Anti-Cataract Effect of the Traditional Aqueous Extract of Yerba Mate (Ilex paraguariensis A. St.-Hil.): An In Ovo Perspective
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Plant
2.2. Plant Extraction
2.3. Determination of Phenolic Compounds
2.4. Experimental Animals and Study Design
2.5. Biochemical Analysis
2.6. Immunohistochemical Analysis
2.7. Genetic Analysis
2.8. Statistical Analysis
3. Results
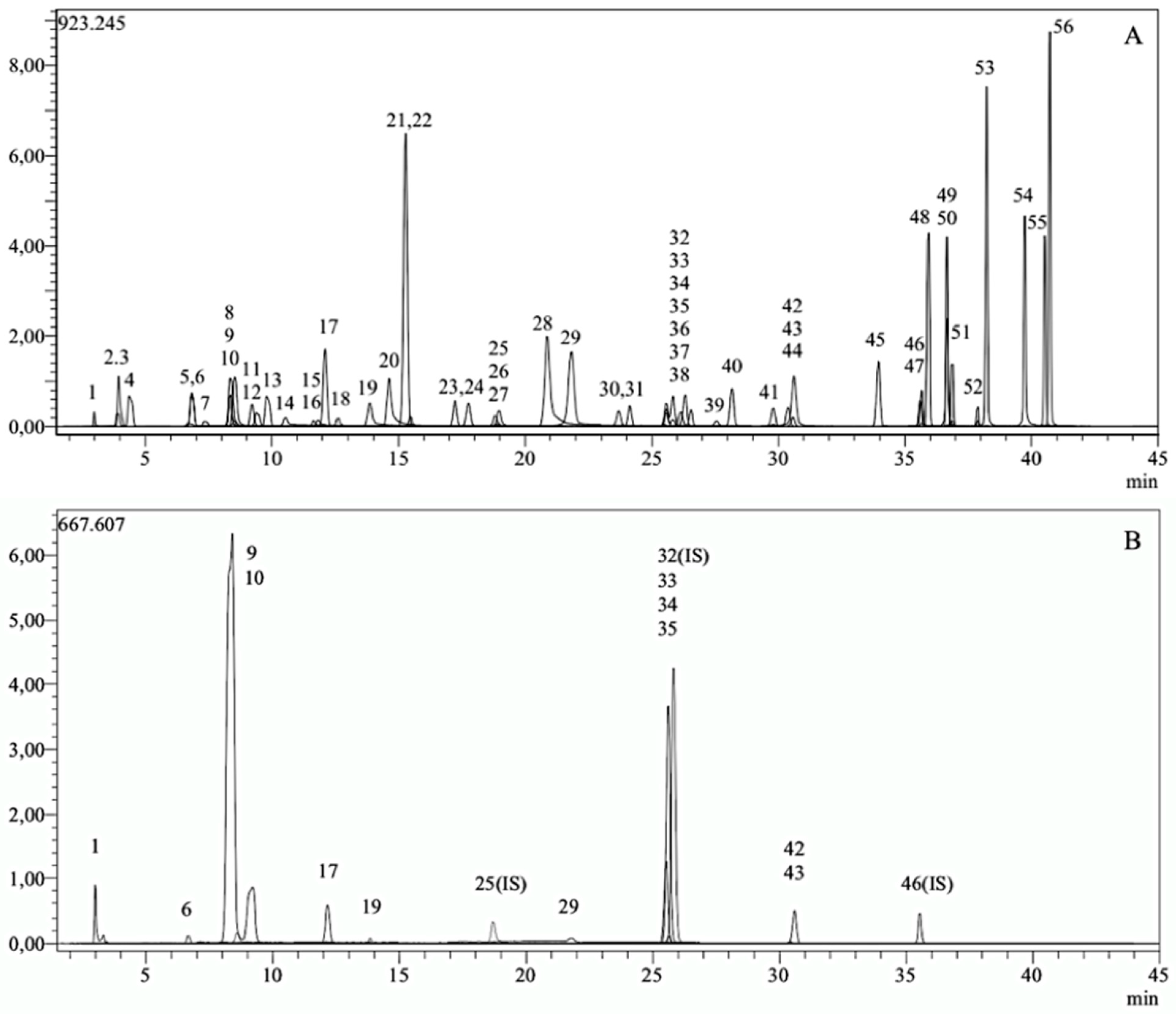
3.1. Phytochemical Compounds of YM Extract Using LC-MS/MS
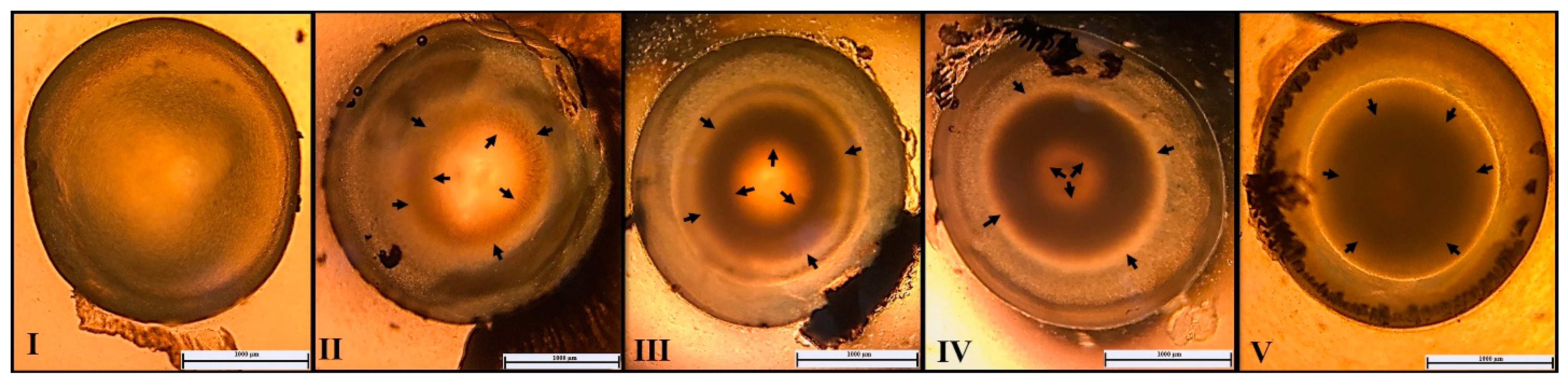
3.2. Grading of Cataracts
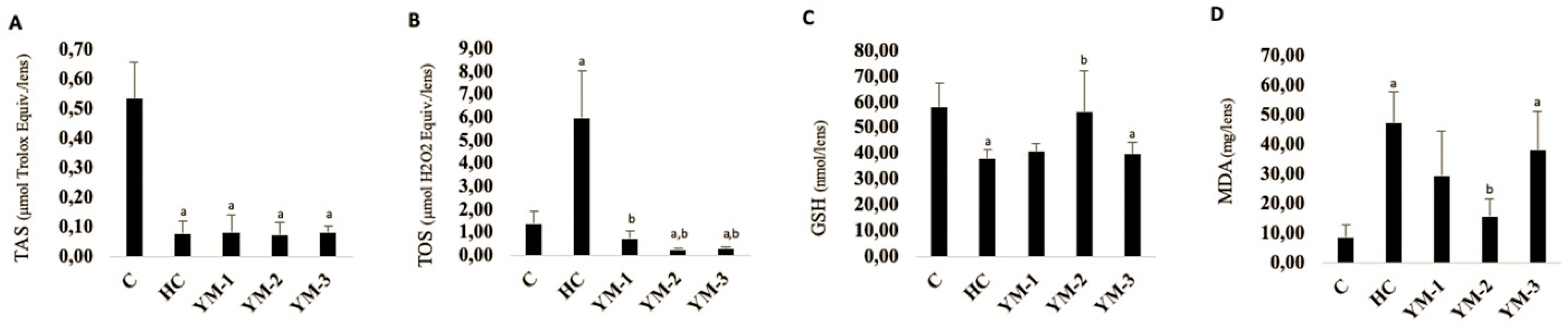
3.3. Biochemical Analysis
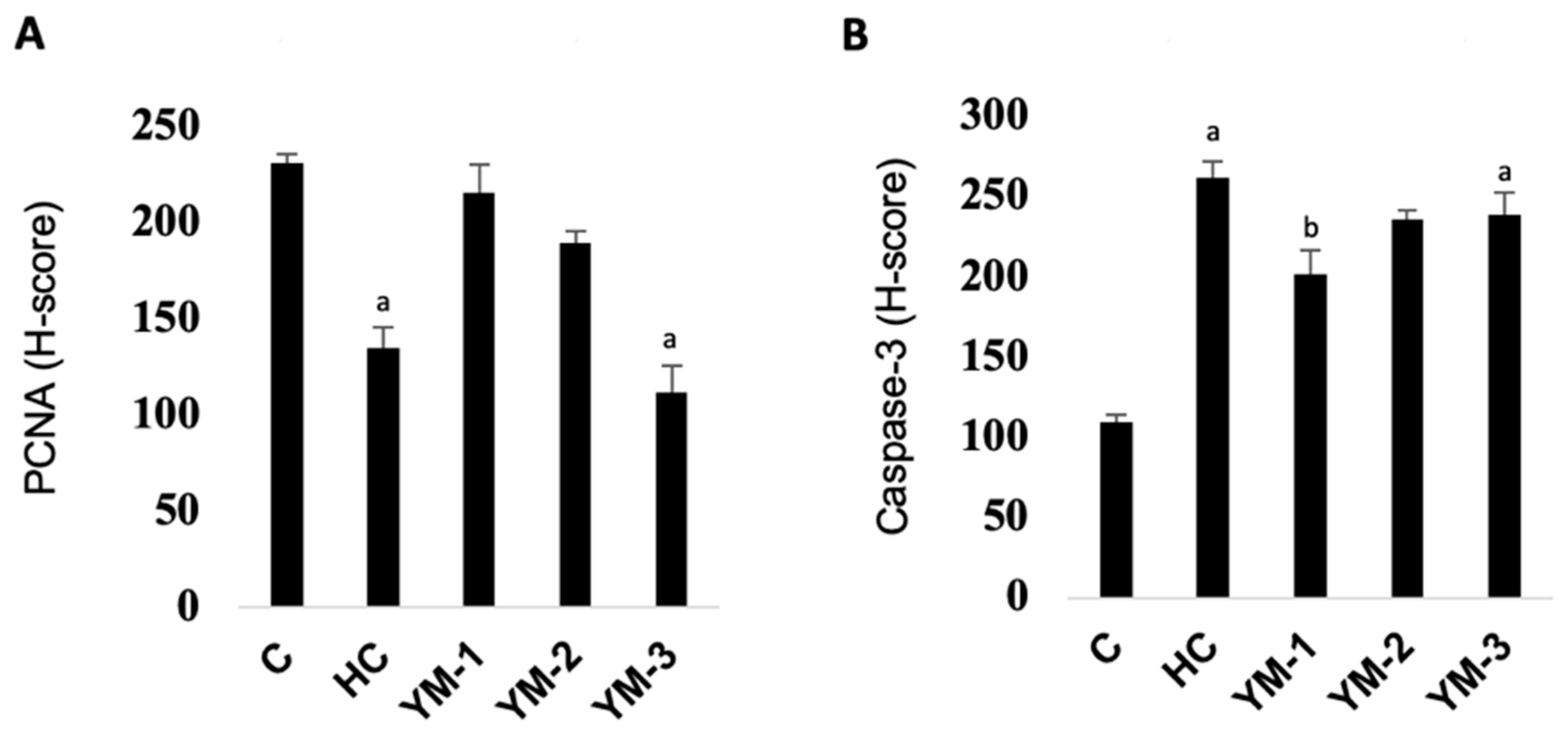
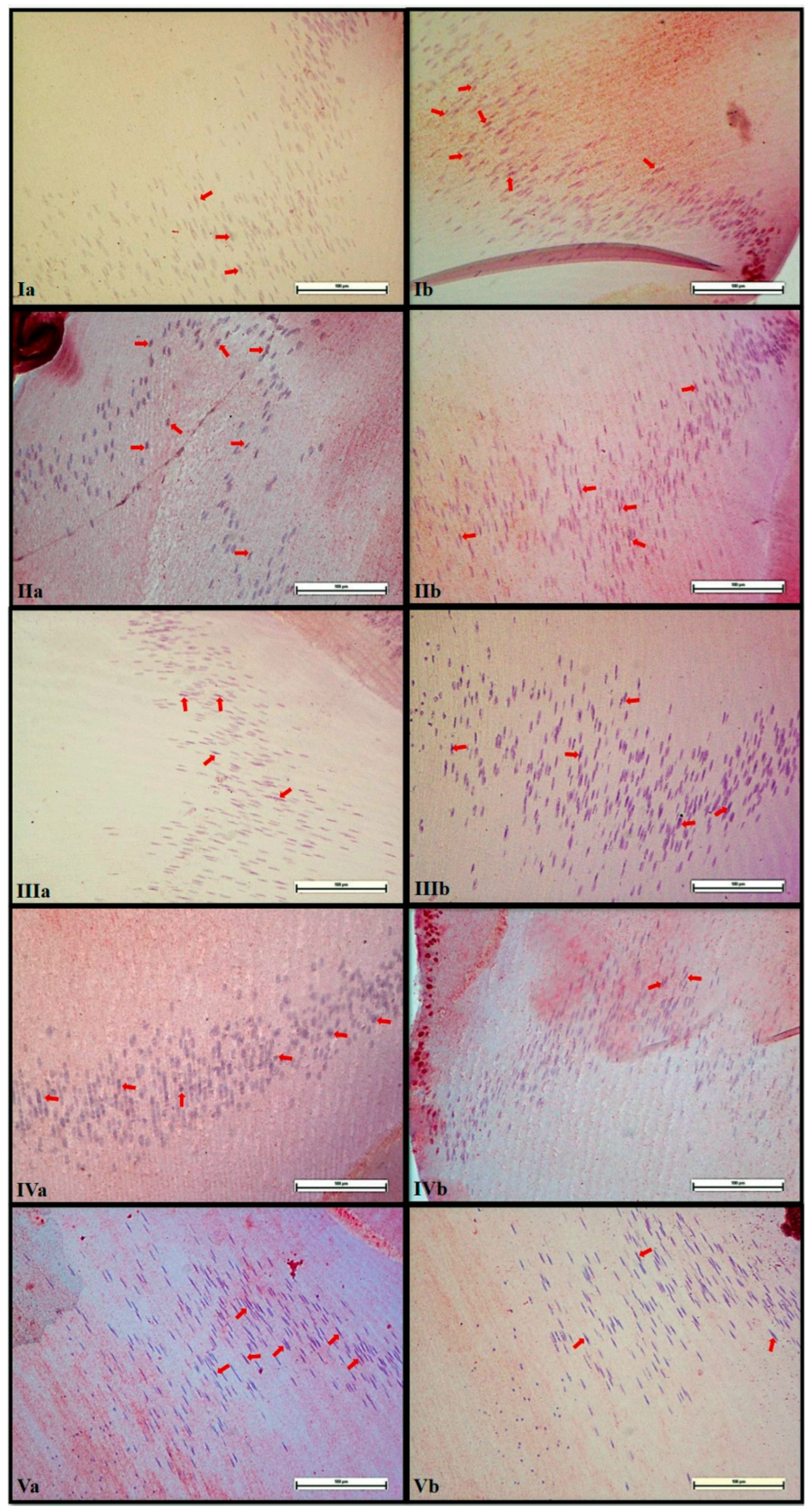
3.4. Immunohistochemical Analysis
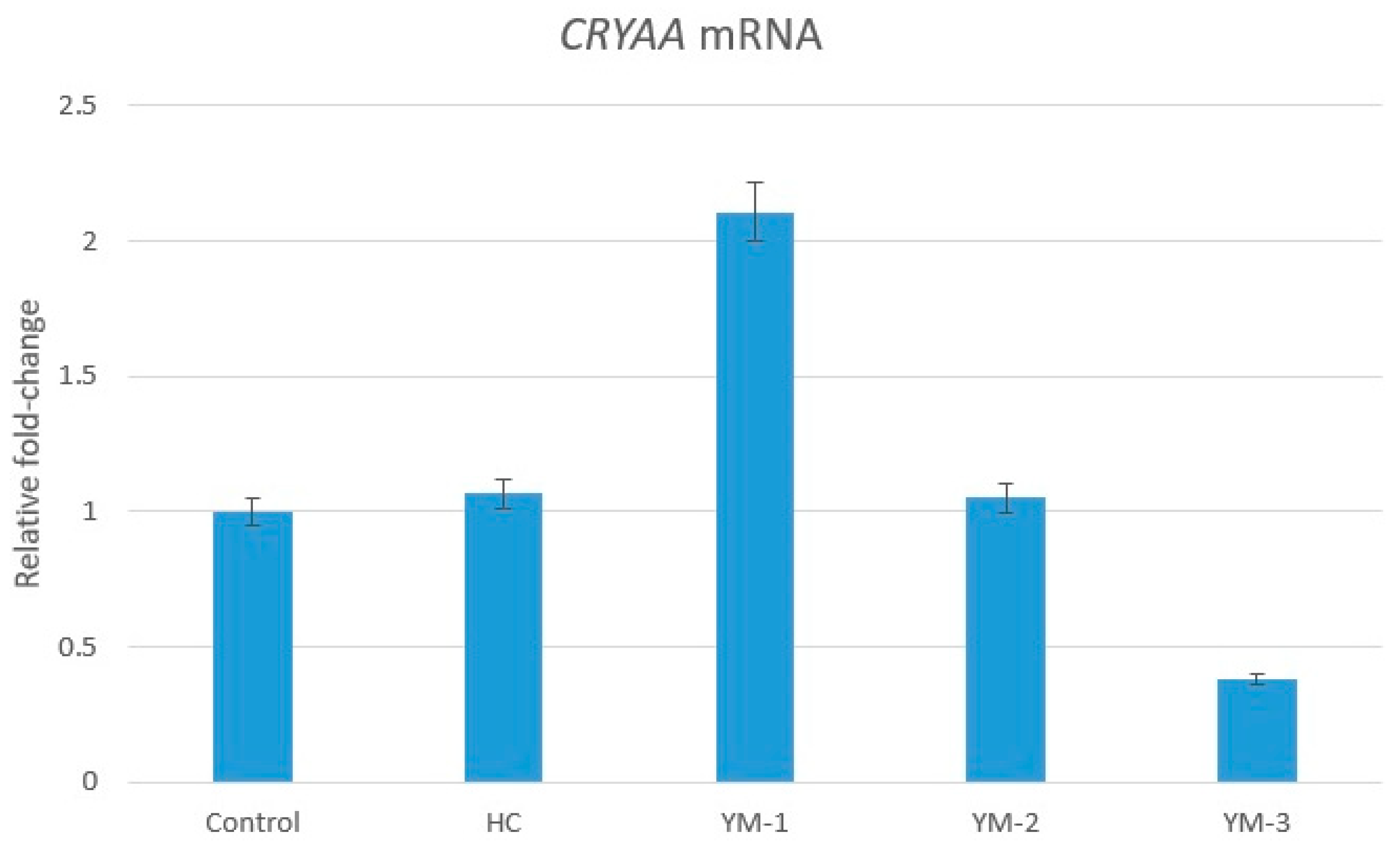
3.5. Genetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreau, K.L.; King, J.A. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol. Med. 2012, 18, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Vurmaz, A.; Duman, R.; Sabaner, M.C.; Ertekin, T.; Bilir, A. Antioxidant effects of piperine in in-vivo chick embryo cataract model induced by steroids. Cutan. Ocul. Toxicol. 2019, 38, 182–189. [Google Scholar] [CrossRef]
- Hashemi, H.; Pakzad, R.; Yekta, A.; Aghamirsalim, M.; Pakbin, M.; Ramin, S.; Khabazkhoob, M. Global and regional prevalence of age-related cataract: A comprehensive systematic review and meta-analysis. Eye 2020, 34, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Mann, S.N.; Mandal, N.A. Botanical compounds: Effects on major eye diseases. Evid. Based Complement. Alternat. Med. 2013, 2013, 549174. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Samoilă, O.; Gocan, D.; Mocan, A.; Moldovan, C.; Devkota, H.P.; Atanasov, A.G.; Zengin, G.; Echeverría, J.; Vodnar, D.; et al. Medicinal Plants and Natural Products Used in Cataract Management. Front. Pharmacol. 2019, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhou, Y.; Li, W.; Zheng, M.; Zhong, R.; Jin, X.; Lin, Y. Effect of Moringa oleifera stem extract on hydrogen peroxide-induced opacity of cultured mouse lens. BMC Complement. Altern. Med. 2019, 19, 144. [Google Scholar] [CrossRef]
- Tsai, C.F.F.; Wu, J.Y.Y.; Hsu, Y.W. Protective Effects of Rosmarinic Acid against Selenite-Induced Cataract and Oxidative Damage in Rats. Int. J. Med. Sci. 2019, 16, 729–740. [Google Scholar] [CrossRef]
- Gonzalez de Mejia, E.E.; Song, Y.S.S.; Ramirez-Mares, M.V.; Kobayashi, H. Effect of Yerba Mate (Ilex paraguariensis) tea on topoisomerase inhibition and oral carcinoma cell proliferation. J. Agric. Food Chem. 2005, 53, 1966–1973. [Google Scholar] [CrossRef]
- Lutomski, P.; Goździewska, M.; Florek-Łuszczki, M. Health properties of Yerba Mate. Ann. Agric. Environ. Med. 2020, 27, 310–313. [Google Scholar] [CrossRef]
- Murakami, A.N.N.; Amboni, R.D.d.M.C.; Prudêncio, E.S.; Amante, E.R.; Fritzen-Freire, C.B.; Boaventura, B.C.B.; Muñoz, I.d.B.; Branco, C.d.S.; Salvador, M.; Maraschin, M. Concentration of biologically active compounds extracted from Ilex paraguariensis St. Hil. by nanofiltration. Food Chem. 2013, 141, 60–65. [Google Scholar] [CrossRef]
- Lorini, A.; Damin, F.M.; de Oliveira, D.N.; Crizel, R.L.; Godoy, H.T.; Galli, V.; Meinhart, A.D. Characterization and quantification of bioactive compounds from Ilex paraguariensis residue by HPLC-ESI-QTOF-MS from plants cultivated under different cultivation systems. J. Food Sci. 2021, 86, 1599–1619. [Google Scholar] [CrossRef] [PubMed]
- Rząsa-Duran, E.; Kryczyk-Poprawa, A.; Drabicki, D.; Podkowa, A.; Sułkowska-Ziaja, K.; Szewczyk, A.; Kała, K.; Opoka, W.; Zięba, P.; Fidurski, M.; et al. Yerba Mate as a Source of Elements and Bioactive Compounds with Antioxidant Activity. Antioxidants 2022, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Schiefer, E.M.; Surek, M.; Martins, C.A.F.; Worfel, P.R.; Sassaki, G.L.; de Souza, L.M.; Nowacki, L.C.; de Souza, W.M. Chemical, biological, and pharmacological evaluation of the aqueous extract of Ilex paraguariensis, St. Hill. (Aqualifoliaceae). Res. Soc. Dev. 2022, 11, e3011225335. [Google Scholar] [CrossRef]
- Ghimire, S.; Zhang, X.; Zhang, J.; Wu, C. Use of Chicken Embryo Model in Toxicity Studies of Endocrine-Disrupting Chemicals and Nanoparticles. Chem. Res. Toxicol. 2022, 35, 550–568. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, H.; Lee, J.W.; Iwatsuru, M. An animal model for cataract research: Cataract formation in developing chick embryo by glucocorticoid. Exp. Eye Res. 1983, 36, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Kosano, H.; Watanabe, H.; Nishigori, H. Suppressive effects of thyroxine on glucocorticoid (gc)-induced metabolic changes and cataract formation on developing chick embryos. Exp. Eye Res. 2001, 72, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Hashizume, K.; Nishigori, H.; Tezuka, Y.; Sanbe, A.; Kurosaka, D. Effect of astaxanthin on cataract formation induced by glucocorticoids in the chick embryo. Curr. Eye Res. 2015, 40, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Vurmaz, A.; Ertekin, A.; Sabaner, M.C.; Atay, E.; Bozkurt, E.; Bilir, A. Effects of vitamin E in a glucocorticoid induced cataract model in chicken embryos. Biotech. Histochem. 2021, 96, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.; Ertekin, T.; Duman, R.; Vurmaz, A.; Çetinkaya, E.; Güzel, H. Anticataractogenic effect of betaine in chick embryo hydrocortisone-induced cataract model. Indian J. Med. Res. 2019, 150, 407–411. [Google Scholar] [CrossRef]
- Kundakci, Y.E.; Bilir, A.; Atay, E.; Vurmaz, A.; Firat, F.; Arikan, E.S. Protective Effects of Different Doses of Ginsenoside-Rb1 Experimental Cataract Model That in Chick Embryos. Curr. Eye Res. 2023, 48, 817–825. [Google Scholar] [CrossRef]
- Schinella, G.; Fantinelli, J.C.; Mosca, S.M. Cardioprotective effects of Ilex paraguariensis extract: Evidence for a nitric oxide-dependent mechanism. Clin. Nutr. 2005, 24, 360–366. [Google Scholar] [CrossRef]
- Yilmaz, M.A. Simultaneous quantitative screening of 53 phytochemicals in 33 species of medicinal and aromatic plants: A detailed, robust and comprehensive LC–MS/MS method validation. Ind. Crop. Prod. 2020, 149, 112347. [Google Scholar] [CrossRef]
- Duman, R.; Vurmaz, A. Role of innate immunity and oxidative stress in steroid-induced cataracts in developing chick embryos. Cutan. Ocul. Toxicol. 2018, 37, 281–285. [Google Scholar] [CrossRef]
- Becit-Kizilkaya, M.; Oncu, S.; Bilir, A.; Koca, H.B.; Firat, F.; Arikan-Soylemez, E.S.; Albas-Kurt, G. Effect of Stevia rebaudiana Bertoni aqueous extract on steroid-induced cataract in chick embryo model. Food. Biosci. 2024, 58, 103685. [Google Scholar] [CrossRef]
- Turan, G.; Turan, M. The Evaluation of TUNEL, PCNA and SOX2 Expressions in Lens Epithelial Cells of Cataract Patients with Pseudoexfoliation Syndrome. Curr. Eye Res. 2020, 45, 12–16. [Google Scholar] [CrossRef]
- Gawron-Gzella, A.; Chanaj-Kaczmarek, J.; Cielecka-Piontek, J. Yerba mate-A long but current history. Nutrients 2021, 13, 3706. [Google Scholar] [CrossRef] [PubMed]
- Gerber, T.; Nunes, A.; Moreira, B.R.; Maraschin, M. Yerba mate (Ilex paraguariensis A. St.-Hil.) for new therapeutic and nutraceutical interventions: A review of patents issued in the last 20 years (2000–2020). Phytother. Res. 2023, 37, 527–548. [Google Scholar] [CrossRef]
- Cardozo, J.E.L.; Morand, C. Interest of mate (Ilex paraguariensis A. St.-Hil.) as a new natural functional food to preserve human cardiovascular health-A review. J. Funct. Foods 2016, 21, 440–454. [Google Scholar] [CrossRef]
- Bisognin, D.A.; da Luz, L.V.; Lencina, K.H.; dos Santos, C.O.; Sautter, C.K. Contents of total phenolics and flavonoids and antioxi- dant activity of Ilex paraguariensis leaves. Pesqui. Agropecu. Bras. 2019, 54, e00856. [Google Scholar] [CrossRef]
- Wu, T.W.; Lee, C.C.; Hsu, W.H.; Hengel, M.; Shibamoto, T. Antioxidant activity of natural plant extracts from mate (Ilex paraguariensis), lotus plumule (Nelumbo nucifera Gaertn.) and rhubarb (Rheum rhabarbarum L.). J. Food Nutr. Disord. 2017, 6, 1000316. [Google Scholar] [CrossRef]
- Luz, A.B.G.; da Silva, C.H.B.; Nascimento, M.V.P.S.; de Campos Facchin, B.M.; Baratto, B.; Fröde, T.S.; Reginatto, F.H.; Dalmarco, E.M. The anti-inflammatory effect of Ilex paraguariensis A. St. Hil (Mate) in a murine model of pleurisy. Int. Immunopharmacol. 2016, 36, 165–172. [Google Scholar] [CrossRef]
- Cittadini, M.C.; Repossi, G.; Albrecht, C.; Naranjo, R.D.P.; Miranda, A.R.; de Pascual-Teresa, S.; Soria, E.A. Effects of bioavailable phenolic compounds from Ilex paraguariensis on the brain of mice with lung adenocarcinoma. Phytother. Res. 2019, 33, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-J.; Chen, Y.-N.; Tsao, Y.-T.; Cheng, C.-M.; Wu, W.-C.; Chen, H.-C. The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases. Int. J. Mol. Sci. 2022, 23, 1255. [Google Scholar] [CrossRef] [PubMed]
- Javadzadeh, A.; Ghorbanihaghjo, A.; Arami, S.; Rashtchizadeh, N.; Mesgari, M.; Rafeey, M.; Omidi, Y. Prevention of selenite-induced cataractogenesis in Wistar albino rats by aqueous extract of garlic. J. Ocul. Pharmacol. Ther. 2009, 25, 395–400. [Google Scholar] [CrossRef]
- Dubey, S.; Deep, P.; Singh, A.K. Phytochemical characterization and evaluation of anticataract potential of seabuckthorn leaf extract. Vet. Ophthalmol. 2016, 19, 144–148. [Google Scholar] [CrossRef]
- Colpo, A.C.; Rosa, H.; Lima, M.E.; Pazzini, C.E.F.; de Camargo, V.B.; Bassante, F.E.; Puntel, R.; Ávila, D.S.; Mendez, A.; Folmer, V. Yerba Mate (Ilex paraguariensis St. Hill.)-Based Beverages: How Successive Extraction Influences the Extract Composition and Its Capacity to Chelate Iron and Scavenge Free Radicals. Food Chem. 2016, 209, 185–195. [Google Scholar] [CrossRef]
- Bravo, L.; Goya, L.; Lecumberri, E. LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis, St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Res. Int. 2007, 40, 393–405. [Google Scholar] [CrossRef]
- Bastos, D.H.; Saldanha, L.A.; Catharino, R.R.; Sawaya, A.; Cunha, I.B.; Carvalho, P.O.; Eberlin, M.N. Phenolic Antioxidants Identified by ESI-MS from Yerba Maté (Ilex paraguariensis) and Green Tea (Camelia sinensis) Extracts. Molecules 2007, 12, 423–432. [Google Scholar] [CrossRef]
- Bastos, D.H.M.; Ishimoto, E.Y.; Marques, M.O.M.; Ferri, A.F.; Torres, E.A.F.S. Essential oil and antioxidant activity of green mate and mate tea (Ilex paraguariensis) infusions. J. Food Compos. Anal. 2006, 19, 538–543. [Google Scholar] [CrossRef]
- Heck, C.I.; Schmalko, M.; De Mejia, E.G. Effect of growing and drying conditions on the phenolic composition of mate teas (Ilex paraguariensis). J. Agric. Food Chem. 2008, 56, 8394–8403. [Google Scholar] [CrossRef]
- Becker, A.M.; Cunha, H.P.; Lindenberg, A.C.; de Andrade, F.; de Carvalho, T.; Boaventura, B.C.B.; da Silva, E.L. Spray-Dried Yerba Mate Extract Capsules: Clinical Evaluation and Antioxidant Potential in Healthy Individuals. Plant Foods Hum. Nutr. 2019, 74, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J.; Friedrich, M.G. The etiology of human age-related cataract. Proteins don’t last forever. Biochim. Biophys. Acta 2016, 1860, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, C.; Carrière, I.; Ponton-Sanchez, A.; Lacroux, A.; Covacho, M.-J.; Papoz, L. Light exposure and the risk of cortical, nuclear, and posterior subcapsular cataracts: The Pathologies Oculaires Liées à l’Age (POLA) study. Arch. Ophthalmol. 2000, 118, 385–392. [Google Scholar] [CrossRef] [PubMed]
- McCarty, C.A.; Taylor, H.R. A review of the epidemiologic evidence linking ultraviolet radiation and cataracts. Dev. Ophthalmol. 2002, 35, 21–31. [Google Scholar] [CrossRef]
- Gupta, S.K.; Trivedi, D.; Srivastava, S.; Joshi, S.; Halder, N.; Verma, S.D. Lycopene attenuates oxidative stress induced experimental cataract development: An in vitro and in vivo study. Nutrition 2003, 19, 794–799. [Google Scholar] [CrossRef]
- Matsumoto, R.L.T.; Bastos, D.H.M.; Mendonça, S.; Nunes, V.S.; Bartchewsky, W.; Ribeiro, M.L.; Carvalho, P.d.O. Effects of Mate Tea (Ilex paraguariensis) Ingestion on MRNA Expression of Antioxidant Enzymes, Lipid Peroxidation, and Total Antioxidant Status in Healthy Young Women. J. Agric. Food Chem. 2009, 57, 1775–1780. [Google Scholar] [CrossRef]
- Riachi, L.G.; Bastos de Maria, C.A.B. Yerba Mate: An overview of physiological effects in humans. J. Funct. Foods 2017, 38, 308–320. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Drinking Coffee, Mate, and Very Hot Beverages; Bookshelf ID: NBK543953; International Agency for Research on Cancer: Lyon, France, 2018. [PubMed]
- Szymańska, K.; Matos, E.; Hung, R.J.; Wünsch-Filho, V.; Eluf-Neto, J.; Menezes, A.; Daudt, A.W.; Brennan, P.; Boffetta, P. Drinking of maté and the risk of cancers of the upper aerodigestive tract in Latin America: A case-control study. Cancer Causes Control 2010, 21, 1799–1806. [Google Scholar] [CrossRef]
- Vieira, M.A.; Maraschin, M.; Rovaris, A.A.; Amboni, R.D.d.M.C.; Pagliosa, C.M.; Xavier, J.J.M.; Amante, E.R. Occurrence of Polycyclic Aromatic Hydrocarbons throughout the Processing Stages of Erva-Mate (Ilex paraguariensis). Food Addit. Contam. Part A 2010, 27, 776–782. [Google Scholar] [CrossRef]
- Menoni, C.; Donangelo, C.M.; Rufo, C. Polycyclic Aromatic Hydrocarbons in Yerba Mate (Ilex paraguariensis) Infusions and Probabilistic Risk Assessment of Exposure. Toxicol. Rep. 2021, 8, 324–330. [Google Scholar] [CrossRef]
- Holowaty, S.A.; Thea, A.E.; Alegre, C.; Schmalko, M.E. Differences in physicochemical properties of Yerba Maté (Ilex paraguariensis) obtained using traditional and alternative manufacturing methods. J. Food Process Eng. 2018, 41, e12911. [Google Scholar] [CrossRef]
- Tate, P.S.; Marazita, M.C.; Marquioni-Ramella, M.D.; Suburo, A.M. Ilex paraguariensis extracts and its polyphenols prevent oxidative damage and senescence of human retinal pigment epithelium cells. J. Funct. Foods 2020, 67, 103833. [Google Scholar] [CrossRef]
- Tate, P.S.; Marquioni-Ramella, M.D.; Cerchiaro, C.; Suburo, A.M. Ilex paraguariensis extracts prevent oxidative damage in a mouse model of age-related macular degeneration. Mol. Nutr. Food Res. 2022, 66, e2100807. [Google Scholar] [CrossRef] [PubMed]
- Colpo, A.C.; de Lima, M.E.; Maya-López, M.; Rosa, H.; Márquez-Curiel, C.; Galván-Arzate, S.; Santamaría, A.; Folmer, V. Compounds from Ilex paraguariensis extracts have antioxidant effects in the brains of rats subjected to chronic immobilization stress. Appl. Physiol. Nutr. Metab. 2017, 42, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.A.F.; Tirapeli, K.G.; Chaves-Neto, A.H.; Brasilino, M.d.S.; da Rocha, C.Q.; Belló-Klein, A.; Llesuy, S.F.; Dornelles, R.C.M.; Nakamune, A.C.d.M.S. Ilex paraguariensis supplementation may be an effective nutritional approach to modulate oxidative stress during perimenopause. Exp. Gerontol. 2017, 90, 14–18. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, H. The Role of DNA Methylation in Lens Development and Cataract Formation. Cell Mol. Neurobiol. 2017, 37, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Graw, J. Genetics of crystallins: Cataract and beyond. Exp. Eye Res. 2009, 88, 173–189. [Google Scholar] [CrossRef]
- Su, S.; Liu, P.; Zhang, H.; Li, Z.; Song, Z.; Zhang, L.; Chen, S. Proteomic analysis of human age-related nuclear cataracts and normal lens nuclei. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4182–4191. [Google Scholar] [CrossRef] [PubMed]
- Bloemendal, H.; de Jong, W.; Jaenicke, R.; Lubsen, N.H.; Slingsby, C.; Tardieu, A. Ageing and vision: Structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 2004, 86, 407–485. [Google Scholar] [CrossRef]
- Yonova-Doing, E.; Zhao, W.; Igo, R.P., Jr.; Wang, C.; Sundaresan, P.; Lee, K.E.; Jun, G.R.; Alves, A.C.; Chai, X.; Chan, A.S.Y.; et al. Common variants in SOX-2 and congenital cataract genes contribute to age-related nuclear cataract. Commun. Biol. 2020, 3, 755. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-R.; Lee, H.-Y.; Kim, J.-H.; Moon, D.-I.; Seo, M.-Y.; Park, S.-H.; Choi, K.-H.; Kim, C.-R.; Kim, S.-H.; Oh, J.-H.; et al. Anti-obesity and anti-diabetic effects of Yerba Mate (Ilex paraguariensis) in C57BL/6J mice fed a high-fat diet. Lab. Anim. Res. 2012, 28, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.S.; Casagrande, L.; Model, J.F.A.; dos Santos, J.T.; Hoefel, A.L.; Kucharski, L.C. Effect of yerba mate (Ilex paraguariensis) extract on the metabolism of diabetic rats. Biomed. Pharmacother. 2018, 105, 370–376. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequences (5′ → 3′) |
|---|---|
| CRYAA-Forward | GACCACGGCTACATCTCTCG |
| CRYAA-Reverse | TGGTGTTACTCGTGCACTCC |
| GAPDH-Forward | CTCAACGGATTTGGCCGTAT |
| GAPDH-Reverse | AATGCCAAAGTTGTCATGGATG |
| No. | Analyte | YM (mg/g) | No. | Analyte | YM (mg/g) | No. | Analyte | YM (mg/g) | No. | Analyte | YM (mg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid | 6.847 | 15 | Epicatechin | n.d. | 29 | Salicylic acid | 0.051 | 43 | Nicotiflorin | 2.368 |
| 2 | Fumaric aid | n.d. | 16 | Vanilic acid | n.d. | 30 | Cyranoside | n.d. | 44 | Fisetin | n.d. |
| 3 | Aconitic acid | n.d. | 17 | Caffeic acid | 0.403 | 31 | Miquelianin | n.d. | 45 | Daidzein | n.d. |
| 4 | Gallic acid | n.d. | 18 | Syringic acid | n.d. | 32 | Rutin-D3-IS a | n.a. | 46 | Quercetin-D3-IS a | n.a. |
| 5 | Epigallocatechin | n.d. | 19 | Vanillin | 0.035 | 33 | Rutin | 7.619 | 47 | Quercetin | n.d. |
| 6 | Protocatechuic acid | 0.084 | 20 | Syringic aldehyde | n.d. | 34 | Isoquercitrin | 0.450 | 48 | Naringenin | n.d. |
| 7 | Catechin | n.d. | 21 | Daidzin | n.d. | 35 | Hesperidin | 8.166 | 49 | Hesperetin | n.d. |
| 8 | Gentisic acid | n.d. | 22 | Epicatechin gallate | n.d. | 36 | O-Coumaric acid | n.d. | 50 | Luteolin | n.d. |
| 9 | Chlorogenic acid | 23.723 | 23 | Piceid | n.d. | 37 | Genistin | n.d. | 51 | Genistein | n.d. |
| 10 | Protocatechuic aldehyde | 0.210 | 24 | p-Coumaric acid | n.d. | 38 | Rosmarinic acid | n.d. | 52 | Kaempferol | n.d. |
| 11 | Tannic acid | n.d. | 25 | Ferulic acid-D3-IS a | n.a. | 39 | Ellagic acid | n.d. | 53 | Apigenin | n.d. |
| 12 | Epigallocatechin gallate | n.d. | 26 | Ferulic acid | n.d. | 40 | Cosmosiin | n.d. | 54 | Amentoflavone | n.d. |
| 13 | 1,5-dicaffeoylquinic acid | n.d. | 27 | Sinapic acid | n.d. | 41 | Quercitrin | n.d. | 55 | Chrysin | n.d. |
| 14 | 4-OH benzoic acid | n.d. | 28 | Coumarin | n.d. | 42 | Astragalin | 0.094 | 56 | Acacetin | n.d. |
| Groups | Cataract Grades (n) | n | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| C | 16 | 0 | 0 | 0 | 0 | 16 | <0.05 |
| HC | 0 | 0 | 3 | 11 | 2 | 16 | |
| YM-1 | 0 | 0 | 3 | 11 | 2 | 16 | |
| YM-2 | 0 | 13 | 3 | 0 | 0 | 16 | |
| YM-3 | 0 | 3 | 10 | 3 | 0 | 16 | |
| Total | 16 | 23 | 20 | 19 | 2 | 80 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oncu, S.; Becit-Kizilkaya, M.; Bilir, A.; Saritas, A.; Arikan-Soylemez, E.S.; Koca, H.B.; Firat, F.; Ugur-Kaplan, A.B.; Yilmaz, M.A. Anti-Cataract Effect of the Traditional Aqueous Extract of Yerba Mate (Ilex paraguariensis A. St.-Hil.): An In Ovo Perspective. Life 2024, 14, 994. https://doi.org/10.3390/life14080994
Oncu S, Becit-Kizilkaya M, Bilir A, Saritas A, Arikan-Soylemez ES, Koca HB, Firat F, Ugur-Kaplan AB, Yilmaz MA. Anti-Cataract Effect of the Traditional Aqueous Extract of Yerba Mate (Ilex paraguariensis A. St.-Hil.): An In Ovo Perspective. Life. 2024; 14(8):994. https://doi.org/10.3390/life14080994
Chicago/Turabian StyleOncu, Seyma, Merve Becit-Kizilkaya, Abdulkadir Bilir, Alperen Saritas, Evrim Suna Arikan-Soylemez, Halit Bugra Koca, Fatma Firat, Afife Busra Ugur-Kaplan, and Mustafa Abdullah Yilmaz. 2024. "Anti-Cataract Effect of the Traditional Aqueous Extract of Yerba Mate (Ilex paraguariensis A. St.-Hil.): An In Ovo Perspective" Life 14, no. 8: 994. https://doi.org/10.3390/life14080994
APA StyleOncu, S., Becit-Kizilkaya, M., Bilir, A., Saritas, A., Arikan-Soylemez, E. S., Koca, H. B., Firat, F., Ugur-Kaplan, A. B., & Yilmaz, M. A. (2024). Anti-Cataract Effect of the Traditional Aqueous Extract of Yerba Mate (Ilex paraguariensis A. St.-Hil.): An In Ovo Perspective. Life, 14(8), 994. https://doi.org/10.3390/life14080994






