.42 (1.03–1.96) | 3.2 × 10−2 | ||||||||||
| Severe | 1.22 (0.96–1.53) | 9.9 × 10−2 | 0.93 (0.66–1.32) | 6.9 × 10−1 | 1.52 (1.11–2.09) | 9.4 × 10−3 | ||||||||||
| rs6047844 | LINC01432 | Intron | T | All | 1.34 (1.09–1.66) | 6.6 × 10−3 | 1.48 (1.05–2.07) | 2.4 × 10−2 | 1.26 (0.96–1.66) | 9.8 × 10−2 | 1.60 | 2.0 × 10−39 | 1.59 | 2.4 × 10−39 | 0.66 | 0 |
| Early | 1.25 (0.95–1.63) | 1.0 × 10−1 | 1.47 (0.95–2.26) | 8.2 × 10−2 | 1.13 (0.80–1.6) | 4.9 × 10−1 | ||||||||||
| Severe | 1.47 (1.14–1.89) | 2.6 × 10−3 | 1.59 (1.09–2.31) | 1.6 × 10−2 | 1.39 (0.99–1.94) | 5.8 × 10−2 | ||||||||||
| rs1160312 | LINC01432 | Intron | A | All | 1.35 (1.09–1.67) | 5.4 × 10−3 | 1.45 (1.04–2.03) | 3.1 × 10−2 | 1.29 (0.98–1.69) | 7.0 × 10−2 | 1.60 | 1.0 × 10−14 | 1.58 | 1.3 × 10−15 | 0.59 | 0 |
| Early | 1.24 (0.94–1.62) | 1.3 × 10−1 | 1.41 (0.91–2.17) | 1.2 × 10−1 | 1.14 (0.80–1.61) | 4.7 × 10−1 | ||||||||||
| Severe | 1.48 (1.16–1.90) | 1.9 × 10−3 | 1.60 (1.10–2.32) | 1.5 × 10−2 | 1.41 (1.01–1.96) | 4.6 × 10−2 | ||||||||||
| rs10888690 | FAF1 | Intron | C | All | 1.46 (1.08–1.97) | 1.5 × 10−2 | 1.43 (0.88–2.33) | 1.5 × 10−1 | 1.47 (1.00–2.16) | 5.1 × 10−2 | 1.11 | 6.0 × 10−13 | 1.11 | 1.6 × 10−13 | 0.31 | 4.81 |
| Early | 1.41 (0.96–2.07) | 7.8 × 10−2 | 1.20 (0.64–2.22) | 5.7 × 10−1 | 1.55 (0.96–2.51) | 7.2 × 10−2 | ||||||||||
| Severe | 1.41 (0.99–2.00) | 5.8 × 10−2 | 1.53 (0.90–2.62) | 1.2 × 10-1 | 1.32 (0.82–2.12) | 2.5 × 10−1 | ||||||||||
| rs13021718 | DPY30 | Intron | A | All | 0.63 (0.46–0.86) | 3.3 × 10−3 | 0.58 (0.36–0.94) | 2.6 × 10−2 | 0.67 (0.45–0.99) | 4.6 × 10−2 | 0.81 | 2.0 × 10−26 | 0.81 | 2.0 × 10−32 | 0.18 | 43.81 |
| Early | 0.73 (0.50–1.08) | 1.2 × 10−1 | 0.77 (0.42–1.43) | 4.2 × 10−1 | 0.71 (0.43–1.17) | 1.8 × 10−1 | ||||||||||
| Severe | 0.54 (0.36–0.80) | 2.4 × 10−3 | 0.47 (0.26–0.84) | 1.1 × 10−2 | 0.61 (0.36–1.04) | 6.9 × 10−2 | ||||||||||
| rs7976269 | FAR2 | 5′ Upstream | A | All | 1.23 (0.96–1.56) | 9.6 × 10−2 | 1.58 (1.07–2.33) | 2.3 × 10−2 | 1.04 (0.77–1.42) | 8.0 × 10−1 | 1.15 | 6.0 × 10−14 | 1.15 | 2.4 × 10−13 | 0.11 | 60.58 |
| Early | 1.15 (0.84–1.57) | 3.8 × 10−1 | 1.22 (0.72–2.07) | 4.6 × 10−1 | 1.11 (0.75–1.63) | 6.1 × 10−1 | ||||||||||
| Severe | 1.29 (0.98–1.71) | 6.9 × 10−2 | 1.72 (1.14–2.61) | 1.0 × 10−2 | 1.00 (0.68–1.48) | 9.8 × 10−1 | ||||||||||
| CHR | BP | SNP | Mapped Gene | Function | A1 | Effect Allele (A1) Frequency | Case Type | All Subjects | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| This Study | EAS | EUR | AMR | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |||||||
| Significant in Both Sexes | ||||||||||||||||
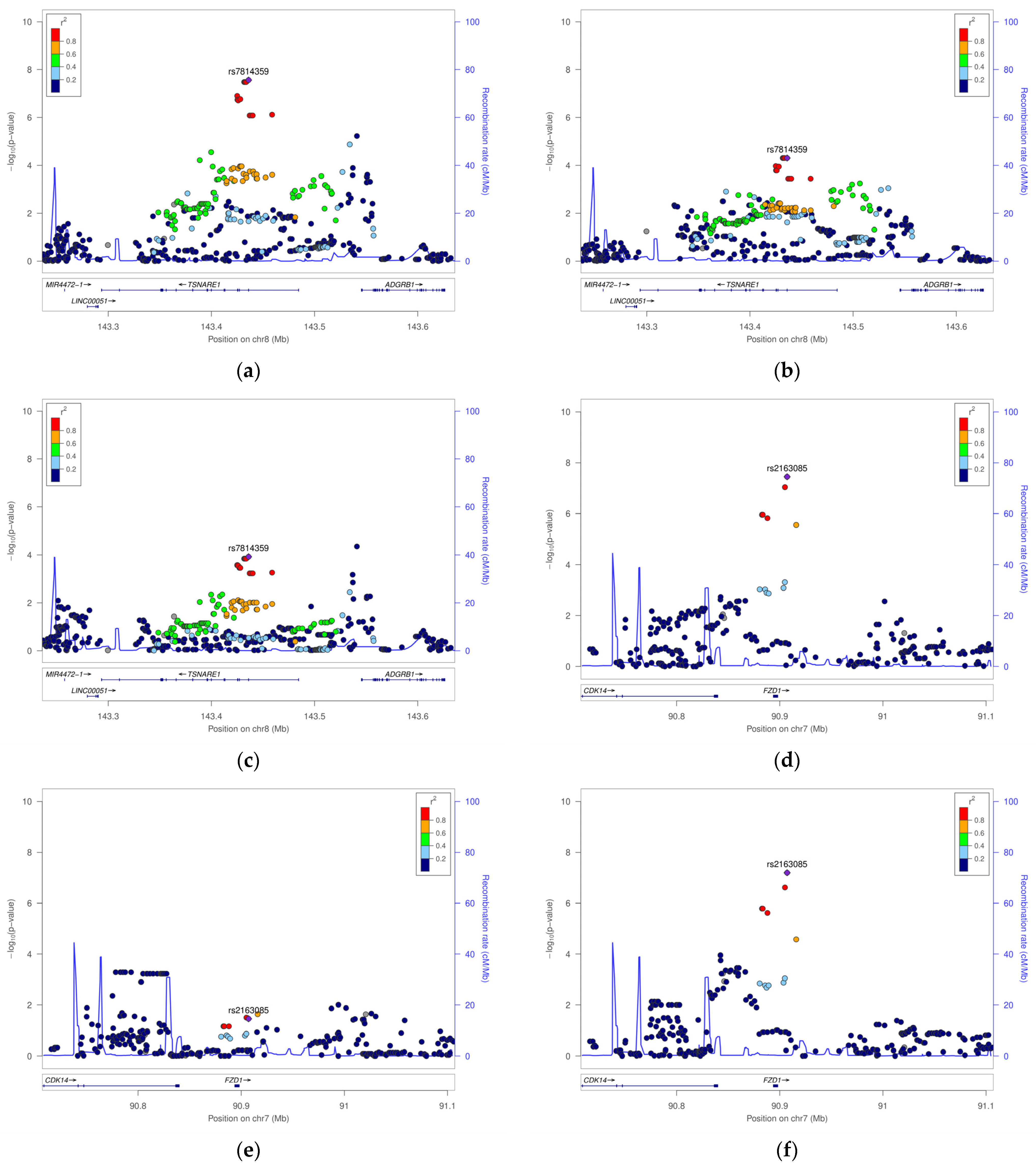
| 8 | 142354673 | rs7814359 | TSNARE1 | Missense p.(Phe18Leu) | G | 0.34 | 0.35 | 0.20 | 0.29 | All cases | 0.57 (0.46–0.69) | 2.7 × 10−8 | 0.53 (0.39–0.72) | 4.9 × 10−5 | 0.60 (0.46–0.78) | 1.2 × 10−4 |
| Early cases | 0.59 (0.45–0.76) | 4.6 × 10−5 | 0.53 (0.36–0.79) | 2.0 × 10−3 | 0.63 (0.45–0.88) | 6.5 × 10−3 | ||||||||||
| Severe cases | 0.54 (0.43–0.7) | 1.4 × 10−6 | 0.52 (0.36–0.74) | 3.1 × 10−4 | 0.57 (0.40–0.80) | 1.1 × 10−3 | ||||||||||
| Female Specific | ||||||||||||||||
| 7 | 91277819 | rs2163085 | FZD1 | Flanking | C | 0.25 | 0.22 | 0.36 | 0.46 | All cases | 1.88 (1.50–2.35) | 3.6 × 10−8 | 1.45 (1.03–2.05) | 3.5 × 10−2 | 2.24 (1.67–3.01) | 6.4 × 10−8 |
| Early cases | 1.86 (1.40–2.47) | 1.6 × 10−5 | 1.64 (1.05–2.56) | 3.0 × 10−2 | 2.05 (1.42–2.95) | 1.1 × 10−4 | ||||||||||
| Severe cases | 1.74 (1.34–2.26) | 2.8 × 10−5 | 1.26 (0.86–1.85) | 2.4 × 10−1 | 2.24 (1.58–3.17) | 5.4 × 10−6 | ||||||||||
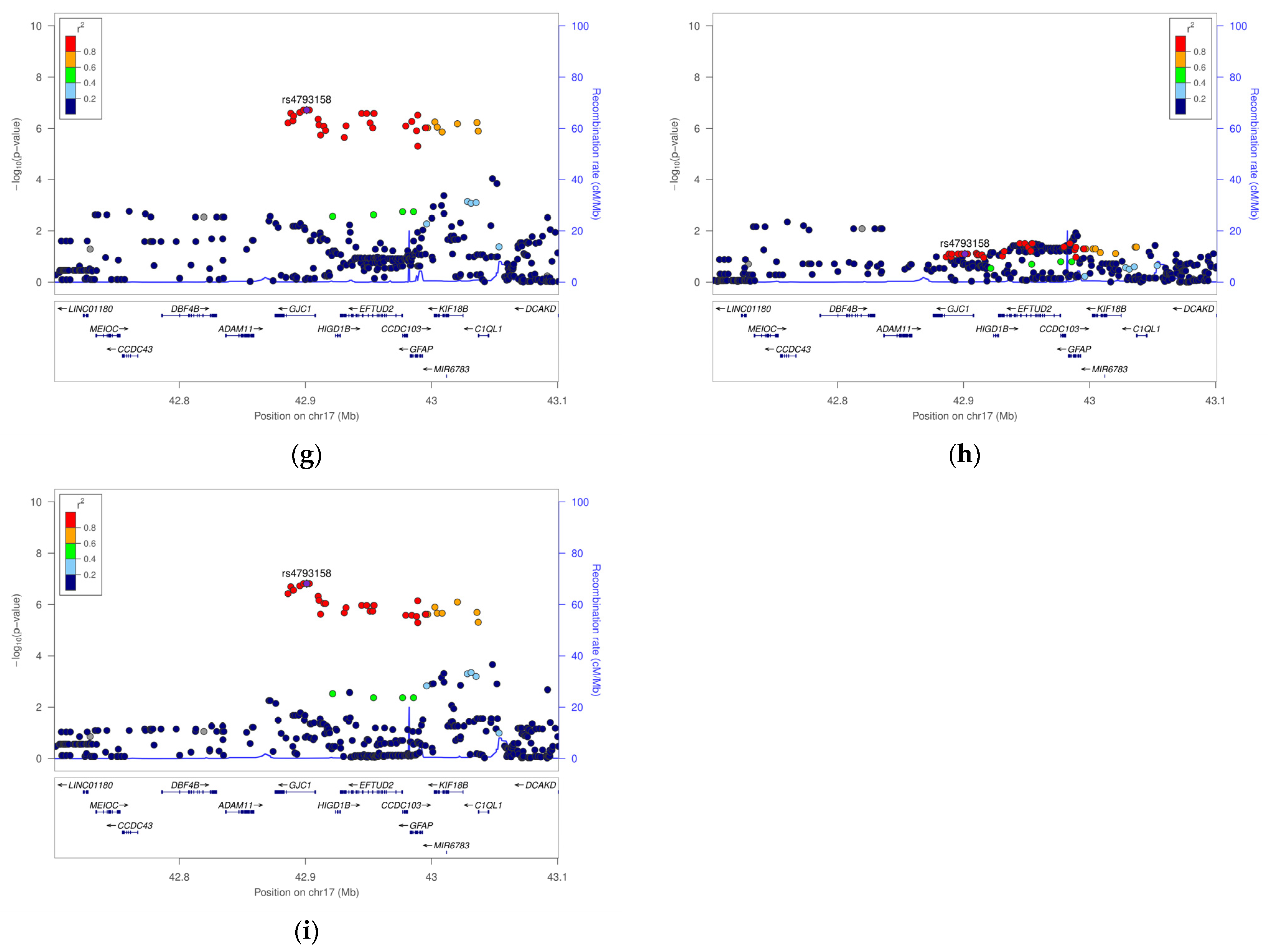
| 17 | 44823618 | rs4793158 | GJC1 (EFTUD2) | Intron | C | 0.14 | 0.13 | 0.13 | 0.10 | All cases | 2.15 (1.61–2.87) | 1.9 × 10−7 | 1.49 (0.95–2.34) | 8.0 × 10−2 | 2.70 (1.86–3.91) | 1.5 × 10−7 |
| Early cases | 2.38 (1.67–3.40) | 1.6 × 10−6 | 1.74 (0.98–3.09) | 5.9 × 10−2 | 2.88 (1.84–4.49) | 3.4 × 10−6 | ||||||||||
| Severe cases | 1.77 (1.26–2.48) | 9.6 × 10−4 | 1.21 (0.74–1.98) | 4.5 × 10−1 | 2.39 (1.53–3.75) | 1.5 × 10−4 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Choi, J.-E.; Ha, J.; Kim, Y.; Lee, C.; Hong, K.-W. Genetic Differences between Male and Female Pattern Hair Loss in a Korean Population. Life 2024, 14, 939. https://doi.org/10.3390/life14080939
Lee J, Choi J-E, Ha J, Kim Y, Lee C, Hong K-W. Genetic Differences between Male and Female Pattern Hair Loss in a Korean Population. Life. 2024; 14(8):939. https://doi.org/10.3390/life14080939
Chicago/Turabian StyleLee, Jihyun, Ja-Eun Choi, Joohun Ha, Youngjoo Kim, Changhyun Lee, and Kyung-Won Hong. 2024. "Genetic Differences between Male and Female Pattern Hair Loss in a Korean Population" Life 14, no. 8: 939. https://doi.org/10.3390/life14080939
APA StyleLee, J., Choi, J.-E., Ha, J., Kim, Y., Lee, C., & Hong, K.-W. (2024). Genetic Differences between Male and Female Pattern Hair Loss in a Korean Population. Life, 14(8), 939. https://doi.org/10.3390/life14080939






