Effects of High-Intensity Swimming Interval Training on Area, Perimeter, Circularity Index and Phenotype of Cardiac Mitochondrial Ultrastructure in Sprague Dawley Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Animal Model
2.3. High-Intensity Swimming Interval Training (HIIT-Swim) Protocol
2.4. Transmission Electron Microscopy Protocol
2.5. Mitochondrial Ultrastructural Evaluation of the Cardiac Muscle
2.6. Statistical Analysis
3. Results
3.1. Mitochondrial Morphological Parameters
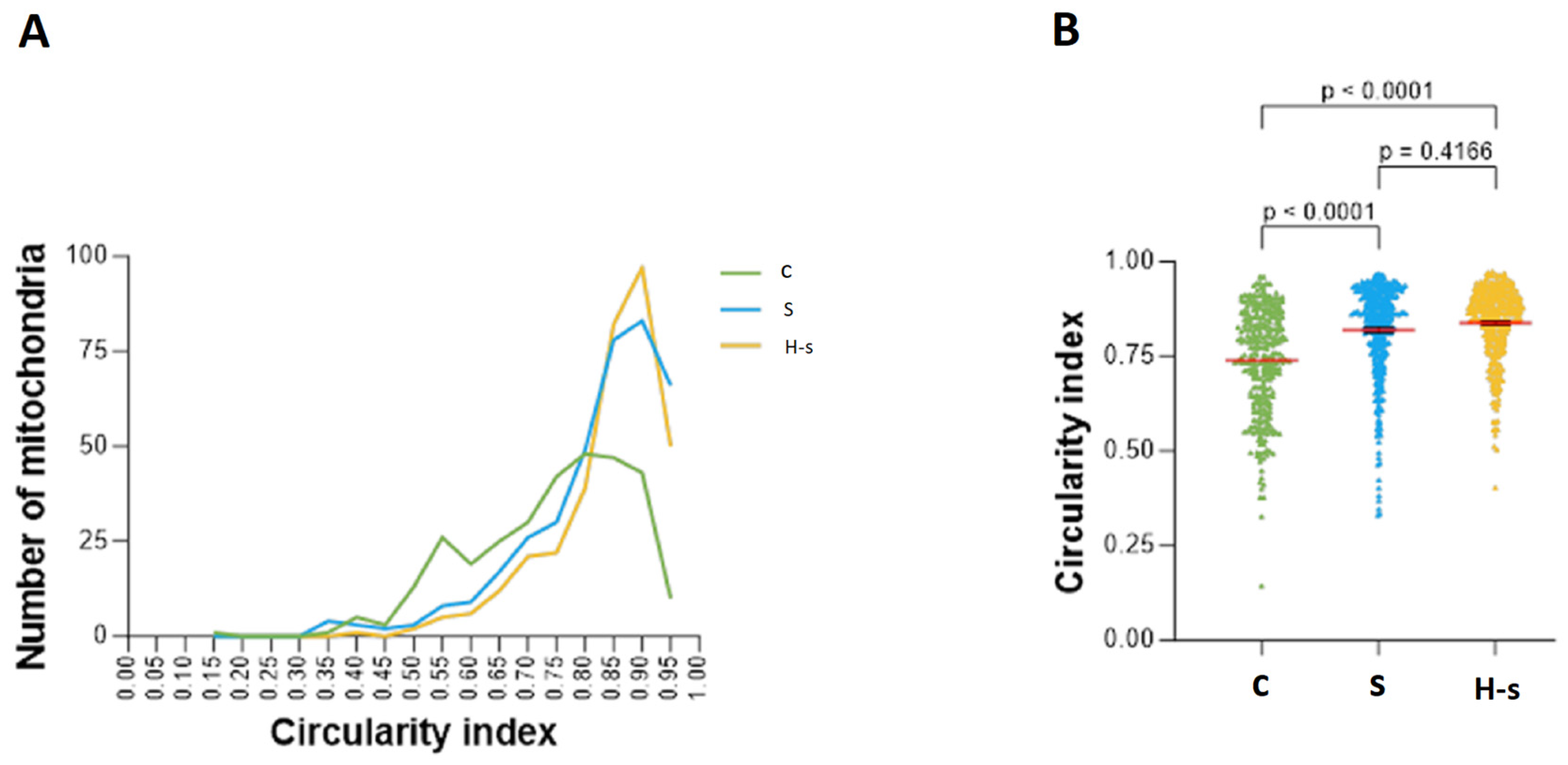
3.1.1. Mitochondrial Shape: Mitochondrial Circularity Index
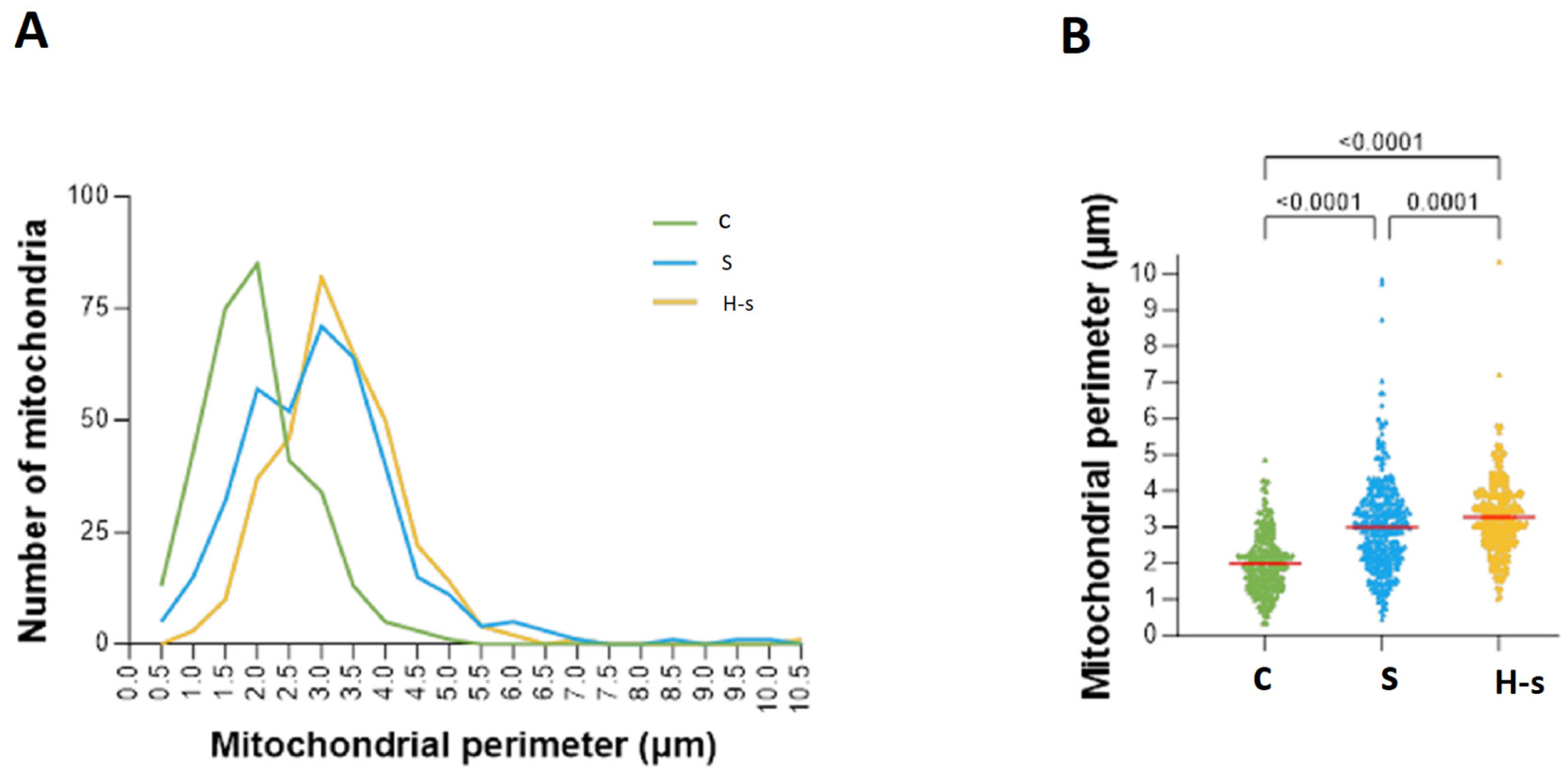
3.1.2. Mitochondrial Size: Area and Perimeter
Mitochondrial Area
Mitochondrial Perimeter
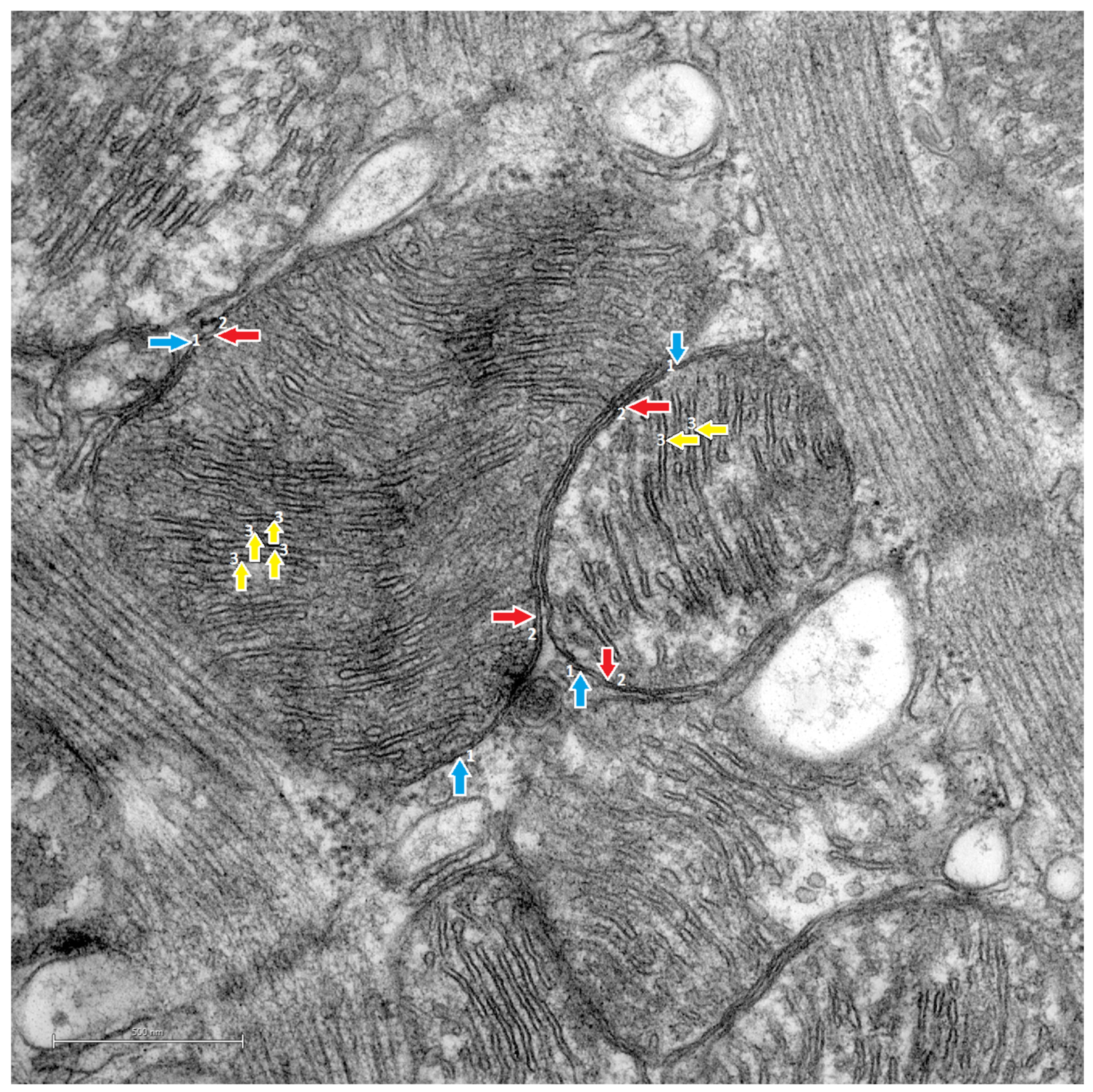
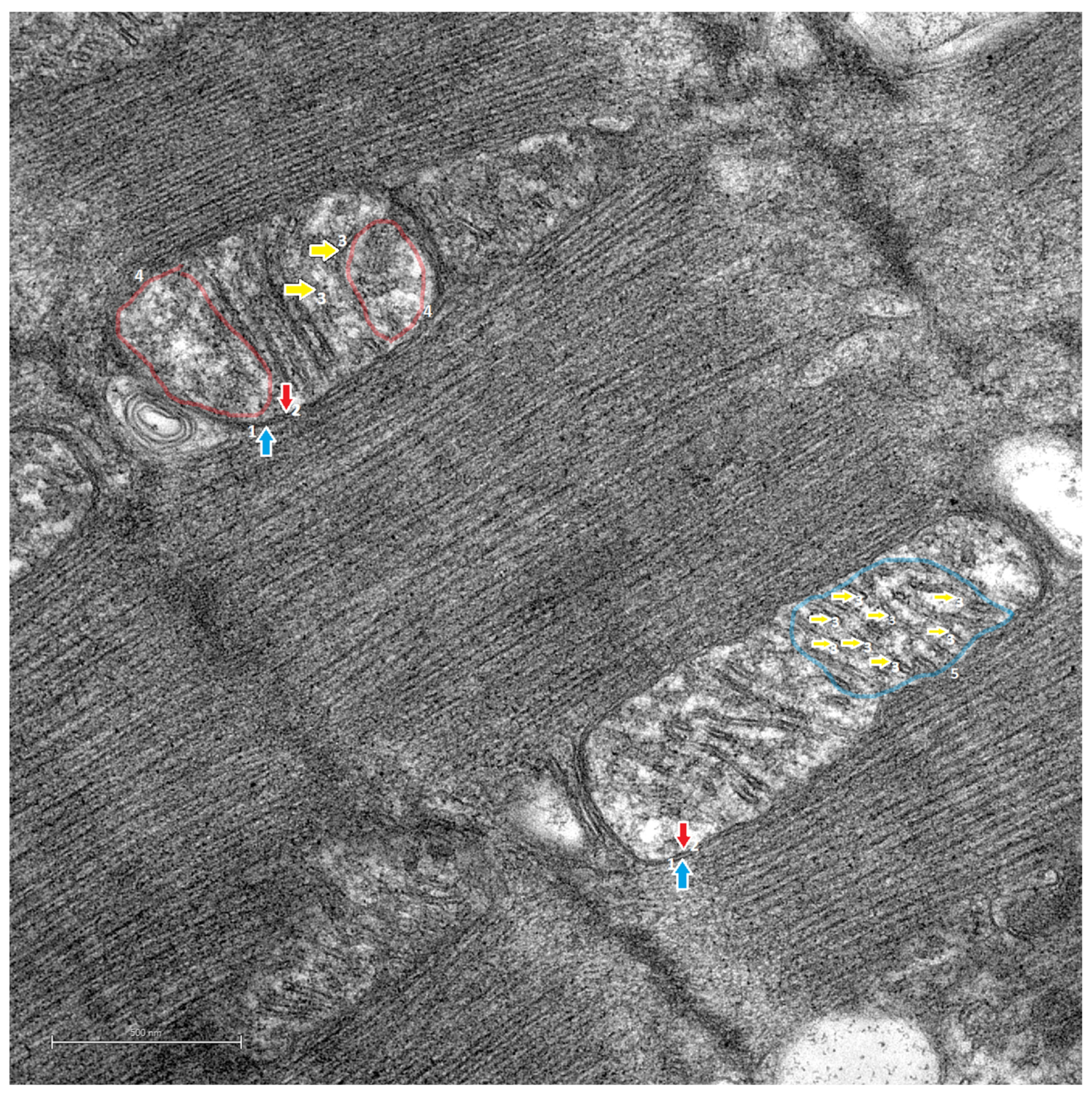
3.2. Transmission Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forrest, C.B.; Riley, A.W.; Berwick, D.M.; Nolan, T.W.; Whittington, J.; Frieden, T.R.; Dietz, W.; Collins, J.; Adler, N.E.; Newman, K.; et al. Childhood Origins of Adult Health: A Basis for Life-Course Health Policy. Health Aff. 2004, 23, 155–164. [Google Scholar] [CrossRef]
- Mora, S.; Cook, N.; Buring, J.E.; Ridker, P.M.; Lee, I.-M. Physical Activity and Reduced Risk of Cardiovascular Events. Circulation 2007, 116, 2110–2118. [Google Scholar] [CrossRef]
- Booth, F.W.; Gordon, S.E.; Carlson, C.J.; Hamilton, M.T.; Hesketh, K.; Shepherd, S.O.; Strauss, J.A.; Low, D.A.; Cooper, R.J.; Wagenmakers, A.J.M.; et al. Waging war on modern chronic diseases: Primary prevention through exercise biology. J. Appl. Physiol. 2000, 88, 774–787. [Google Scholar] [CrossRef]
- Raitakan, O.T.; Porkka, K.V.K.; Taimela, S.; Telama, R.; Räsänen, L.; Vllkari, J.S. Effects of Persistent Physical Activity and Inactivity on Coronary Risk Factors in Children and Young Adults. The Cardiovascular Risk in Young Finns Study. Am. J. Epidemiology 1994, 140, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Correa-Bautista, J.E.; Mota, J.; Garcia-Hermoso, A. Comparison of different maximal oxygen uptake equations to discriminate the cardiometabolic risk in children and adolescents. J. Pediatr. 2018, 194, 152–157.e1. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hermoso, A.; Alonso-Martinez, A.M.; Ramirez-Velez, R.; Perez-Sousa, M.A.; Ramirez-Campillo, R.; Izquierdo, M. Association of physical education with improvement of health-related physical fitness outcomes and fundamental motor skills among youths: A systematic review and meta-analysis. JAMA Pediatr. 2020, 174, e200223. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.P.; Kaminsky, L.A.; Arena, R.; Blair, S.N.; Franklin, B.A.; Myers, J.; Ross, R. Impact of cardiorespiratory fitness on all-cause and disease-specific mortality: Advances since 2009. Prog Cardiovasc Dis. 2017, 60, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.A.; Cushman, M. Recent Advances in Preventive Cardiology and Lifestyle Medicine. Circ. 2011, 123, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Kim, S.P.; Hsu, I.R.; Catalano, K.J.; Chiu, J.D.; Kabir, M.; Richey, J.M.; Ader, M. Abdominal Obesity: Role in the Pathophysiology of Metabolic Disease and Cardiovascular Risk. Am. J. Med. 2007, 120, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Physical Activity 2022; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240059160. [Google Scholar]
- Belardinelli, R.; Georgiou, D.; Cianci, G.; Purcaro, A. 10-Year exercise training in chronic heart failure. J. Am. Coll. Cardiol. 2012, 60, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Din, S.; Konstandin, M.H.; Johnson, B.; Emathinger, J.; Völkers, M.; Toko, H.; Collins, B.; Ormachea, L.; Samse, K.; Kubli, D.A.; et al. Metabolic dysfunction consistent with premature aging results from deletion of pim kinases. Circ. Res. 2014, 115, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, J.; Goldenthal, M.J. La mitocondria y el corazón. Rev. Esp. Cardiol. 2002, 55, 1293–1310. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.; Khan, M.; Nguyen, J.; Alkatib, M.; Siddiqi, S.; Hariharan, N.; Wallach, K.; Monsanto, M.; Dembitsky, W.; Sussman, M.A.; et al. Rejuvenation of human cardiac progenitor cells with pim-1 kinase. Circ. Res. 2013, 113, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac Metabolism in Heart Failure. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Duranova, H.; Valkova, V.; Knazicka, Z.; Olexikova, L.; Vasicek, J. Mitochondria: A worthwhile object for ultrastructural qualitative characterization and quantification of cells at physiological and pathophysiological states using conventional transmission electron microscopy. Acta Histochem. 2020, 122, 151646. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.A.; Eijsvogels, T.M.; Pandey, A.; Quindry, J.; Toth, P.P. Physical activity, cardiorespiratory fitness, and cardiovascular health: A clinical practice statement of the ASPC Part I: Bioenergetics, contemporary physical activity recommendations, benefits, risks, extreme exercise regimens, potential maladaptations. Am. J. Prev. Cardiol. 2022, 12, 100424. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.-P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Sciarretta, S.; Nagarajan, N.; Rubattu, S.; Volpe, M.; Frati, G.; Sadoshima, J. New insights into the role of mitochondrial dynamics and autophagy during oxidative stress and aging in the heart. Oxidative Med. Cell. Longev. 2014, 2014, 210934. [Google Scholar] [CrossRef]
- Can, S.; Soyadı, Y.A.Y.; ARSLAN, E.; Ersöz, G. Chronic Diseases and Exercise. Int. Ref. Acad. J. Sports 2015, 5, 136. [Google Scholar] [CrossRef]
- Parra, V.; Verdejo, H.; del Campo, A.; Pennanen, C.; Kuzmicic, J.; Iglewski, M.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. The complex interplay between mitochondrial dynamics and cardiac metabolism. J. Bioenerg. Biomembr. 2011, 43, 47–51. [Google Scholar] [CrossRef]
- Hackenbrock, C.R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. J. Cell Biol. 1966, 30, 269–297. [Google Scholar] [CrossRef]
- El’darov, C.M.; Vays, V.B.; Vangeli, I.M.; Kolosova, N.G.; Bakeeva, L.E. Morphometric examination of mitochondrial ultrastructure in aging cardiomyocytes. Biochemistry 2015, 80, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Acin-Perez, R.; Lechuga-Vieco, A.V.; Muñoz, M.d.M.; Nieto-Arellano, R.; Torroja, C.; Sánchez-Cabo, F.; Jiménez, C.; González-Guerra, A.; Carrascoso, I.; Benincá, C.; et al. Ablation of the stress protease OMA1 protects against heart failure in mice. Sci. Transl. Med. 2018, 10, eaan4935. [Google Scholar] [CrossRef] [PubMed]
- Gidlund, E.K. Exercise and the mitochondria. In Cardiorespiratory Fitness in Cardiometabolic Diseases; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 23–48. [Google Scholar] [CrossRef]
- Bou-Teen, D.; Fernandez-Sanz, C.; Miro-Casas, E.; Nichtova, Z.; Bonzon-Kulichenko, E.; Casós, K.; Inserte, J.; Rodriguez-Sinovas, A.; Benito, B.; Sheu, S.; et al. Defective dimerization of FoF1-ATP synthase secondary to glycation favors mitochondrial energy deficiency in cardiomyocytes during aging. Aging Cell 2022, 21, e13564. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Komuro, I. Heart Failure as an Aging-Related Phenotype. Int. Heart J. 2018, 59, 6–13. [Google Scholar] [CrossRef]
- Searls, Y.M.; Smirnova, I.V.; Fegley, B.R.; Stehno-Bittel, L. Exercise attenuates diabetes-induced ultrastructural changes in rat cardiac tissue. Med. Sci. Sports Exerc. 2004, 36, 1863–1870. [Google Scholar] [CrossRef]
- Rector, R.S.; Uptergrove, G.M.; Borengasser, S.J.; Mikus, C.R.; Morris, E.M.; Naples, S.P.; Laye, M.J.; Laughlin, M.H.; Booth, F.W.; Ibdah, J.A.; et al. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am. J. Physiol. Metab. 2010, 298, E1179–E1187. [Google Scholar] [CrossRef]
- Hafstad, A.D.; Lund, J.; Hadler-Olsen, E.; Höper, A.C.; Larsen, T.S.; Aasum, E. High- and moderate-intensity training normalizes ventricular function and mechanoenergetics in mice with diet-induced Obesity. Diabetes 2013, 62, 2287–2294. [Google Scholar] [CrossRef]
- Tomczak, C.R.; Thompson, R.B.; Paterson, I.; Schulte, F.; Cheng-Baron, J.; Haennel, R.G.; Haykowsky, M.J. Effect of acute high-intensity interval exercise on postexercise biventricular function in mild heart failure. J. Appl. Physiol. 2011, 110, 398–406. [Google Scholar] [CrossRef]
- Wisloff, U.; Stoylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, O.; Haram, P.M.; Tjonna, A.E.; Helgerud, J.; Slordahl, S.A.; Lee, S.J.; et al. Superior Cardiovascular Effect of Aerobic Interval Training versus Moderate Continuous Training in Heart Failure Patients—A Randomized Study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; Institute for Laboratory Animal Research; Division on Earth and Life Studies; National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: New York, NY, USA, 2010.
- Morton, D.; Griffiths, P. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Veter Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Amirazodi, F.; Mehrabi, A.; Amirazodi, M.; Parsania, S.; Rajizadeh, M.A.; Esmaeilpour, K. The combination effects of resveratrol and swimming HIIT exercise on novel object recognition and open-field tasks in aged rats. Exp. Aging Res. 2020, 46, 336–358. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Paukov, V.S.; Kazanskaya, T.A.; Frolov, V.A. Quantitative analysis of some components of myocardial electron micrographs. Bull. Exp. Biol. Med. 1971, 71, 469–472. [Google Scholar] [CrossRef]
- Li, Y.-J.; Yang, L.-P.; Hou, J.-L.; Li, X.-M.; Chen, L.; Zhu, J.-H.; Wang, Q.-Y.; Li, G.; Zhao, P.-Y.; Liu, X.-H.; et al. Prenatal stress impairs postnatal learning and memory development via disturbance of the cgmp–pkg pathway and oxidative phosphorylation in the hippocampus of rats. Front. Mol. Neurosci. 2020, 13, 158. [Google Scholar] [CrossRef]
- Lam, J.; Katti, P.; Biete, M.; Mungai, M.; AshShareef, S.; Neikirk, K.; Lopez, E.G.; Vue, Z.; Christensen, T.A.; Beasley, H.K.; et al. A universal approach to analyzing transmission electron microscopy with Imagej. Cells 2021, 10, 2177. [Google Scholar] [CrossRef]
- Scholtes, C.; Bellemin, S.; Martin, E.; Carre-Pierrat, M.; Mollereau, B.; Gieseler, K.; Walter, L. DRP-1-mediated apoptosis induces muscle degeneration in dystrophin mutants. Sci. Rep. 2018, 8, 7354. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Joubert, F.; Puff, N. Mitochondrial Cristae Architecture and Functions: Lessons from Minimal Model Systems. Membranes 2021, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann-Freestone, M.; Madsen, P.L.; Manners, D.; Blamire, A.M.; Buckingham, R.E.; Styles, P.; Radda, G.K.; Neubauer, S.; Clarke, K. Abnormal Cardiac and Skeletal Muscle Energy Metabolism in Patients with Type 2 Diabetes. Circulation 2003, 107, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Mourier, A.; Tain, L.S.; Partridge, L.; Larsson, N.-G.; Kühlbrandt, W. Changes of mitochondrial ultrastructure and function during ageing in mice and Drosophila. Elife 2017, 6, e24662. [Google Scholar] [CrossRef]
- Li, Y.M. Effectiveness of high-intensity interval training on different training populations. Sport. Sci. 2015, 35, 59–75. [Google Scholar]
- You, Y.; Li, W.; Liu, J.; Li, X.; Fu, Y.; Ma, X. Bibliometric Review to Explore Emerging High-Intensity Interval Training in Health Promotion: A New Century Picture. Front. Public. Health 2021, 9, 697633. [Google Scholar] [CrossRef]
- Wewege, M.A.; Ahn, D.; Yu, J.; Liou, K.; Keech, A. High-Intensity Interval Training for Patients with Cardiovascular Disease—Is It Safe? A Systematic Review. J. Am. Heart Assoc. 2018, 7, e009305. [Google Scholar] [CrossRef]
- Kramps, K.; Lane-Cordova, A. High-intensity interval training in cardiac rehabilitation. Sport Sci. Health 2021, 17, 269–278. [Google Scholar] [CrossRef]
- Maestu, E.; Harro, J.; Veidebaum, T.; Kurrikoff, T.; Jurimae, J.; Maestu, J. Changes in cardiorespiratory fitness through adolescence predictmetabolic syndrome in young adults. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 701–708. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, R.; Wang, Y.; Liu, X.; Shou, X.; Yang, Y.; Yang, C.; Tong, Q.; Mao, G.; Wu, Q. Effect of High-Intensity Interval Training on Cardiac Structure and Function in Rats with Acute Myocardial Infarct. Biomed. Pharmacother. 2020, 131, 110690. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Paumard, P.; Vaillier, J.; Coulary, B.; Schaeffer, J.; Soubannier, V.; Mueller, D.M.; Brèthes, D.; di Rago, J.-P.; Velours, J. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002, 21, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.; Mathai, J.P.; McBride, H.M.; Shore, G.C. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J. 2005, 24, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, L.; Kim, E.; Tran, D.; Phinney, B.S.; Knowlton, A.A. Mitochondrial proteome remodeling in ischemic heart failure. Life Sci. 2014, 101, 27–36. [Google Scholar] [CrossRef]
- Ong, S.-B.; Hausenloy, D.J. Mitochondrial morphology and cardiovascular disease. Cardiovasc. Res. 2010, 88, 16–29. [Google Scholar] [CrossRef]
- Perry, C.G.R.; Lally, J.; Holloway, G.P.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 2010, 588, 4795–4810. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Joseph, A.-M. Mitochondrial assembly: Protein import. Proc. Nutr. Soc. 2004, 63, 293–300. [Google Scholar] [CrossRef]
- Zuurbier, C.J.; Bertrand, L.; Beauloye, C.R.; Andreadou, I.; Ruiz-Meana, M.; Jespersen, N.R.; Kula-Alwar, D.; Prag, H.A.; Eric Botker, H.; Dambrova, M.; et al. Cardiac metabolism as a driver and therapeutic target of myocardial infarction. J. Cell. Mol. Med. 2020, 24, 5937–5954. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, S.E.; Calvani, R.; Marzetti, E. The interplay between autophagy and mitochondrial dysfunction in oxidative stress-induced cardiac aging and pathology. J. Mol. Cell. Cardiol. 2014, 71, 62–70. [Google Scholar] [CrossRef]
- Yan, Z.; Lira, V.A.; Greene, N.P. Exercise Training-Induced Regulation of Mitochondrial Quality. Exerc. Sport Sci. Rev. 2012, 40, 159–164. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Morphometric Parameters | C | S | H-s |
|---|---|---|---|---|
| Mitochondrial shape | Circularity index | 0.738 ± 0.007 | 0.818 ± 0.006 * | 0.837 ± 0.005 * |
| Mitochondrial size | Mitochondrial area (µm2) | 0.262 ± 0.011 | 0.645 ± 0.024 * | 0.761 ± 0.024 *# |
| Perimeter (µm) | 1.982 ± 0.045 | 2.992 ± 0.065 * | 3.264 ± 0.054 *# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasmiño, G.; Paredes, M.; Silva, H. Effects of High-Intensity Swimming Interval Training on Area, Perimeter, Circularity Index and Phenotype of Cardiac Mitochondrial Ultrastructure in Sprague Dawley Rats. Life 2024, 14, 984. https://doi.org/10.3390/life14080984
Pasmiño G, Paredes M, Silva H. Effects of High-Intensity Swimming Interval Training on Area, Perimeter, Circularity Index and Phenotype of Cardiac Mitochondrial Ultrastructure in Sprague Dawley Rats. Life. 2024; 14(8):984. https://doi.org/10.3390/life14080984
Chicago/Turabian StylePasmiño, Grace, Marco Paredes, and Héctor Silva. 2024. "Effects of High-Intensity Swimming Interval Training on Area, Perimeter, Circularity Index and Phenotype of Cardiac Mitochondrial Ultrastructure in Sprague Dawley Rats" Life 14, no. 8: 984. https://doi.org/10.3390/life14080984
APA StylePasmiño, G., Paredes, M., & Silva, H. (2024). Effects of High-Intensity Swimming Interval Training on Area, Perimeter, Circularity Index and Phenotype of Cardiac Mitochondrial Ultrastructure in Sprague Dawley Rats. Life, 14(8), 984. https://doi.org/10.3390/life14080984






