Integrated Transcriptomic Analysis Reveals Reciprocal Interactions between SARS-CoV-2 Infection and Multi-Organ Dysfunction, Especially the Correlation of Renal Failure and COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Information of Transcriptomics Analysis
2.2. Gene Set Enrichment Analysis
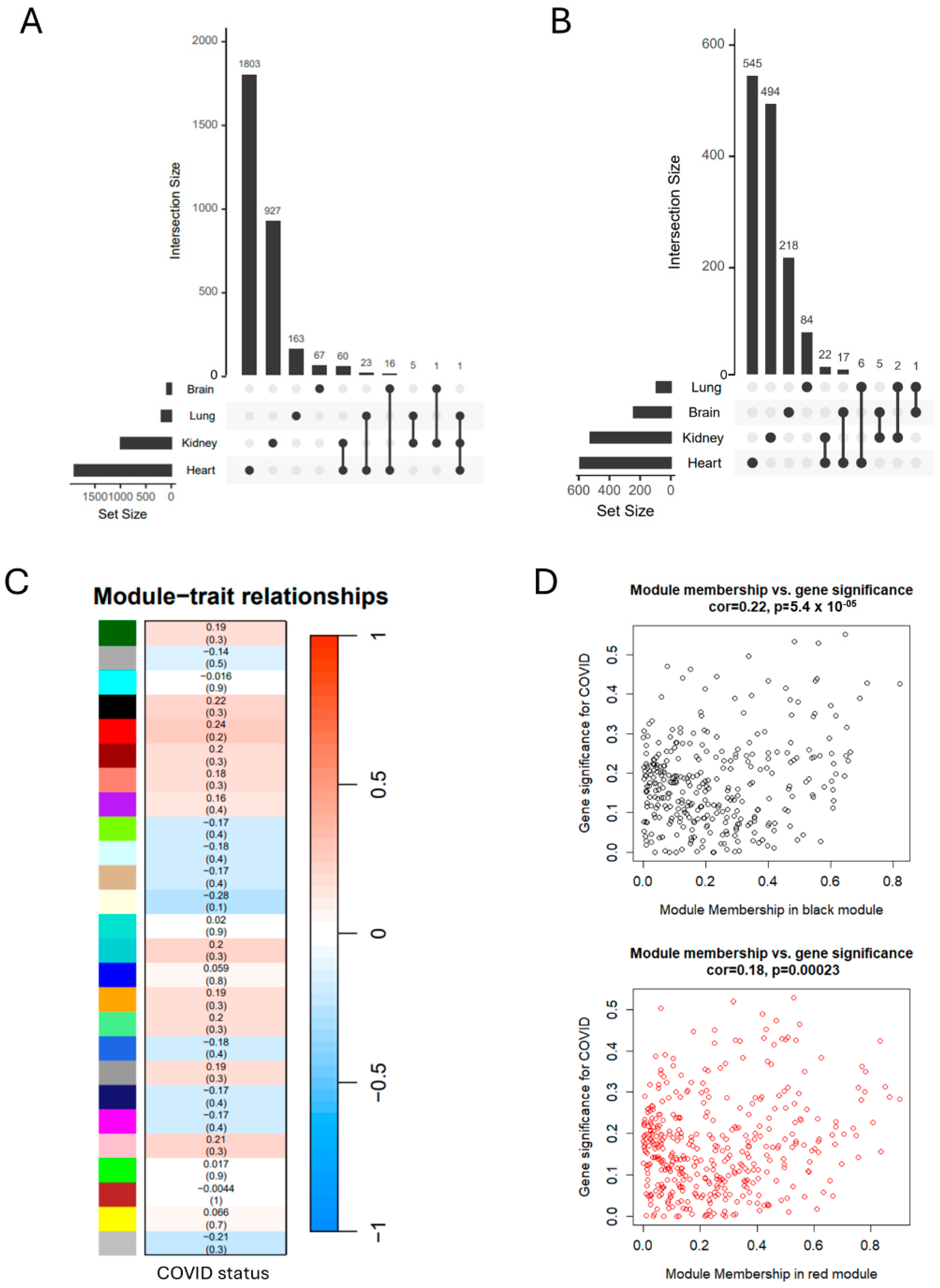
2.3. Weighted Gene Co-Expression Network Analysis (WGCNA)
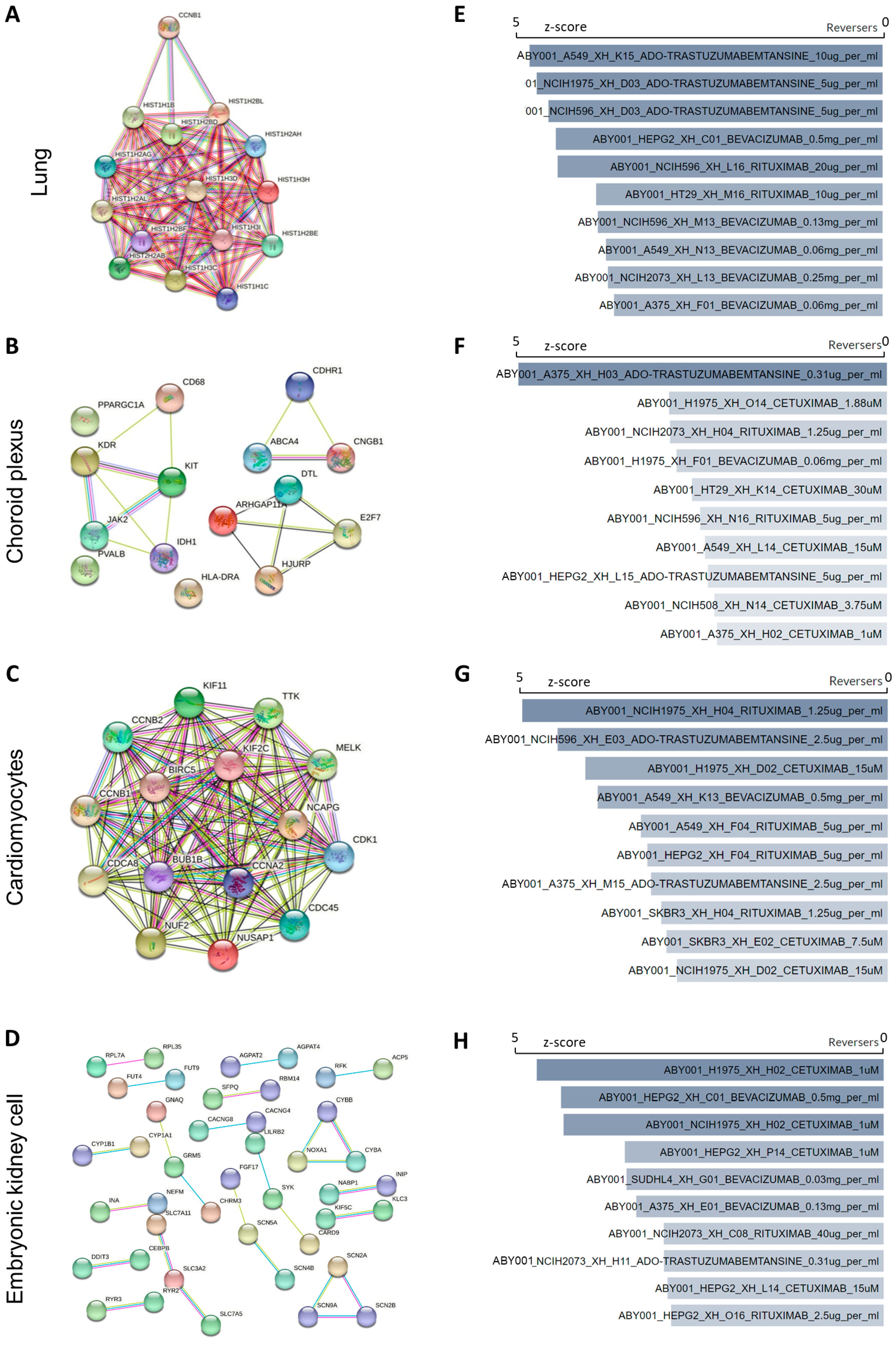
2.4. Protein–Protein Interaction Network
2.5. Identification Hub Genes
2.6. Gene–Disease Association and Candidate Drugs Prediction
2.7. Prediction of Candidate Antibody by LINCS L1000 Antibody Perturbations Database
2.8. Single-Nucleus and Single-Cell RNA Sequencing Data Processing
2.9. Single-Cell Metabolic Pathway Enrichment Calculation
2.10. Ligand-Receptor Analysis on Single-Cell Level
2.11. Subcluster and Trajectory Analysis
3. Results
3.1. Rapid Immune Responses across Different Organs after SARS-CoV-2 Infection
3.2. SARS-CoV-2 Infection Induces Organ-Specific Transcriptional Profiling
3.3. Characterization of Hub Genes–Disease Association and Prediction of Candidate Drugs and Antibodies
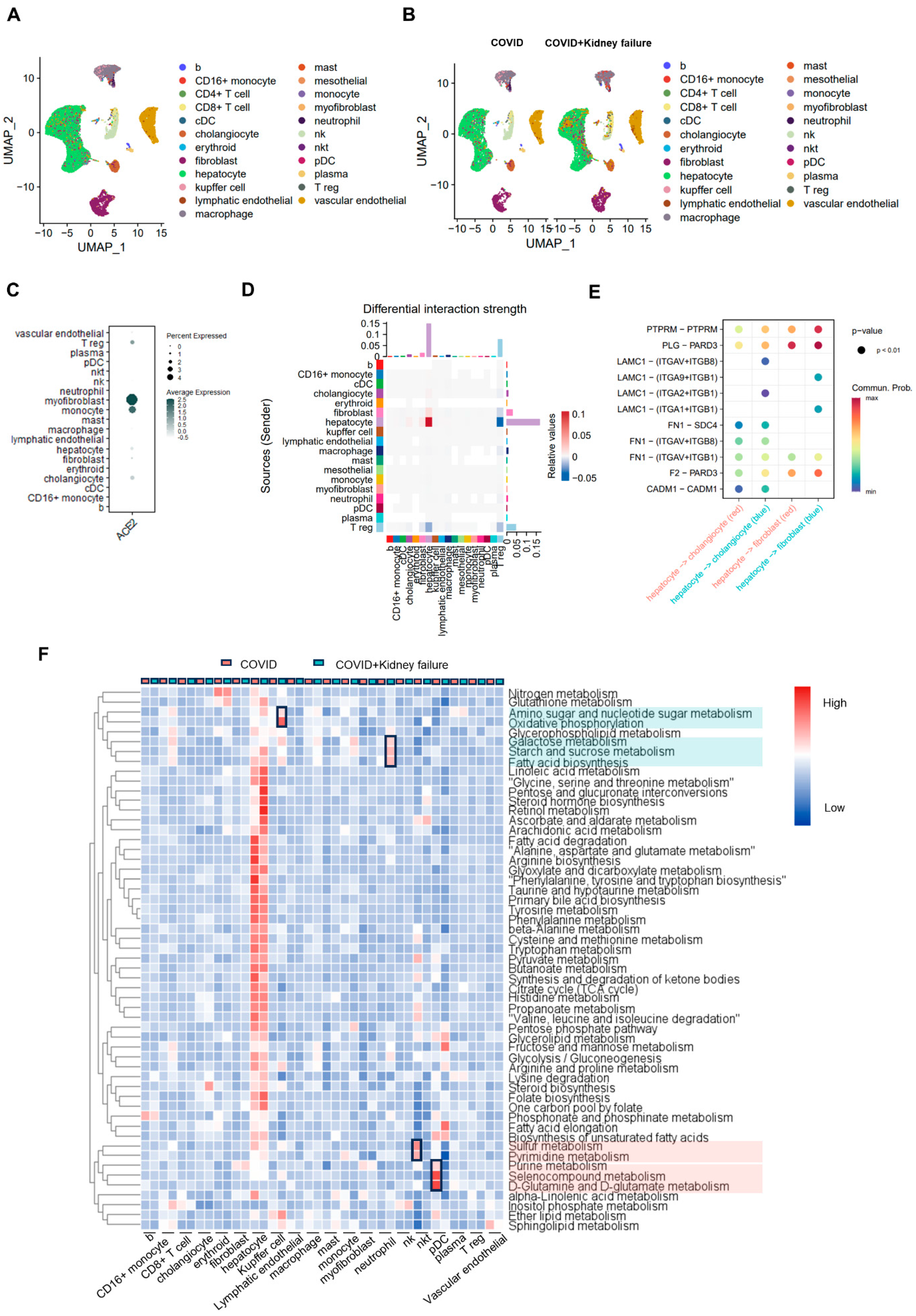
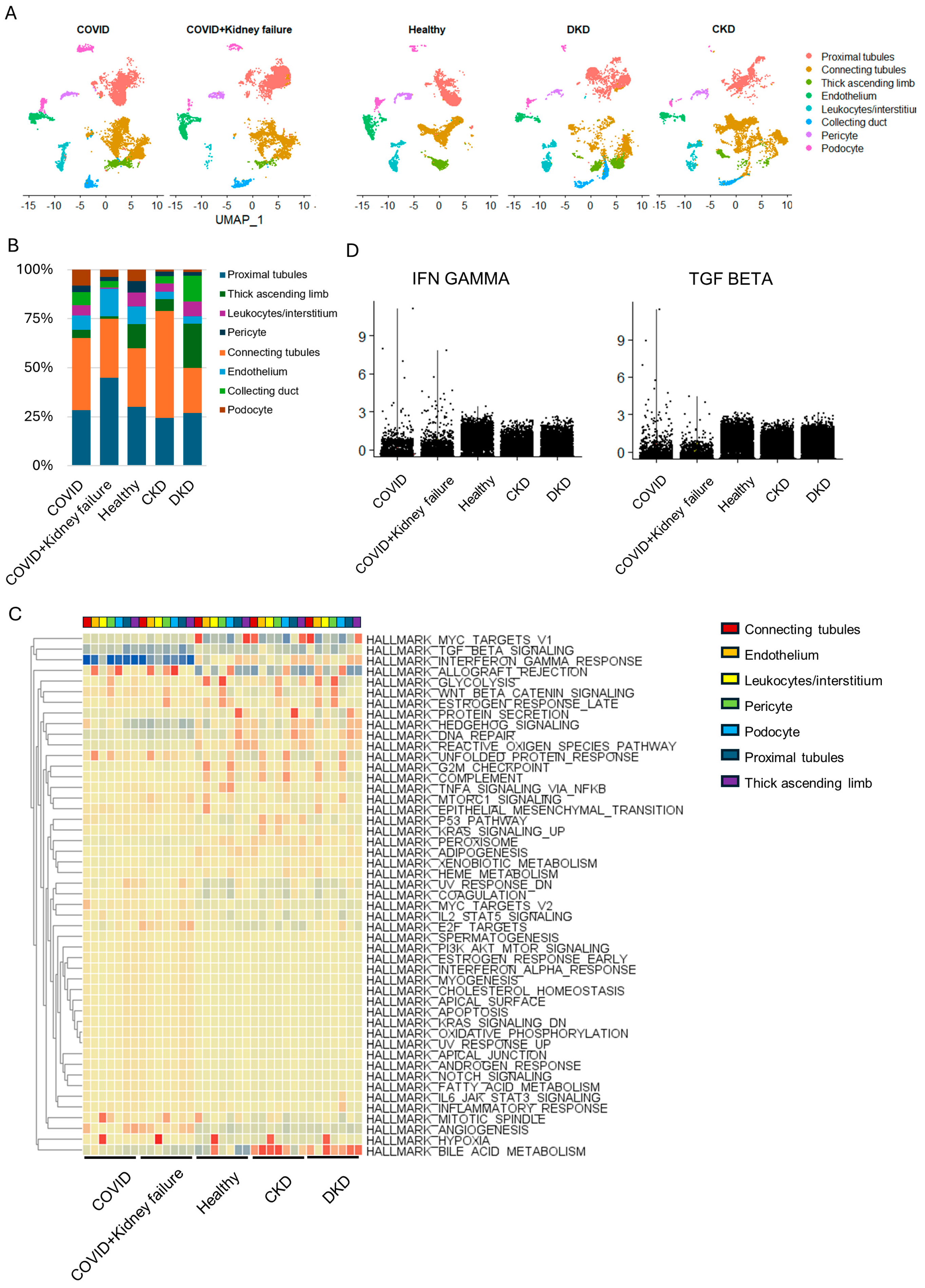
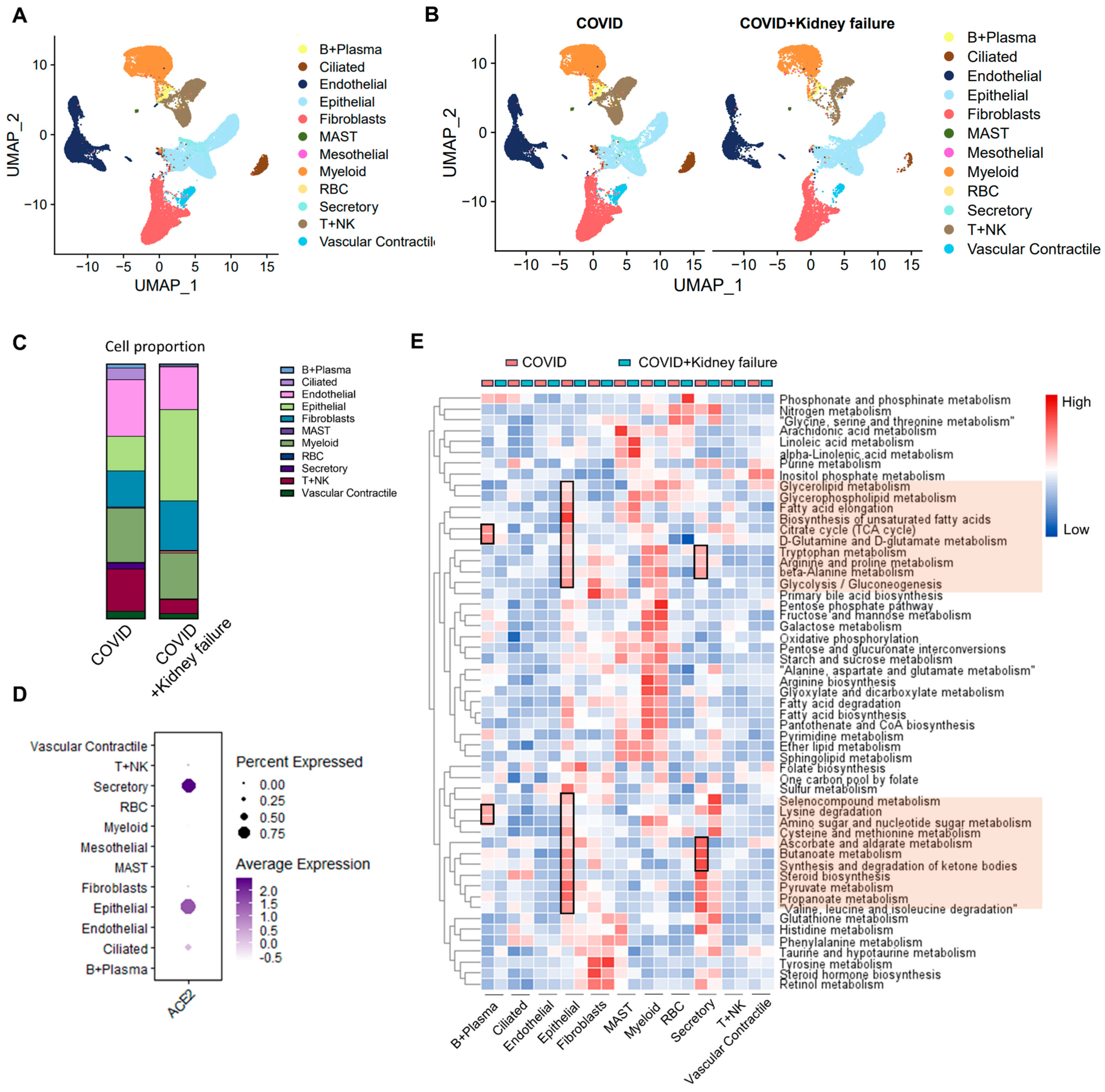
3.4. Kidney Dysfunction Results in Compromised Immune Response
3.5. Evaluate the Correlation of Kidney Failure on Lung Function in COVID-19 Patients
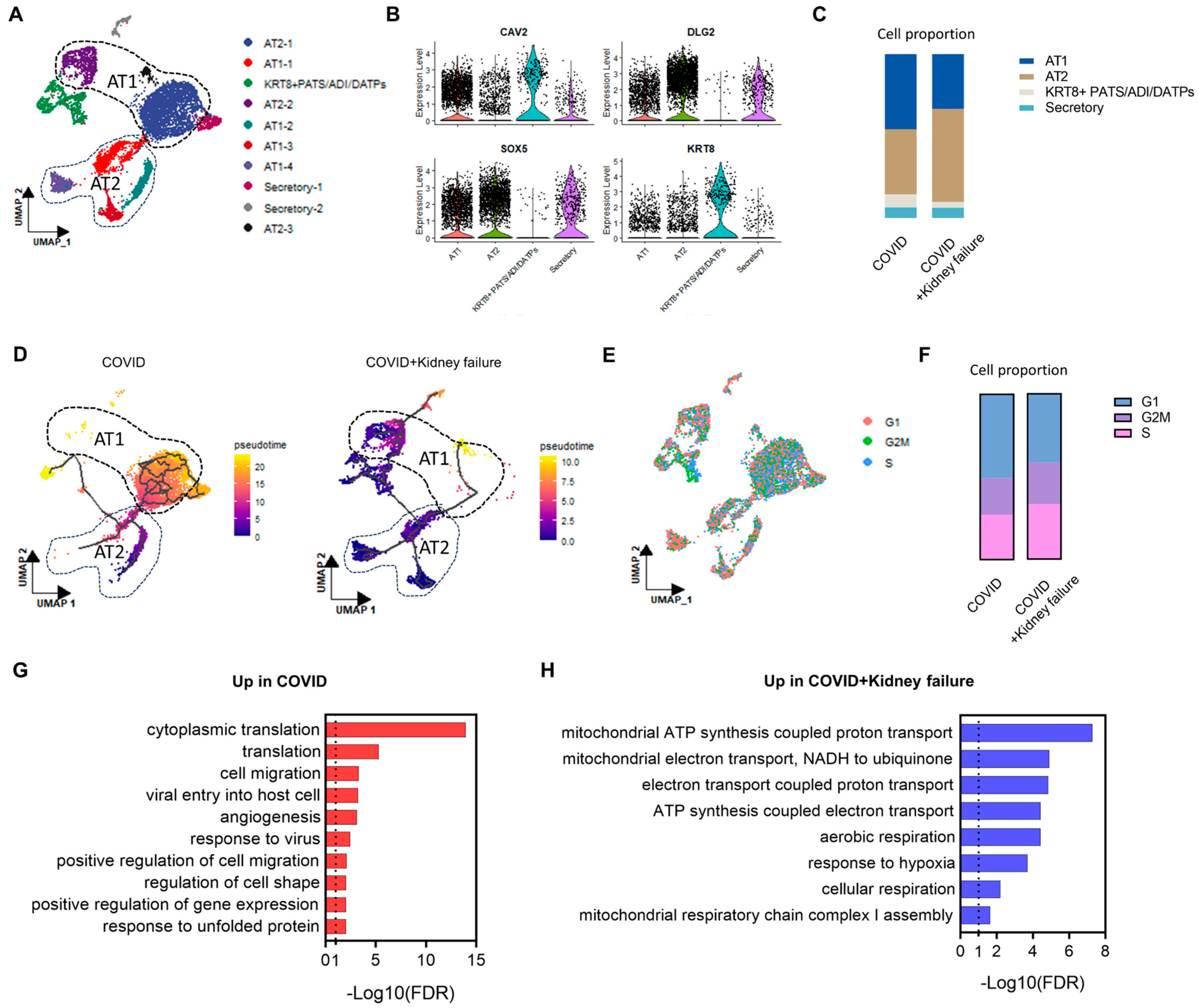
3.6. COVID-19 Patients with Kidney Failure Exhibited De-Differentiation of AT1 Cells
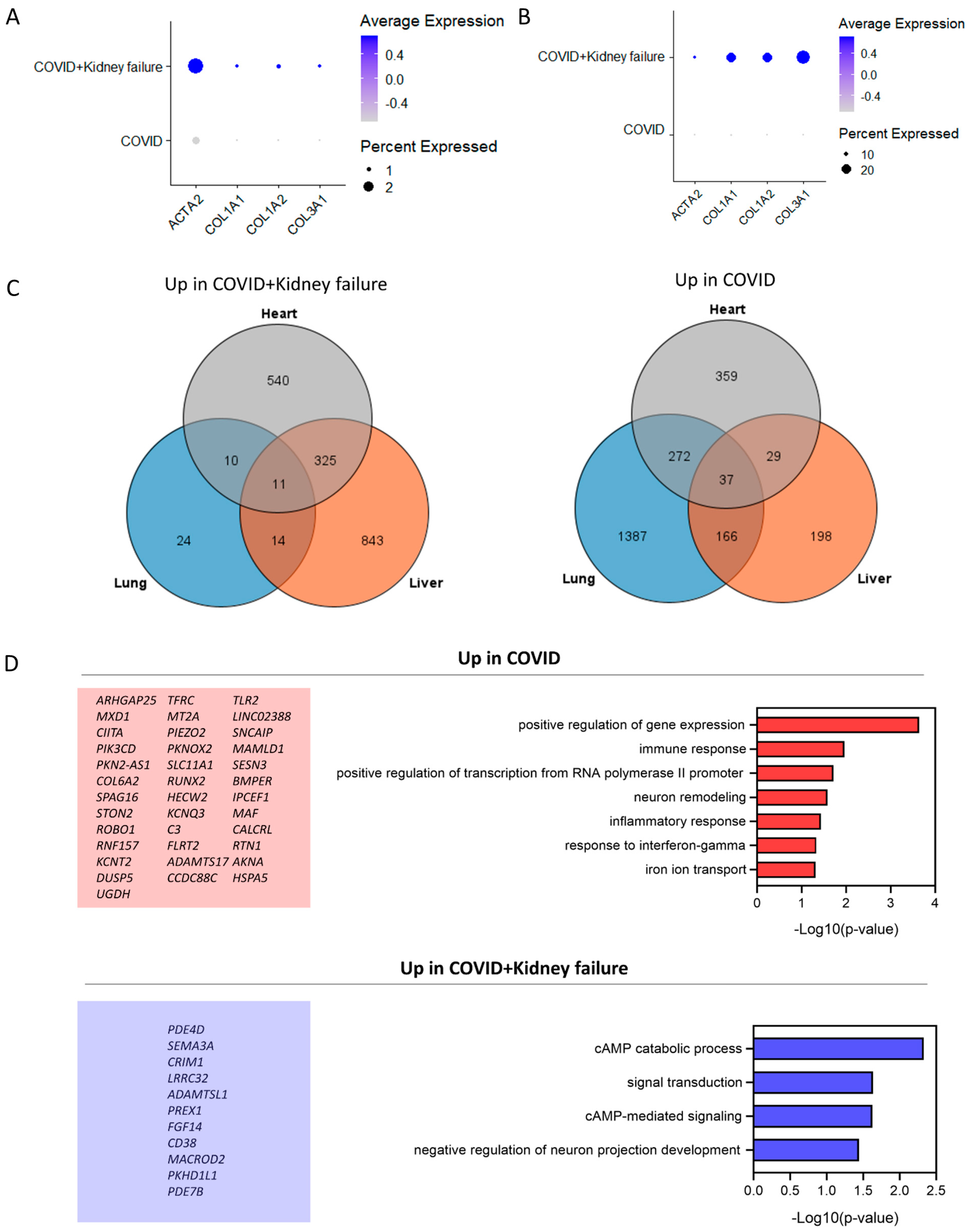
3.7. COVID-19 Patients with Kidney Failure Exhibited Increased Fibrosis in Multiple Organs

4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.S.; Ho, R.C. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-nCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the Novel Coronavirus from the Ongoing Wuhan Outbreak and Modeling of Its Spike Protein for Risk of Human Transmission. Sci. China Life Sci. 2020, 63, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-W.; Liu, X.-F.; Jia, Z.-F. 2019-nCoV Transmission through the Ocular Surface Must Not Be Ignored. Lancet 2020, 395, e39. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological Inflammation in Patients with COVID-19: A Key Role for Monocytes and Macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Meyerowitz, E.A.; Scott, J.; Richterman, A.; Male, V.; Cevik, M. Clinical Course and Management of COVID-19 in the Era of Widespread Population Immunity. Nat. Rev. Microbiol. 2023, 22, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.L.; Goßling, A.; Adam, G.; Aepfelbacher, M.; Behrendt, C.-A.; Cavus, E.; Cheng, B.; Fischer, N.; Gallinat, J.; Kühn, S.; et al. Multi-Organ Assessment in Mainly Non-Hospitalized Individuals after SARS-CoV-2 Infection: The Hamburg City Health Study COVID Programme. Eur. Heart J. 2022, 43, 1124–1137. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.F. Impact of the COVID-19 Pandemic on Mental Health and Quality of Life among Local Residents in Liaoning Province, China: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 2381. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, L.; Wang, F.-S. Liver Injury in COVID-19: Management and Challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, N.; Dinkar, A. Demographic, Clinical, and Investigational Characteristics of COVID-19- Related Guillain-Barré Syndrome with Differences from Typical and Another Virus-Related Guillain-Barré Syndrome. Infect. Disord. Drug Targets 2022, 22, 27–38. [Google Scholar] [CrossRef]
- Knížatová, N.; Massányi, M.; Roychoudhury, S.; Guha, P.; Greifová, H.; Tokárová, K.; Jambor, T.; Massányi, P.; Lukáč, N. Is There Impact of the SARS-CoV-2 Pandemic on Steroidogenesis and Fertility? Physiol. Res. 2021, 70, S161–S175. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dinkar, A.; Kumar, N.; Kumar, K. COVID-19 Associated Pancytopenia (CAP): A Clinical Impact. Recent. Adv. Inflamm. Allergy Drug Discov. 2023, 17, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, N.; Kumar, K.; Dinkar, A. COVID-19 Associated Acute Pancreatitis: A Case Series from India. Infect. Disord. Drug Targets 2022, 22, 99–103. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; Mariette, X. Systemic and Organ-Specific Immune-Related Manifestations of COVID-19. Nat. Rev. Rheumatol. 2021, 17, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Yuan, S.; Chu, H.; Sridhar, S.; Yuen, K.-Y. COVID-19 Drug Discovery and Treatment Options. Nat. Rev. Microbiol. 2024, 22, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Aklilu, A.M.; Kumar, S.; Nugent, J.; Yamamoto, Y.; Coronel-Moreno, C.; Kadhim, B.; Faulkner, S.C.; O’Connor, K.D.; Yasmin, F.; Greenberg, J.H.; et al. COVID-19—Associated Acute Kidney Injury and Longitudinal Kidney Outcomes. JAMA Intern. Med. 2024, 184, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Mahalingasivam, V.; Su, G.; Iwagami, M.; Davids, M.R.; Wetmore, J.B.; Nitsch, D. COVID-19 and Kidney Disease: Insights from Epidemiology to Inform Clinical Practice. Nat. Rev. Nephrol. 2022, 18, 485–498. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, X.; Li, C.; Liu, Y.; Cheng, J.; Adhikari, B.K.; Wang, Y. COVID-19 and the Cardiovascular System: A Study of Pathophysiology and Interpopulation Variability. Front. Microbiol. 2023, 14, 1213111. [Google Scholar] [CrossRef]
- Fedorowski, A.; Fanciulli, A.; Raj, S.R.; Sheldon, R.; Shibao, C.A.; Sutton, R. Cardiovascular Autonomic Dysfunction in Post-COVID-19 Syndrome: A Major Health-Care Burden. Nat. Rev. Cardiol. 2024, 21, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, V.; Arya, R.; Anand, U.; Priyadarshi, R.N. Association of COVID-19 with Hepatic Metabolic Dysfunction. World J. Virol. 2022, 11, 237–251. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence That Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Andrade Silva, M.; da Silva, A.R.P.A.; do Amaral, M.A.; Fragas, M.G.; Câmara, N.O.S. Metabolic Alterations in SARS-CoV-2 Infection and Its Implication in Kidney Dysfunction. Front. Physiol. 2021, 12, 624698. [Google Scholar] [CrossRef]
- Desai, N.; Neyaz, A.; Szabolcs, A.; Shih, A.R.; Chen, J.H.; Thapar, V.; Nieman, L.T.; Solovyov, A.; Mehta, A.; Lieb, D.J.; et al. Temporal and Spatial Heterogeneity of Host Response to SARS-CoV-2 Pulmonary Infection. medRxiv 2020, 11, 6319. [Google Scholar] [CrossRef]
- Jacob, F.; Pather, S.R.; Huang, W.-K.; Zhang, F.; Wong, S.Z.H.; Zhou, H.; Cubitt, B.; Fan, W.; Chen, C.Z.; Xu, M.; et al. Human Pluripotent Stem Cell-Derived Neural Cells and Brain Organoids Reveal SARS-CoV-2 Neurotropism Predominates in Choroid Plexus Epithelium. Cell Stem Cell 2020, 27, 937–950.e9. [Google Scholar] [CrossRef]
- Chen, S.; Yang, L.; Nilsson-Payant, B.; Han, Y.; Jaffré, F.; Zhu, J.; Wang, P.; Zhang, T.; Redmond, D.; Houghton, S.; et al. SARS-CoV-2 Infected Cardiomyocytes Recruit Monocytes by Secreting CCL2. preprint 2020. [Google Scholar] [CrossRef]
- Zhao, Y.; Sui, L.; Wu, P.; Li, L.; Liu, L.; Ma, B.; Wang, W.; Chi, H.; Wang, Z.-D.; Wei, Z.; et al. EGR1 Functions as a New Host Restriction Factor for SARS-CoV-2 to Inhibit Virus Replication through the E3 Ubiquitin Ligase MARCH8. J. Virol. 2023, 97, e0102823. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.J.; Courau, T.; Kuhn, N.F.; Hu, K.H.; Ray, A.; Chen, W.S.; Chew, N.W.; Cleary, S.J.; Kushnoor, D.; Reeder, G.C.; et al. Global Absence and Targeting of Protective Immune States in Severe COVID-19. Nature 2021, 591, 124–130. [Google Scholar] [CrossRef]
- Lake, B.B.; Menon, R.; Winfree, S.; Hu, Q.; Melo Ferreira, R.; Kalhor, K.; Barwinska, D.; Otto, E.A.; Ferkowicz, M.; Diep, D.; et al. An Atlas of Healthy and Injured Cell States and Niches in the Human Kidney. Nature 2023, 619, 585–594. [Google Scholar] [CrossRef]
- Delorey, T.M.; Ziegler, C.G.K.; Heimberg, G.; Normand, R.; Yang, Y.; Segerstolpe, Å.; Abbondanza, D.; Fleming, S.J.; Subramanian, A.; Montoro, D.T.; et al. COVID-19 Tissue Atlases Reveal SARS-CoV-2 Pathology and Cellular Targets. Nature 2021, 595, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-Responsive Genes Involved in Oxidative Phosphorylation Are Coordinately Downregulated in Human Diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A Comprehensive Platform Integrating Information on Human Disease-Associated Genes and Variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.; Shin, J.; Kim, J.; Ryall, K.A.; Lee, K.; Lee, S.; Jeon, M.; Kang, J.; Tan, A.C. DSigDB: Drug Signatures Database for Gene Set Analysis. Bioinformatics 2015, 31, 3069–3071. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, J.E.; Clarke, D.J.B.; Xie, Z.; Lachmann, A.; Jeon, M.; Chen, K.; Jagodnik, K.M.; Jenkins, S.L.; Kuleshov, M.V.; Wojciechowicz, M.L.; et al. SigCom LINCS: Data and Metadata Search Engine for a Million Gene Expression Signatures. Nucleic Acids Res. 2022, 50, W697–W709. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated Analysis of Multimodal Single-Cell Data. Cell 2021, 184, 3573–3587. [Google Scholar] [CrossRef]
- DeTomaso, D.; Jones, M.G.; Subramaniam, M.; Ashuach, T.; Ye, C.J.; Yosef, N. Functional Interpretation of Single Cell Similarity Maps. Nat. Commun. 2019, 10, 4376. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.-H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and Analysis of Cell-Cell Communication Using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Spielmann, M.; Qiu, X.; Huang, X.; Ibrahim, D.M.; Hill, A.J.; Zhang, F.; Mundlos, S.; Christiansen, L.; Steemers, F.J.; et al. The Single-Cell Transcriptional Landscape of Mammalian Organogenesis. Nature 2019, 566, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.; Hossain, N.; Kashem, M.A.; Shahid, M.A.; Alam, A. Immune Response in COVID-19: A Review. J. Infect. Public Health 2020, 13, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Hu, B.; Zhang, Y.; Wang, H.; Zhou, X.; Hu, W.; Cheng, Y.; Yan, J.; Ping, H.; Zhou, Q. Suspected Myocardial Injury in Patients with COVID-19: Evidence from Front-Line Clinical Observation in Wuhan, China. Int. J. Cardiol. 2020, 311, 116–121. [Google Scholar] [CrossRef]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.-Q.; Wang, Q.; Miao, H. Lymphopenia Predicts Disease Severity of COVID-19: A Descriptive and Predictive Study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef]
- Woodruff, M.C.; Bonham, K.S.; Anam, F.A.; Walker, T.A.; Faliti, C.E.; Ishii, Y.; Kaminski, C.Y.; Ruunstrom, M.C.; Cooper, K.R.; Truong, A.D.; et al. Chronic Inflammation, Neutrophil Activity, and Autoreactivity Splits Long COVID. Nat. Commun. 2023, 14, 4201. [Google Scholar] [CrossRef]
- Li, X.; Ye, Y.; Peng, K.; Zeng, Z.; Chen, L.; Zeng, Y. Histones: The Critical Players in Innate Immunity. Front. Immunol. 2022, 13, 1030610. [Google Scholar] [CrossRef]
- Lee, C.-C.; Lin, J.-C.; Hwang, W.-L.; Kuo, Y.-J.; Chen, H.-K.; Tai, S.-K.; Lin, C.-C.; Yang, M.-H. Macrophage-Secreted Interleukin-35 Regulates Cancer Cell Plasticity to Facilitate Metastatic Colonization. Nat. Commun. 2018, 9, 3763. [Google Scholar] [CrossRef]
- Charbel Issa, P.; Reuter, P.; Kühlewein, L.; Birtel, J.; Gliem, M.; Tropitzsch, A.; Whitcroft, K.L.; Bolz, H.J.; Ishihara, K.; MacLaren, R.E.; et al. Olfactory Dysfunction in Patients With CNGB1-Associated Retinitis Pigmentosa. JAMA Ophthalmol. 2018, 136, 761–769. [Google Scholar] [CrossRef]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.-G.; Neidleman, J.; Andrew, A.; Young, K.C.; Ma, T.; Hoh, R.; et al. Long COVID Manifests with T Cell Dysregulation, Inflammation and an Uncoordinated Adaptive Immune Response to SARS-CoV-2. Nat. Immunol. 2024, 25, 218–225. [Google Scholar] [CrossRef]
- Wang, G.; Xiao, B.; Deng, J.; Gong, L.; Li, Y.; Li, J.; Zhong, Y. The Role of Cytochrome P450 Enzymes in COVID-19 Pathogenesis and Therapy. Front. Pharmacol. 2022, 13, 791922. [Google Scholar] [CrossRef]
- Marshall, M. COVID and the Brain: Researchers Zero in on How Damage Occurs. Nature 2021, 595, 484–485. [Google Scholar] [CrossRef]
- Thaweerat, W. Current Evidence on Pancreatic Involvement in SARS-CoV-2 Infection. Pancreatology 2020, 20, 1013–1014. [Google Scholar] [CrossRef]
- Gęca, T.; Wojtowicz, K.; Guzik, P.; Góra, T. Increased Risk of COVID-19 in Patients with Diabetes Mellitus—Current Challenges in Pathophysiology, Treatment and Prevention. Int. J. Environ. Res. Public Health 2022, 19, 6555. [Google Scholar] [CrossRef]
- Cho, A.; Saunders, O.L.; Butler, T.; Zhang, L.; Xu, J.; Vela, J.E.; Feng, J.Y.; Ray, A.S.; Kim, C.U. Synthesis and Antiviral Activity of a Series of 1′-Substituted 4-Aza-7,9-Dideazaadenosine C-Nucleosides. Bioorg. Med. Chem. Lett. 2012, 22, 2705–2707. [Google Scholar] [CrossRef]
- Patel, M.; Dominguez, E.; Sacher, D.; Desai, P.; Chandar, A.; Bromberg, M.; Caricchio, R.; Criner, G.J. Etoposide as Salvage Therapy for Cytokine Storm Due to Coronavirus Disease 2019. Chest 2021, 159, e7–e11. [Google Scholar] [CrossRef]
- Singh, S.; Weiss, A.; Goodman, J.; Fisk, M.; Kulkarni, S.; Lu, I.; Gray, J.; Smith, R.; Sommer, M.; Cheriyan, J. Niclosamide-A Promising Treatment for COVID-19. Br. J. Pharmacol. 2022, 179, 3250–3267. [Google Scholar] [CrossRef]
- de Ligt, M.; Hesselink, M.K.C.; Jorgensen, J.; Jocken, J.W.E.; Blaak, E.E.; Goossens, G.H. The Angiotensin II Type 1 Receptor Blocker Valsartan in the Battle against COVID-19. Obesity 2021, 29, 1423–1426. [Google Scholar] [CrossRef]
- Abruzzese, E.; Luciano, L.; D’Agostino, F.; Trawinska, M.M.; Pane, F.; De Fabritiis, P. SARS-CoV-2 (COVID-19) and Chronic Myeloid Leukemia (CML): A Case Report and Review of ABL Kinase Involvement in Viral Infection. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020031. [Google Scholar] [CrossRef]
- Shionoya, K.; Yamasaki, M.; Iwanami, S.; Ito, Y.; Fukushi, S.; Ohashi, H.; Saso, W.; Tanaka, T.; Aoki, S.; Kuramochi, K.; et al. Mefloquine, a Potent Anti-Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2) Drug as an Entry Inhibitor in Vitro. Front. Microbiol. 2021, 12, 651403. [Google Scholar] [CrossRef]
- Kindrachuk, J.; Ork, B.; Hart, B.J.; Mazur, S.; Holbrook, M.R.; Frieman, M.B.; Traynor, D.; Johnson, R.F.; Dyall, J.; Kuhn, J.H.; et al. Antiviral Potential of ERK/MAPK and PI3K/AKT/mTOR Signaling Modulation for Middle East Respiratory Syndrome Coronavirus Infection as Identified by Temporal Kinome Analysis. Antimicrob. Agents Chemother. 2015, 59, 1088–1099. [Google Scholar] [CrossRef]
- Lin, H.; Singla, B.; Ghoshal, P.; Faulkner, J.L.; Cherian-Shaw, M.; O’Connor, P.M.; She, J.; Belin de Chantemele, E.J.; Csányi, G. Identification of Novel Macropinocytosis Inhibitors Using a Rational Screen of Food and Drug Administration-approved Drugs. Br. J. Pharmacol. 2018, 175, 3640–3655. [Google Scholar] [CrossRef]
- Glebov, O.O. Understanding SARS-CoV-2 Endocytosis for COVID-19 Drug Repurposing. FEBS J. 2020, 287, 3664–3671. [Google Scholar] [CrossRef]
- Mostafa, A.; Kandeil, A.; Elshaier, Y.A.M.M.; Kutkat, O.; Moatasim, Y.; Rashad, A.A.; Shehata, M.; Gomaa, M.R.; Mahrous, N.; Mahmoud, S.H.; et al. FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2. Pharmaceuticals 2020, 13, 443. [Google Scholar] [CrossRef]
- Salonia, A.; Pontillo, M.; Capogrosso, P.; Gregori, S.; Carenzi, C.; Ferrara, A.M.; Rowe, I.; Boeri, L.; Larcher, A.; Ramirez, G.A.; et al. Testosterone in Males with COVID-19: A 7-Month Cohort Study. Andrology 2022, 10, 34–41. [Google Scholar] [CrossRef]
- Dhindsa, S.; Champion, C.; Deol, E.; Lui, M.; Campbell, R.; Newman, J.; Yeggalam, A.; Nadella, S.; Ahir, V.; Shrestha, E.; et al. Association of Male Hypogonadism With Risk of Hospitalization for COVID-19. JAMA Netw. Open 2022, 5, e2229747. [Google Scholar] [CrossRef]
- Pang, J.; Xu, F.; Aondio, G.; Li, Y.; Fumagalli, A.; Lu, M.; Valmadre, G.; Wei, J.; Bian, Y.; Canesi, M.; et al. Efficacy and Tolerability of Bevacizumab in Patients with Severe COVID-19. Nat. Commun. 2021, 12, 814. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Syed-Ahmed, M.; Narayanan, M. Immune Dysfunction and Risk of Infection in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 8–15. [Google Scholar] [CrossRef]
- Kong, L.; Andrikopoulos, S.; MacIsaac, R.J.; Mackay, L.K.; Nikolic-Paterson, D.J.; Torkamani, N.; Zafari, N.; Marin, E.C.S.; Ekinci, E.I. Role of the Adaptive Immune System in Diabetic Kidney Disease. J. Diabetes Investig. 2022, 13, 213–226. [Google Scholar] [CrossRef]
- Xiao, Z.; Dai, Z.; Locasale, J.W. Metabolic Landscape of the Tumor Microenvironment at Single Cell Resolution. Nat. Commun. 2019, 10, 3763. [Google Scholar] [CrossRef]
- de Vries, L.S.; Pistorius, L.; Lichtenbelt, K.D.; Koopman, C.; Meuwissen, M.E.C.; Mancini, G.M.S. COL4A1 Mutation: Expansion of the Phenotype. Pediatr. Res. 2011, 70, 181. [Google Scholar] [CrossRef]
- Mishra, A.; Malik, R.; Hachiya, T.; Jürgenson, T.; Namba, S.; Posner, D.C.; Kamanu, F.K.; Koido, M.; Le Grand, Q.; Shi, M.; et al. Stroke Genetics Informs Drug Discovery and Risk Prediction across Ancestries. Nature 2022, 611, 115–123. [Google Scholar] [CrossRef]
- Romero-González, G.; González, A.; López, B.; Ravassa, S.; Díez, J. Heart Failure in Chronic Kidney Disease: The Emerging Role of Myocardial Fibrosis. Nephrol. Dial. Transpl. 2022, 37, 817–824. [Google Scholar] [CrossRef]
- López, B.; González, A.; Hermida, N.; Laviades, C.; Díez, J. Myocardial Fibrosis in Chronic Kidney Disease: Potential Benefits of Torasemide. Kidney Int. Suppl. 2008, 74, S19–S23. [Google Scholar] [CrossRef]
- Giacca, M.; Shah, A.M. The Pathological Maelstrom of COVID-19 and Cardiovascular Disease. Nat. Cardiovasc. Res. 2022, 1, 200–210. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 Is Associated with Changes in Brain Structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical Characteristics and Intrauterine Vertical Transmission Potential of COVID-19 Infection in Nine Pregnant Women: A Retrospective Review of Medical Records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef]
- Ma, Y.; Diao, B.; Lv, X.; Zhu, J.; Chen, C.; Liu, L.; Zhang, S.; Shen, B.; Wang, H. Epidemiological, Clinical, and Immunological Features of a Cluster of COVID-19–Contracted Hemodialysis Patients. Kidney Int. Rep. 2020, 5, 1333–1341. [Google Scholar] [CrossRef]
- Han, Y.; Duan, X.; Yang, L.; Nilsson-Payant, B.E.; Wang, P.; Duan, F.; Tang, X.; Yaron, T.M.; Zhang, T.; Uhl, S.; et al. Identification of SARS-CoV-2 Inhibitors Using Lung and Colonic Organoids. Nature 2021, 589, 270–275. [Google Scholar] [CrossRef]
- Krüger, J.; Groß, R.; Conzelmann, C.; Müller, J.A.; Koepke, L.; Sparrer, K.M.J.; Weil, T.; Schütz, D.; Seufferlein, T.; Barth, T.F.E.; et al. Drug Inhibition of SARS-CoV-2 Replication in Human Pluripotent Stem Cell-Derived Intestinal Organoids. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 935–948. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Liu, M.; He, W.-M. Integrated Transcriptomic Analysis Reveals Reciprocal Interactions between SARS-CoV-2 Infection and Multi-Organ Dysfunction, Especially the Correlation of Renal Failure and COVID-19. Life 2024, 14, 960. https://doi.org/10.3390/life14080960
Li P, Liu M, He W-M. Integrated Transcriptomic Analysis Reveals Reciprocal Interactions between SARS-CoV-2 Infection and Multi-Organ Dysfunction, Especially the Correlation of Renal Failure and COVID-19. Life. 2024; 14(8):960. https://doi.org/10.3390/life14080960
Chicago/Turabian StyleLi, Pai, Meng Liu, and Wei-Ming He. 2024. "Integrated Transcriptomic Analysis Reveals Reciprocal Interactions between SARS-CoV-2 Infection and Multi-Organ Dysfunction, Especially the Correlation of Renal Failure and COVID-19" Life 14, no. 8: 960. https://doi.org/10.3390/life14080960




