Acetazolamide as an Add-on Therapy Following Barbed Reposition Pharyngoplasty in Obstructive Sleep Apnea: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Randomization
2.4. Intervention
2.5. Outcome Measurement
2.6. Statistical Analysis
3. Results
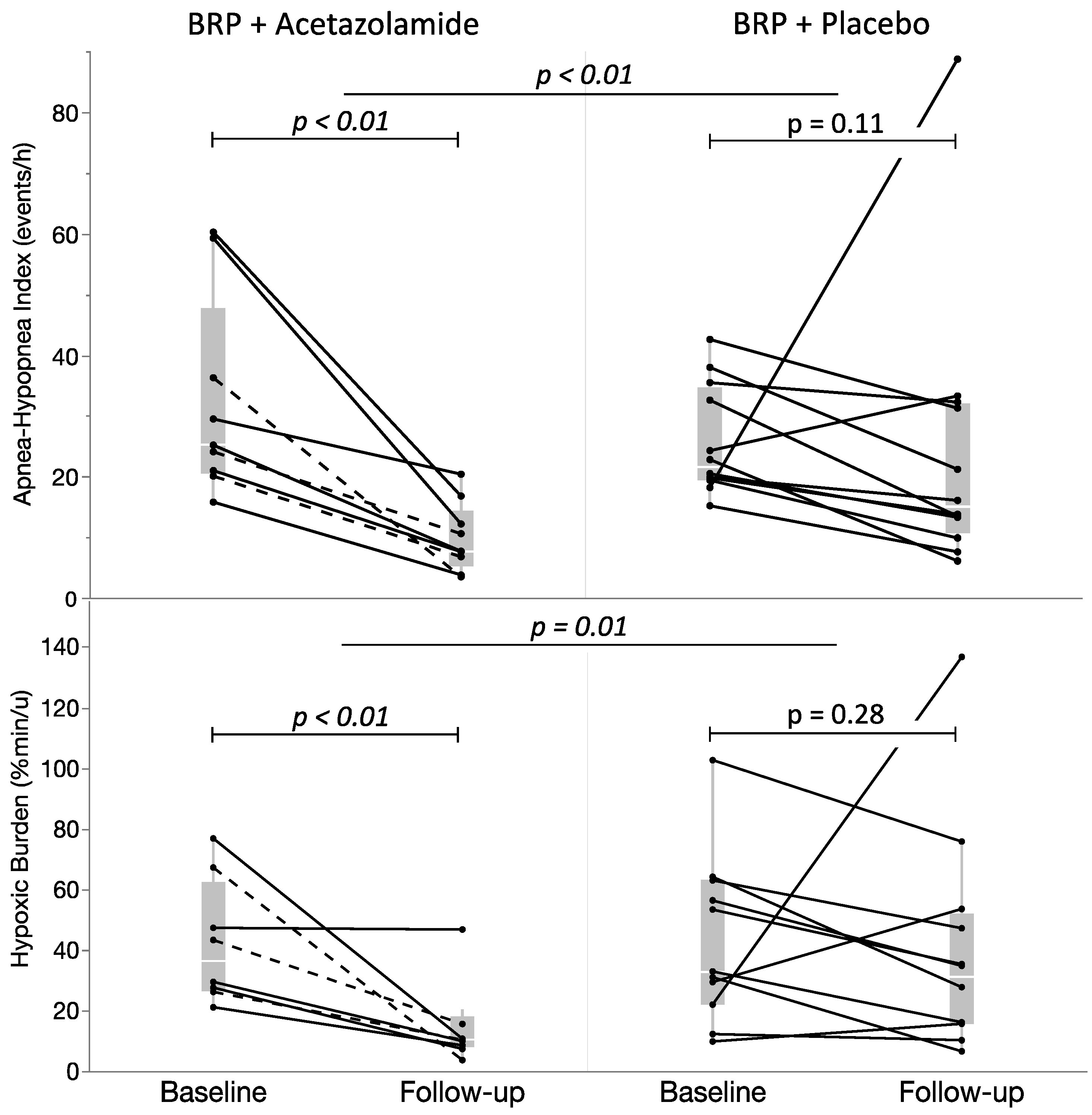
3.1. Primary Outcome
3.2. Secondary Outcomes
3.3. OSA Endotypes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef] [PubMed]
- White, D.P.; Younes, M.K. Obstructive sleep apnea. Compr. Physiol. 2012, 2, 2541–2594. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Cistulli, P.A.; Armitstead, J.; Pepin, J.L.; Woehrle, H.; Nunez, C.M.; Benjafield, A.; Malhotra, A. Short-term CPAP adherence in obstructive sleep apnea: A big data analysis using real world data. Sleep. Med. 2019, 59, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Weaver, T.E.; Sawyer, A.M. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: Implications for future interventions. Indian J. Med. Res. 2010, 131, 245–258. [Google Scholar]
- Ravesloot, M.J.; de Vries, N.; Stuck, B.A. Treatment adherence should be taken into account when reporting treatment outcomes in obstructive sleep apnea. Laryngoscope 2014, 124, 344–345. [Google Scholar] [CrossRef]
- Pang, K.P.; Woodson, B.T. Expansion sphincter pharyngoplasty: A new technique for the treatment of obstructive sleep apnea. Otolaryngol. Head Neck Surg. 2007, 137, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Cahali, M.B. Lateral pharyngoplasty: A new treatment for obstructive sleep apnea hypopnea syndrome. Laryngoscope 2003, 113, 1961–1968. [Google Scholar] [CrossRef]
- Friedman, M.; Ibrahim, H.Z.; Vidyasagar, R.; Pomeranz, J.; Joseph, N.J. Z-palatoplasty (ZPP): A technique for patients without tonsils. Otolaryngol. Head Neck Surg. 2004, 131, 89–100. [Google Scholar] [CrossRef]
- Vicini, C.; Hendawy, E.; Campanini, A.; Eesa, M.; Bahgat, A.; AlGhamdi, S.; Meccariello, G.; DeVito, A.; Montevecchi, F.; Mantovani, M. Barbed reposition pharyngoplasty (BRP) for OSAHS: A feasibility, safety, efficacy and teachability pilot study. “We are on the giant’s shoulders”. Eur. Arch. Otorhinolaryngol. 2015, 272, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Conway, W.; Zorick, F.; Roth, T. Surgical correction of anatomic azbnormalities in obstructive sleep apnea syndrome: Uvulopalatopharyngoplasty. Otolaryngol. Head Neck Surg. 1981, 89, 923–934. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Woodson, B.T.; Koka, V.; Cammaroto, G.; Iannella, G.; Bosi, M.; Pelucchi, S.; Filograna-Pignatelli, G.R.; El Chater, P.; Vicini, C. OSA Upper Airways Surgery: A Targeted Approach. Medicina 2021, 57, 690. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G.; Magliulo, G.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Pelucchi, S.; Ciorba, A.; Maniaci, A.; Cocuzza, S.; Gulotta, G.; et al. Effectiveness of drug-induced sleep endoscopy in improving outcomes of barbed pharyngoplasty for obstructive sleep apnea surgery: A prospective randomized trial. Sleep. Breath. 2022, 26, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G.; Lechien, J.R.; Perrone, T.; Meccariello, G.; Cammaroto, G.; Cannavicci, A.; Burgio, L.; Maniaci, A.; Cocuzza, S.; Di Luca, M.; et al. Barbed reposition pharyngoplasty (BRP) in obstructive sleep apnea treatment: State of the art. Am. J. Otolaryngol. 2022, 43, 103197. [Google Scholar] [CrossRef]
- Joosten, S.A.; Leong, P.; Landry, S.A.; Sands, S.A.; Terrill, P.I.; Mann, D.; Turton, A.; Rangaswamy, J.; Andara, C.; Burgess, G.; et al. Loop Gain Predicts the Response to Upper Airway Surgery in Patients With Obstructive Sleep Apnea. Sleep 2017, 40, zsx094. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, J.; Han, D.; Cao, X.; Ding, X.; Zhang, Y.; Xu, W.; Orr, J.; Jen, R.; Sands, S.; et al. Physiology-Based Modeling May Predict Surgical Treatment Outcome for Obstructive Sleep Apnea. J. Clin. Sleep Med. 2017, 13, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Schmickl, C.N.; Landry, S.; Orr, J.E.; Nokes, B.; Edwards, B.A.; Malhotra, A.; Owens, R.L. Effects of acetazolamide on control of breathing in sleep apnea patients: Mechanistic insights using meta-analyses and physiological model simulations. Physiol. Rep. 2021, 9, e15071. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, M.; Teppema, L.; Berkenbosch, A.; Olievier, C.; Folgering, H. Effect of low-dose acetazolamide on the ventilatory CO2 response during hypoxia in the anaesthetized cat. Eur. Respir. J. 1998, 12, 1271–1277. [Google Scholar] [CrossRef]
- Edwards, B.A.; Sands, S.A.; Eckert, D.J.; White, D.P.; Butler, J.P.; Owens, R.L.; Malhotra, A.; Wellman, A. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J. Physiol. 2012, 590, 1199–1211. [Google Scholar] [CrossRef]
- De Vito, A.; Carrasco Llatas, M.; Ravesloot, M.J.; Kotecha, B.; De Vries, N.; Hamans, E.; Maurer, J.; Bosi, M.; Blumen, M.; Heiser, C.; et al. European position paper on drug-induced sleep endoscopy: 2017 Update. Clin. Otolaryngol. 2018, 43, 1541–1552. [Google Scholar] [CrossRef]
- Statement on ASA Physical Status Classification System. Available online: https://www.asahq.org/standards-and-practice-parameters/statement-on-asa-physical-status-classification-system (accessed on 14 July 2024).
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.E.; Schechtman, K.B.; Piccirillo, J.F. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996, 19, 156–177. [Google Scholar] [CrossRef]
- Azarbarzin, A.; Sands, S.A.; Stone, K.L.; Taranto-Montemurro, L.; Messineo, L.; Terrill, P.I.; Ancoli-Israel, S.; Ensrud, K.; Purcell, S.; White, D.P.; et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur. Heart J. 2019, 40, 1149–1157. [Google Scholar] [CrossRef]
- Sands, S.A.; Edwards, B.A.; Terrill, P.I.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.; Hess, L.B.; White, D.P.; Wellman, A. Phenotyping Pharyngeal Pathophysiology using Polysomnography in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2018, 197, 1187–1197. [Google Scholar] [CrossRef]
- Sands, S.A.; Terrill, P.I.; Edwards, B.A.; Taranto Montemurro, L.; Azarbarzin, A.; Marques, M.; de Melo, C.M.; Loring, S.H.; Butler, J.P.; White, D.P.; et al. Quantifying the Arousal Threshold Using Polysomnography in Obstructive Sleep Apnea. Sleep 2018, 41, zsx183. [Google Scholar] [CrossRef]
- Terrill, P.I.; Edwards, B.A.; Nemati, S.; Butler, J.P.; Owens, R.L.; Eckert, D.J.; White, D.P.; Malhotra, A.; Wellman, A.; Sands, S.A. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur. Respir. J. 2015, 45, 408–418. [Google Scholar] [CrossRef]
- Browaldh, N.; Nerfeldt, P.; Lysdahl, M.; Bring, J.; Friberg, D. SKUP3 randomised controlled trial: Polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea. Thorax 2013, 68, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Sommer, U.J.; Heiser, C.; Gahleitner, C.; Herr, R.M.; Hörmann, K.; Maurer, J.T.; Stuck, B.A. Tonsillectomy with Uvulopalatopharyngoplasty in Obstructive Sleep Apnea. Dtsch. Arztebl. Int. 2016, 113, 1–8. [Google Scholar] [CrossRef]
- Malhotra, A.; Ayappa, I.; Ayas, N.; Collop, N.; Kirsch, D.; McArdle, N.; Mehra, R.; Pack, A.I.; Punjabi, N.; White, D.P.; et al. Metrics of sleep apnea severity: Beyond the apnea-hypopnea index. Sleep 2021, 44, zsab030. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Takata, K.; Sakamoto, I.; Hazama, H.; Kawahara, R. Clinical efficacy and indication of acetazolamide treatment on sleep apnea syndrome. Psychiatry Clin. Neurosci. 1999, 53, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Vanderveken, O.; Boudewyns, A.; Devolder, A.; Hamans, E.; Verbraecken, J.; Troosters, J.; Van de Heyning, P.; Backer, W. Treatment of mild sleep-disordered breathing by a combined surgical and medical approach (uvulopalatopharyngoplasty and acetazolamide). Sleep-Wake Res. Neth. 2013, 2005, 167–169. [Google Scholar]
- Joosten, S.A.; Tan, M.; Wong, A.M.; Landry, S.A.; Leong, P.; Sands, S.A.; Beatty, C.; Thomson, L.; Stonehouse, J.; Turton, A.; et al. A randomized controlled trial of oxygen therapy for patients who do not respond to upper airway surgery for obstructive sleep apnea. J. Clin. Sleep Med. 2021, 17, 445–452. [Google Scholar] [CrossRef]
- Schmickl, C.N.; Owens, R.L.; Orr, J.E.; Edwards, B.A.; Malhotra, A. Side effects of acetazolamide: A systematic review and meta-analysis assessing overall risk and dose dependence. BMJ Open Respir. Res. 2020, 7, e000557. [Google Scholar] [CrossRef] [PubMed]
- Schmickl, C.N.; Landry, S.A.; Orr, J.E.; Chin, K.; Murase, K.; Verbraecken, J.; Javaheri, S.; Edwards, B.A.; Owens, R.L.; Malhotra, A. Acetazolamide for OSA and Central Sleep Apnea: A Comprehensive Systematic Review and Meta-Analysis. Chest 2020, 158, 2632–2645. [Google Scholar] [CrossRef] [PubMed]
- Redolfi, S.; Yumino, D.; Ruttanaumpawan, P.; Yau, B.; Su, M.C.; Lam, J.; Bradley, T.D. Relationship between overnight rostral fluid shift and Obstructive Sleep Apnea in nonobese men. Am. J. Respir. Crit. Care Med. 2009, 179, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.M.; Landry, S.A.; Joosten, S.A.; Thomson, L.D.J.; Turton, A.; Stonehouse, J.; Mansfield, D.R.; Burgess, G.; Hays, A.; Sands, S.A.; et al. Examining the impact of multilevel upper airway surgery on the obstructive sleep apnoea endotypes and their utility in predicting surgical outcomes. Respirology 2022, 27, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, J.; Han, D.; Zhao, D.; Cao, X.; Orr, J.; Jen, R.; Deacon-Diaz, N.; Sands, S.A.; Owens, R.; et al. The Effect of Upper Airway Surgery on Loop Gain in Obstructive Sleep Apnea. J. Clin. Sleep Med. 2019, 1, 907–913. [Google Scholar] [CrossRef]
- Edwards, B.A.; Sands, S.A.; Owens, R.L.; White, D.P.; Genta, P.R.; Butler, J.P.; Malhotra, A.; Wellman, A. Effects of hyperoxia and hypoxia on the physiological traits responsible for obstructive sleep apnoea. J. Physiol. 2014, 592, 4523–4535. [Google Scholar] [CrossRef]
- Younes, M.; Ostrowski, M.; Thompson, W.; Leslie, C.; Shewchuk, W. Chemical control stability in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2001, 163, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Salloum, A.; Rowley, J.A.; Mateika, J.H.; Chowdhuri, S.; Omran, Q.; Badr, M.S. Increased propensity for central apnea in patients with obstructive sleep apnea: Effect of nasal continuous positive airway pressure. Am. J. Respir. Crit. Care Med. 2010, 181, 189–193. [Google Scholar] [CrossRef] [PubMed]


| Study Population (n = 26) | |
|---|---|
| Demographics and anatomical features | |
| Age, years | 46.0 (36.8–60.3) |
| Gender, male (%) | 23 (88.5) |
| BMI, kg/m2 | 28.5 (26.8–30.0) |
| Tonsil grade, No. (%) | |
| 0 | 3 (11.5) |
| 1 | 14 (53.8) |
| 2 | 6 (23.1) |
| 3 | 2 (7.7) |
| 4 | 1 (3.8) |
| Mallampati grade, No. (%) | |
| 1 | 4 (15.4) |
| 2 | 9 (34.6) |
| 3 | 5 (19.2) |
| 4 | 8 (30.8) |
| Baseline polysomnographic parameters | |
| TST, min | 425.5 (370.5–453.0) |
| SEI, % | 86.1 (74.7–91.6) |
| Mean SpO2, % | 94 (93.1–95.1) |
| T90%, %TST | 2.3 (0.4–7.9) |
| ODI3, events/h | 21.5 (15.2–29.7) |
| AHI, events/h | 23.6 (19.9–35.7) |
| SASHB, (%min/h) | 43.2 (26.4–63.8) |
| BRP + Acetazolamide (n = 9) | BRP + Placebo (n = 12) | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p | Baseline | Follow-Up | p | |
| Sleep characteristics | ||||||
| TST, min | 424 (356–446) | 414 (408–454) | 0.426 | 410 (368–453) | 424 (416–450) | 0.129 |
| SEI, % | 82.8 (73.5–89.6) | 84.7 (81.7–90.5) | 0.426 | 84.0 (73.2–94.0) | 87.1 (84.9–89.5) | 0.147 |
| REM, %TST | 20.2 (14.7–23.3) | 24.5 (18.0–30.2) | 0.059 | 20.1 (17.0–21.6) | 20.4 (16.3–26.6) | 0.176 |
| N1, %TST | 11.5 (5.0–16.0) | 5.2 (3.0–9.5) | 0.020 | 12.4 (2.1–19.6) | 8.7 (4.0–12.6) | 0.240 |
| N2, %TST | 61.8 (46.6–65.1) | 51.6 (49.0–60.9) | 0.129 | 54.3 (46.0–61.3) | 55.9 (50.6–60.0) | 0.309 |
| N3, %TST | 11.7 (6.8–21.3) | 17.2 (12.1–26.0) | 0.164 | 15.7 (3.2–21.0) | 15.8 (9.1–21.1) | 0.229 |
| Mean SpO2, % (*) | 94.1 (92.4–95.5) | 95.2 (93.8–95.7) | 0.008 | 94.0 (93.4–95.1) | 93.9 (92.9–94.4) | 0.373 |
| Nadir SpO2, % | 86 (83.5–89.0) | 89.0 (87.3–90.8) | 0.219 | 84.5 (81.5–88.3) | 86.1 (82.5–88.0) | 0.531 |
| T90%, %TST (*) | 2.3 (0.3–7.8) | 0.2 (0.0–3.3) | 0.016 | 1.8 (0.2–3.7) | 1.6 (0.7–6.5) | 0.451 |
| ODI3, events/h (*) | 22.1 (12.2–39.0) | 9.0 (5.6–15.0) | 0.004 | 17.9 (13.3–25.7) | 16.6 (9.6–26.1) | 0.622 |
| AHI, events/h (*) | 25.2 (20.6–47.8) | 7.7 (5.3–14.5) | 0.004 | 21.7 (19.5–34.8) | 15.0 (10.8–32.1) | 0.110 |
| AHIsupine, events/h | 51.6 (36.9–67.3) | 27.5 (9.1–53.2) | 0.074 | 47.5 (38.8–69.2) | 33.6 (17.1–56.9) | 0.266 |
| AHInonsupine, events/h | 19.1 (2.3–29.0) | 4.1 (2.0–8.8) | 0.055 | 9.1 (1.1–17.8) | 7.5 (3.5–26.6) | 0.684 |
| AI, events/h | 1.8 (0.5–6.8) | 0.2 (0.1–0.8) | 0.008 | 1.3 (0.0–3.4) | 0.5 (0.0–4.6) | 0.920 |
| HI, events/h (*) | 23.3 (20.0–33.2) | 7.4 (5.0–13.8) | 0.004 | 18.8 (17.5–32.4) | 14.7 (8.4–29.0) | 0.151 |
| OAHI, events/h (*) | 23.9 (20.5–46.6) | 7.6 (5.0–13.8) | 0.004 | 20.9 (18.4–34.6) | 14.7 (8.1–31.2) | 0.110 |
| CAHI, events/h | 0.2 (0.0–1.3) | 0.3 (0.0–0.8) | 0.219 | 0.2 (0.0–0.9) | 0.2 (0.0–1.2) | 0.773 |
| SASHB, (%min/h) (*) | 36.3 (26.4–62.2) | 10.3 (7.9–18.0) | 0.008 | 32.9 (21.9–62.9) | 31.2 (15.7–51.9) | 0.278 |
| Patient-reported symptom scores | ||||||
| ESS | 8.0 (4.0–15.5) | 5.0 (2.0–10.5) | 0.164 | 11.0 (5.5–14.8) | 6.0 (3.0–10.0) | 0.113 |
| FOSQ-10 | 18.0 (14.1–19.5) | 19.4 (18.1–20.0) | 0.102 | 15.4 (11.8–18.1) | 17.7 (15.0–18.8) | 0.051 |
| VAS snoring | 8.0 (6.3–9.8) | 3.0 (1.5–4.0) | 0.008 | 8.0 (6.0–10.0) | 4.0 (3.0–9.0) | 0.059 |
| BRP + Acetazolamide (n = 9) | BRP + Placebo (n = 11) | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p | Baseline | Follow-Up | p | |
| Vpassive (*) | 70.3 (64.3–73.8) | 79.8 (76.1–87.0) | 0.195 | 73.8 (67.6–78.3) | 74.9 (72.5–78.8) | 0.322 |
| Muscle compensation (Vcomp) | 0.1 (−8.8–6.3) | 3.8 (1.5–4.3) | 0.313 | 1.3 (−3.9–4.7) | 3.4 (0.4–3.7) | 0.999 |
| Arousal threshold (*) | 137.8 (107.2–156.0) | 117.8 (100.0–131.1) | 0.031 | 134.9 (109.8–154.0) | 119.6 (112.7–131.5) | 0.426 |
| LG1 | 0.62 (0.52–0.76) | 0.56 (0.37–0.78) | 0.641 | 0.53 (0.47–0.67) | 0.60 (0.48–0.73) | 0.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellemans, S.; Van de Perck, E.; Van Loo, D.; Verbraecken, J.; Sands, S.A.; Azarbarzin, A.; Dieltjens, M.; Op De Beeck, S.; Vroegop, A.; Vanderveken, O.M. Acetazolamide as an Add-on Therapy Following Barbed Reposition Pharyngoplasty in Obstructive Sleep Apnea: A Randomized Controlled Trial. Life 2024, 14, 963. https://doi.org/10.3390/life14080963
Hellemans S, Van de Perck E, Van Loo D, Verbraecken J, Sands SA, Azarbarzin A, Dieltjens M, Op De Beeck S, Vroegop A, Vanderveken OM. Acetazolamide as an Add-on Therapy Following Barbed Reposition Pharyngoplasty in Obstructive Sleep Apnea: A Randomized Controlled Trial. Life. 2024; 14(8):963. https://doi.org/10.3390/life14080963
Chicago/Turabian StyleHellemans, Simon, Eli Van de Perck, Dorine Van Loo, Johan Verbraecken, Scott A. Sands, Ali Azarbarzin, Marijke Dieltjens, Sara Op De Beeck, Anneclaire Vroegop, and Olivier M. Vanderveken. 2024. "Acetazolamide as an Add-on Therapy Following Barbed Reposition Pharyngoplasty in Obstructive Sleep Apnea: A Randomized Controlled Trial" Life 14, no. 8: 963. https://doi.org/10.3390/life14080963
APA StyleHellemans, S., Van de Perck, E., Van Loo, D., Verbraecken, J., Sands, S. A., Azarbarzin, A., Dieltjens, M., Op De Beeck, S., Vroegop, A., & Vanderveken, O. M. (2024). Acetazolamide as an Add-on Therapy Following Barbed Reposition Pharyngoplasty in Obstructive Sleep Apnea: A Randomized Controlled Trial. Life, 14(8), 963. https://doi.org/10.3390/life14080963







