Antimicrobial Susceptibility and Genomic Profiles of Multidrug-Resistant Staphylococcus aureus from Nasopharynx of Asymptomatic Children in Dhaka, Bangladesh
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sociodemographic Characteristics and Nutritional Status
2.3. Nasopharyngeal Specimen Collection and Identification of Bacterial Isolates
2.4. Antibacterial Susceptibility Test
2.5. Whole Genome Analysis
2.6. Statistical Analysis
2.7. Ethics Statement
3. Results
3.1. S. aureus Carriage, Patient Demography, Vaccination and Antibiotic Use Profiles
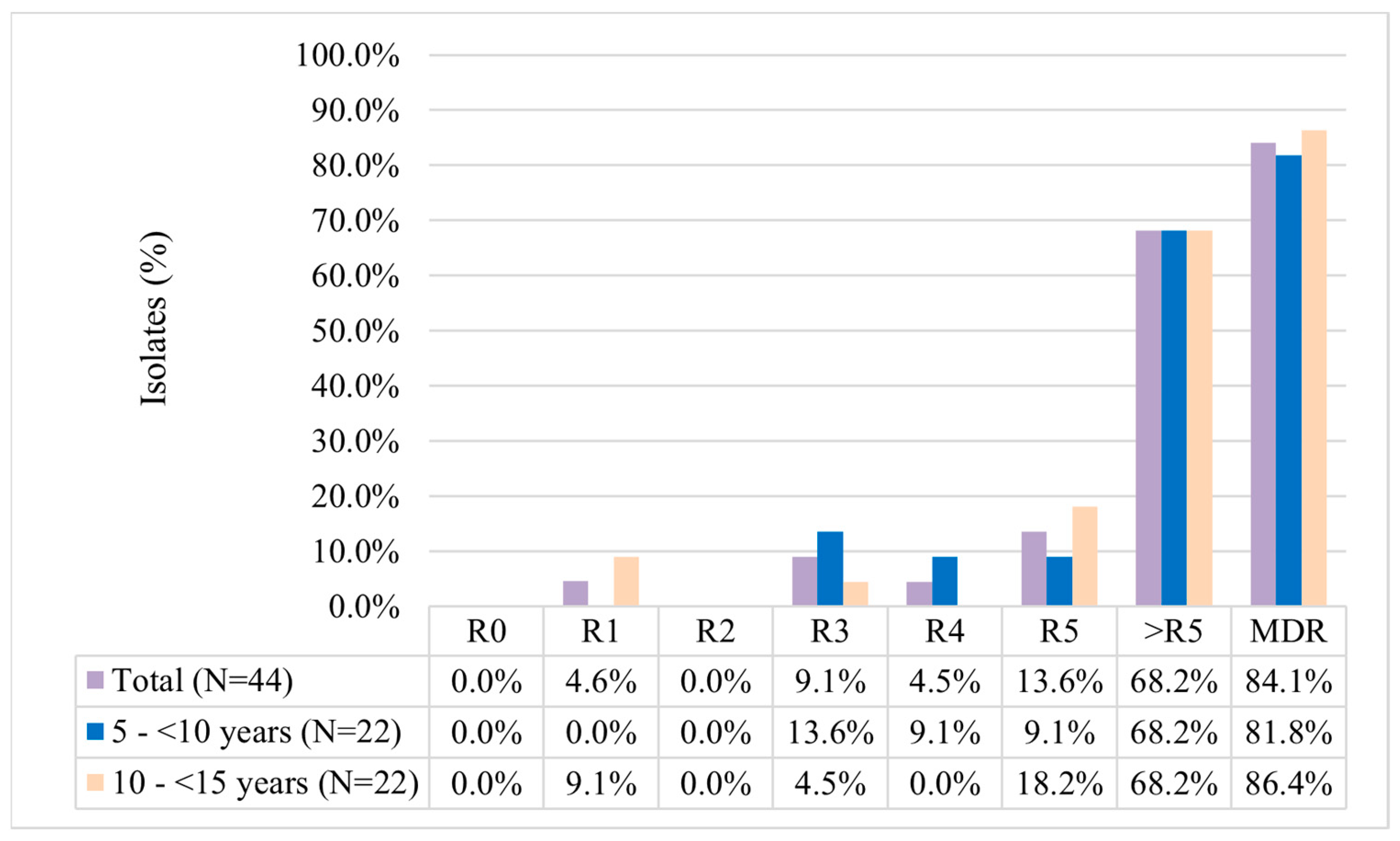
3.2. Antibiotic Resistance Profile
3.3. Whole Genome Analysis and Phenotype–Genotype Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Askarian, M.; Zeinalzadeh, A.; Japoni, A.; Alborzi, A.; Memish, Z.A. Prevalence of nasal carriage of methicillin-resistant Staphylococcus aureus and its antibiotic susceptibility pattern in healthcare workers at Namazi Hospital, Shiraz, Iran. Int. J. Infect. Dis. 2009, 13, e241–e247. [Google Scholar] [CrossRef]
- Hema-Ouangraoua, S.; Tranchot-Diallo, J.; Zongo, I.; Kabore, N.F.; Nikièma, F.; Yerbanga, R.S.; Tinto, H.; Chandramohan, D.; Ouedraogo, G.A.; Greenwood, B.; et al. Impact of mass administration of azithromycin as a preventive treatment on the prevalence and resistance of nasopharyngeal carriage of Staphylococcus aureus. PLoS ONE 2021, 16, e0257190. [Google Scholar] [CrossRef]
- Esposito, S.; Terranova, L.; Zampiero, A.; Ierardi, V.; Rios, W.P.; Pelucchi, C.; Principi, N. Oropharyngeal and nasal Staphylococcus aureus carriage by healthy children. BMC Infect. Dis. 2014, 14, 723. [Google Scholar] [CrossRef]
- Thapa, S.; Gokhale, S.; Sharma, A.L.; Sapkota, L.B.; Ansari, S.; Gautam, R.; Shrestha, S.; Neopane, P. Burden of bacterial upper respiratory tract pathogens in school children of Nepal. BMJ Open Respir. Res. 2017, 4, e000203. [Google Scholar] [CrossRef]
- Van Nguyen, K.; Zhang, T.; Thi Vu, B.N.; Dao, T.T.; Tran, T.K.; Thi Nguyen, D.N.; Thi Tran, H.K.; Thi Nguyen, C.K.; Fox, A.; Horby, P.; et al. Staphylococcus aureus nasopharyngeal carriage in rural and urban northern Vietnam. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 783–790. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Moni, S.C.; Sihan, M.N.; Saha, D.; Akter, S.; Mannan, M.A.; Shahidullah, M. Nasal carriage rate of Staphylococcus aureus and risk factors among healthcare workers and attendants of neonatal intensive care unit in a tertiary care centre in Bangladesh. J. Pediatr. Perinatol. Child Health 2022, 6, 188–199. [Google Scholar] [CrossRef]
- Taz, K.A.; Jobayer, M.; Shamsuzzaman, S.M. Nasal colonization of methicillin resistant Staphylococcus aureus among healthcare providers in a tertiary care hospital, Bangladesh. Mymensingh Med. J. 2019, 28, 627–633. [Google Scholar]
- Chen, C.J.; Huang, Y.C. New epidemiology of Staphylococcus aureus infection in Asia. Clin. Microbiol. Infect. 2014, 20, 605–623. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Ahmad, N.; Ruzan, I.N.; Abd Ghani, M.K.; Hussin, A.; Nawi, S.; Aziz, M.N.; Maning, N.; Eow, V.L.K. Characteristics of community- and hospital-acquired meticillin-resistant Staphylococcus aureus strains carrying SCCmec type IV isolated in Malaysia. J. Med. Microbiol. 2009, 58, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Aung, M.S.; Paul, S.K.; Bari, M.S.; Ahmed, S.; Sarkar, S.R.; Roy, S.; Nasreen, S.A.; Mahmud, M.C.; Hossain, M.A.; et al. Molecular epidemiological characterization of methicillin-susceptible and -resistant Staphylococcus aureus isolated from skin and soft tissue infections in Bangladesh. Microb. Drug Resist. 2019, 25, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Mairi, A.; Touati, A.; Lavigne, J.P. Methicillin-resistant Staphylococcus aureus ST80 clone: A systematic review. Toxins 2020, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.; Brégeon, F.; Mège, J.L.; Rolain, J.M.; Blin, O. Staphylococcus aureus nasal colonization: An update on mechanisms, epidemiology, risk factors, and subsequent infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef]
- Mulcahy, M.E.; McLoughlin, R.M. Host–bacterial crosstalk determines Staphylococcus aureus nasal colonization. Trends Microbiol. 2016, 24, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, V.; Barcellini, L.; Sartorio, M.U.A.; Pendezza, E.; Leone, A.; Meneghin, F.; Dilillo, D.; Zuccotti, G.V. Nutritional status of children and adolescents in three Serbian enclaves in Kosovo and Metohija. BMC Public Health 2021, 21, 794. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Rahman, S.A.; Akter, S.; Shushmita, H.; Ali, M.Y.; Billah, M.A.; Kamal, M.S.; Elahi, M.T.; Paul, D.K. The anthropometric assessment of body composition and nutritional status in children aged 2–15 years: A cross-sectional study from three districts in Bangladesh. PLoS ONE 2021, 16, e0257055. [Google Scholar] [CrossRef]
- Nordin, S.A.; Za’im, N.A.N.; Sahari, N.N.; Jamaluddin, S.F.; Ahmadi, S.; Desa, M.N.M. Staphylococcus aureus nasal carriers among medical students in a medical school. Med. J. Malaysia 2012, 67, 636–638. [Google Scholar]
- Katz, S.D.; Coagulase Test Protocol. American Society for Microbiology. 11 November 2010. Available online: https://asm.org/ASM/media/Protocol-Images/Coagulase-Test-Protocol.pdf?ext=.pdf (accessed on 11 February 2019).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Brown, D.F.J.; Edwards, D.I.; Hawkey, P.M.; Morrison, D.; Ridgway, G.L.; Towner, K.J.; Wren, M.W. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). J. Antimicrob. Chemother. 2005, 56, 1000–1018. [Google Scholar] [CrossRef]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z. The Numbers behind Bangladesh’s Goal of Middle Income Status by 2021. World Bank Blogs. 19 December 2012. Available online: https://blogs.worldbank.org/endpovertyinsouthasia/numbers-behind-bangladesh’s-goal-middle-income-status-2021 (accessed on 25 July 2023).
- Wani, R.T. Socioeconomic status scales-modified Kuppuswamy and Udai Pareekh’s scale updated for 2019. J. Fam. Med. Prim. Care 2019, 8, 1846–1849. [Google Scholar] [CrossRef] [PubMed]
- Awulachew, E.; Diriba, K.; Anja, A.; Wudneh, F. Nasopharyngeal carriage of Staphylococcus aureus and its antimicrobial resistance pattern among healthy people: Systematic review and meta-analysis. J. Bacteriol. Parasitol. 2020, 11, 1000383. [Google Scholar] [CrossRef]
- Assefa, A.; Gelaw, B.; Shiferaw, Y.; Tigabu, Z. Nasopharyngeal carriage rate and antimicrobial susceptibility pattern of potential pathogenic bacteria among paediatrics outpatients at Gondar University Teaching Hospital, Ethiopia. J. Infect. Dis. Ther. 2013, 1, 109. [Google Scholar] [CrossRef]
- Dias, S.P.; Brouwer, M.C.; van de Beek, D. Sex and gender differences in bacterial infections. Infect. Immun. 2022, 90, e0028322. [Google Scholar] [CrossRef] [PubMed]
- Al-Trad, E.I.; Chew, C.H.; Che Hamzah, A.M.; Suhaili, Z.; Rahman, N.I.A.; Ismail, S.; Puah, S.M.; Chua, K.H.; Kwong, S.M.; Yeo, C.C. The plasmidomic landscape of clinical methicillin-resistant Staphylococcus aureus isolates from Malaysia. Antibiotics 2023, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Halablab, M.A.; Hijazi, S.M.; Fawzi, M.A.; Araj, G.F. Staphylococcus aureus nasal carriage rate and associated risk factors in individuals in the community. Epidemiol. Infect. 2010, 138, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Agarwal, L.; Kumar, A.; Sengupta, C.; Singh, R.P. Prevalence of nasal colonization of methicillin-resistant Staphylococcus aureus among schoolchildren of Barabanki district, Uttar Pradesh, India. J. Fam. Med. Prim. Care 2018, 7, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Shamim, A.; Mian, M.S.; Alam, M.J.; Juliana, F.M.; Hossain, N.; Das, B.; Asma, R.; Morshed, M.; Mitra, S. Resistance pattern of cefixime against uropathogens causing urinary tract infection in selected areas of Dhaka City, Bangladesh. Int. J. Eng. Sci. 2018, 7, 33–39. [Google Scholar] [CrossRef]
- Bhatta, D.R.; Gokhale, S.; Sharma, A.L.; Gupta, U.; Gaur, A.; Gowda, S.; Raut, S.; Thapa, S.; Khadka, R. Carrier state of Haemophilus influenzae type b (Hib), Streptococcus pneumoniae, Streptococcus pyogenes, Neisseria meningitidis and Corynebacterium diphtheriae among school children in Pokhara, Nepal. Asian Pac. J. Trop. Dis. 2014, 4, 45–49. [Google Scholar] [CrossRef]
- Fathi, J.; Hashemizadeh, Z.; Dehkordi, R.S.; Bazargani, A.; Javadi, K.; Hosseini-Nave, H.; Hadadi, M. Evaluation of aminoglycoside modifying enzymes, SCCmec, coagulase gene and PCR-RFLP coagulase gene typing of Staphylococcus aureus isolates from hospitals in Shiraz, southwest of Iran. Heliyon 2022, 8, e10230. [Google Scholar] [CrossRef] [PubMed]
- Khoramrooz, S.S.; Alipoor Dolatabad, S.; Mostafapour Dolatabad, F.; Marashifard, M.; Mirzaii, M.; Dabiri, H.; Haddadi, A.; Rabani, S.M.; Shirazi, H.R.G.; Darban-Sarokhalil, D. Detection of tetracycline resistance genes, aminoglycoside modifying enzymes, and coagulase gene typing of clinical isolates of Staphylococcus aureus in the southwest of Iran. Iran. J. Basic Med. Sci. 2017, 20, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Zhang, X.; Liu, Y.; Zhang, Y.; Xu, M.; Wang, S.; Sun, Y.; Zhou, T.; Chen, L. In vitro antimicrobial activity and resistance mechanisms of the new generation tetracycline agents, eravacycline, omadacycline, and tigecycline against clinical Staphylococcus aureus isolates. Front. Microbiol. 2022, 13, 1043736. [Google Scholar] [CrossRef] [PubMed]
- Bruce, S.A.; Smith, J.T.; Mydosh, J.L.; Ball, J.; Needle, D.B.; Gibson, R.; Andam, C.P. Shared antibiotic resistance and virulence genes in Staphylococcus aureus from diverse animal hosts. Sci. Rep. 2022, 12, 4413. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, Y.; Shelenkov, A.; Chernyshkov, A.; Tyumentseva, M.; Saenko, S.; Egorova, A.; Manzeniuk, I.; Akimkin, V. Whole-genome analysis of Staphylococcus aureus isolates from ready-to-eat food in Russia. Foods 2022, 11, 2574. [Google Scholar] [CrossRef]
- Amirsoleimani, A. Investigation into the Presence, Persistence, and Fate of S. aureus, and Its Mobile Genetic Elements, in Wastewater and the Surrounding Environment. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2020. Available online: https://uknowledge.uky.edu/ce_etds/104 (accessed on 14 April 2024).
- Zhu, Z.; Liu, X.; Chen, X.; Zou, G.; Huang, Q.; Meng, X.; Pei, X.; Chen, Z.; Zhou, R.; Hu, D.; et al. Prevalence and virulence determinants of Staphylococcus aureus in wholesale and retail pork in Wuhan, Central China. Foods 2022, 11, 4114. [Google Scholar] [CrossRef]
| Variables | Total (n = 44) | 5–<10 Years 22 (50) | 10–<15 Years 22 (50) | p-Value | |
|---|---|---|---|---|---|
| Sex | Male | 25 (56.8) | 12 (54.5) | 13 (59.1) | 1.000 a |
| Female | 19 (43.2) | 10 (45.5) | 9 (40.9) | ||
| Children BMI | 16.3 ± 3.2 | 16.08 ± 3.8 | 16.56 ± 2.45 | 0.628 c | |
| Family size | 5 (4, 6) | 5 (4, 5) | 6 (5, 10) | 0.008 d | |
| No. of siblings | 3 (1, 3) | 3 (1, 3) | 2 (1, 3) | 0.942 d | |
| Persons living per room | 5 (4, 6) | 5 (4, 5) | 6 (4, 6) | 0.064 d | |
| Monthly family income (1000 BDT) | 12 (10, 15) | 12 (10, 15) | 10 (8, 13) | 0.111 d | |
| Nutritional status | |||||
| BMIZ (BMI for age) | Well-nourished | 38 (86.4) | 19 (86.4) | 19 (86.4) | 1.000 a |
| Malnourished | 6 (13.6) | 3 (13.6) | 3 (13.6) | ||
| Stunting (height for age) | Not stunted | 38 (86.4) | 21 (95.5) | 17 (77.3) | 0.185 a |
| Stunted | 6 (13.6) | 1 (4.5) | 5 (22.7) | ||
| EPI vaccination status (n = 145) | Complete | 28 (68.3) | 20 (95.2) | 8 (40) | <0.001 a |
| Incomplete | 13 (31.7) | 1 (4.8) | 12 (60) | ||
| Hib vaccination status (n = 145) | Complete | 29 (70.7) | 19 (90.5) | 10 (50) | 0.004 b |
| Incomplete | 12 (29.3) | 2 (9.5) | 10 (50) | ||
| PCV vaccination status (n = 145) | Complete | 26 (63.4) | 18 (85.7) | 8 (40) | 0.002 b |
| Incomplete | 15 (36.6) | 3 (14.3) | 12 (60) | ||
| Respiratory episode in last 3 months (n = 145) | No episode | 15 (36.6) | 11 (52.4) | 4 (20) | 0.031 b |
| One or more | 26 (63.4) | 10 (47.6) | 16 (80) | ||
| Antibiotic used in last 6 months (n = 145) | Yes | 5 (12.2) | 3 (14.3) | 2 (10) | 1.000 a |
| No | 36 (87.8) | 18 (85.7) | 18 (90) | ||
| Antibiotic Class | Name of Antibiotic | Staphylococcus aureus | MRSA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 44) | 5–<10 Years (n = 22) | 10–<15 Years (n = 22) | p-Value | Total (n = 19) | 5–<10 Years (n = 10) | 10–<15 Years (n = 9) | p-Value | ||
| Penicillin | Penicillin | 40 (90.9) | 21 (95.5) | 19 (86.4) | 0.607 a | 18 (94.7) | 9 (90) | 9 (100) | 1.000 a |
| Ampicillin | 42 (95.5) | 22 (100) | 20 (90.9) | 0.488 a | 19 (100) | 10 (100) | 9 (100) | - | |
| Amoxyclav | 27 (61.4) | 12 (54.5) | 15 (68.2) | 0.353 b | 13 (68.4) | 5 (50) | 8 (88.9) | 0.141 a | |
| Cephalosporin | Cefixime | 44 (100) | 22 (100) | 22 (100) | - | 19 (100) | 10 (100) | 9 (100) | - |
| Ceftriaxone | 19 (43.2) | 7 (31.8) | 12 (54.5) | 0.128 b | 13 (68.4) | 4 (40) | 9 (100) | 0.011 a | |
| Carbapenem | Meropenem | 21 (47.7) | 9 (40.9) | 12 (54.5) | 0.365 b | 12(63.2) | 4 (40) | 8 (88.9) | 0.057 a |
| Imipenem | 13 (29.5) | 5 (22.7) | 8 (36.4) | 0.322 b | 8 (42.1) | 3 (30) | 5 (55.6) | 0.370 a | |
| Macrolide | Azithromycin | 28 (63.6) | 13 (59.1) | 15 (68.2) | 0.531 b | 12 (63.2) | 5 (50) | 7 (77.8) | 0.350 a |
| Erythromycin | 30 (68.2) | 15 (68.2) | 15 (68.2) | 1.000 b | 12 (63.2) | 5 (50) | 7 (77.8) | 0.350 a | |
| Aminoglycoside | Gentamicin | 0 (0) | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | 0 (0) | - |
| Tobramycin | 1 (2.3) | 1 (4.5) | 0 (0) | 1.000 a | 1 (5.3) | 1 (10) | 0 (0) | 1.000 a | |
| Phenicol | Chloramphenicol | 4 (9.1) | 2 (9.1) | 2 (9.1) | 1.000 a | 2 (10.5) | 2 (20) | 0 (0) | 0.474 a |
| Quinolone | Ciprofloxacin | 20 (45.5) | 9 (40.9) | 11 (50) | 0.545 b | 11 (57.9) | 5 (50) | 6 (66.7) | 0.650 a |
| Levofloxacin | 21 (47.7) | 10 (45.5) | 11 (50) | 0.763 b | 10 (52.6) | 5 (50) | 5 (55.6) | 1.000 a | |
| Sulfa drug | Cotrimoxazole | 3 (6.8) | 1 (4.5) | 2 (9.1) | 1.000 a | 2 (10.5) | 1 (10) | 1 (11.1) | 1.000 a |
| Phenotypes | No. (%) |
|---|---|
| CFM-PEN-AMP-AMC-CTR----- | 17 (45.9) |
| CFM-PEN-AMP-AMC-CIP---- | 6 (16.2) |
| CFM-PEN-AMP-CTR-CIP-LEV--- | 2 (5.4) |
| CFM-PEN-AMP-AMC-AZM-ERY-- | 2 (5.4) |
| CFM-PEN-AMP-LEV-AZM-- | 3 (8.1) |
| CFM-AMP-CIP-LEV-AZM-ERY | 1 (2.7) |
| CFM-PEN-AMP-CIP-LEV | 1 (2.7) |
| CFM-PEN-AMP-AZM-ERY | 2 (5.4) |
| CFM-AMP-IPM-LEV-CHL | 1 (2.7) |
| CFM-PEN-AMP-AMC-ERY | 1 (2.7) |
| CFM-PEN-AMP-ERY | 1 (2.7) |
| Antibiotics | AMR Profiles | Detected AMR Genes | Detected Plasmids | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ID-1 | ID-19 | ID-52 | ID-1 | ID-19 | ID-52 | ID-1 | ID-19 | ID-52 | |
| Beta-lactams | blaI blaZ mecA | blaI blaZ mecA | rep7c (MSSA476) rep5a (pN315) rep16 (pSaa6159) rep10 (pDLK1) | rep7c (MSSA476) | rep7c (MSSA476) rep5a (pN315) rep16 (pSaa6159) rep10 (pDLK1) | ||||
| Penicillin | R | S | R | ||||||
| Ampicillin | R | S | R | ||||||
| Amoxyclav | R | S | R | ||||||
| Ceftriaxone | R | S | R | ||||||
| Cefixime | R | R | R | ||||||
| Meropenem | R | S | R | ||||||
| Imipenem | R | S | S | ||||||
| Aminoglycosides | aph-Stph | aph-Stph | aph-Stph | ||||||
| Gentamycin | S | S | S | ||||||
| Tobramycin | I | S | S | ||||||
| Macrolides | ermC | ermC | |||||||
| Azithromycin | R | S | R | ||||||
| Erythromycin | R | S | R | ||||||
| Lincosamides | ermC lmrS | lmrS | ermC lmrS | ||||||
| Clindamycin | R | - | R | ||||||
| Phenicols | |||||||||
| Chloramphenicol | R | S | S | dha1 | dha1 | dha1 | |||
| Quinolones | norA gyrA_S84L parC_S80F | norA | norA gyrA_S84L parC_S80F | ||||||
| Ciprofloxacin | R | S | R | ||||||
| Levofloxacin | R | S | R | ||||||
| Tetracyclines | |||||||||
| Tetracycline | S | - | S | tet38 | tet38 | tet38 | |||
| Tigecycline | S | - | S | mepA | mepA | mepA | |||
| Sulphonamides | |||||||||
| Cotrimoxazole | I | S | S | ||||||
| Phosphonic | |||||||||
| Fosfomycin | - | - | - | fosB-Saur | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.; Nasrin, N.; Tithi, N.S.; Khatun, F.; Asaduzzaman, M.; Topa, A.F.; Kabir, M.F.; Haque, F.K.M.; Jubair, M.; Rahman, M.; et al. Antimicrobial Susceptibility and Genomic Profiles of Multidrug-Resistant Staphylococcus aureus from Nasopharynx of Asymptomatic Children in Dhaka, Bangladesh. Life 2024, 14, 971. https://doi.org/10.3390/life14080971
Islam S, Nasrin N, Tithi NS, Khatun F, Asaduzzaman M, Topa AF, Kabir MF, Haque FKM, Jubair M, Rahman M, et al. Antimicrobial Susceptibility and Genomic Profiles of Multidrug-Resistant Staphylococcus aureus from Nasopharynx of Asymptomatic Children in Dhaka, Bangladesh. Life. 2024; 14(8):971. https://doi.org/10.3390/life14080971
Chicago/Turabian StyleIslam, Sufia, Nishat Nasrin, Nigar Sultana Tithi, Farjana Khatun, Muhammad Asaduzzaman, Anika Fatema Topa, Md Farhad Kabir, Fahim Kabir Monjurul Haque, Mohammad Jubair, Mustafizur Rahman, and et al. 2024. "Antimicrobial Susceptibility and Genomic Profiles of Multidrug-Resistant Staphylococcus aureus from Nasopharynx of Asymptomatic Children in Dhaka, Bangladesh" Life 14, no. 8: 971. https://doi.org/10.3390/life14080971






