Evolutionary Game-Theoretic Approach to the Population Dynamics of Early Replicators
Abstract
:1. Introduction
2. Non-Structured Populations
2.1. Malthusian vs. Malthusian Replicators
2.2. Hypercyclic vs. Hypercyclic Replicators
2.3. Hypercyclic vs. Malthusian Replicators
3. Structured Populations
3.1. Enzyme-Production as a Public Goods Game
3.2. Stochastic Dynamics
3.3. Deterministic Limit
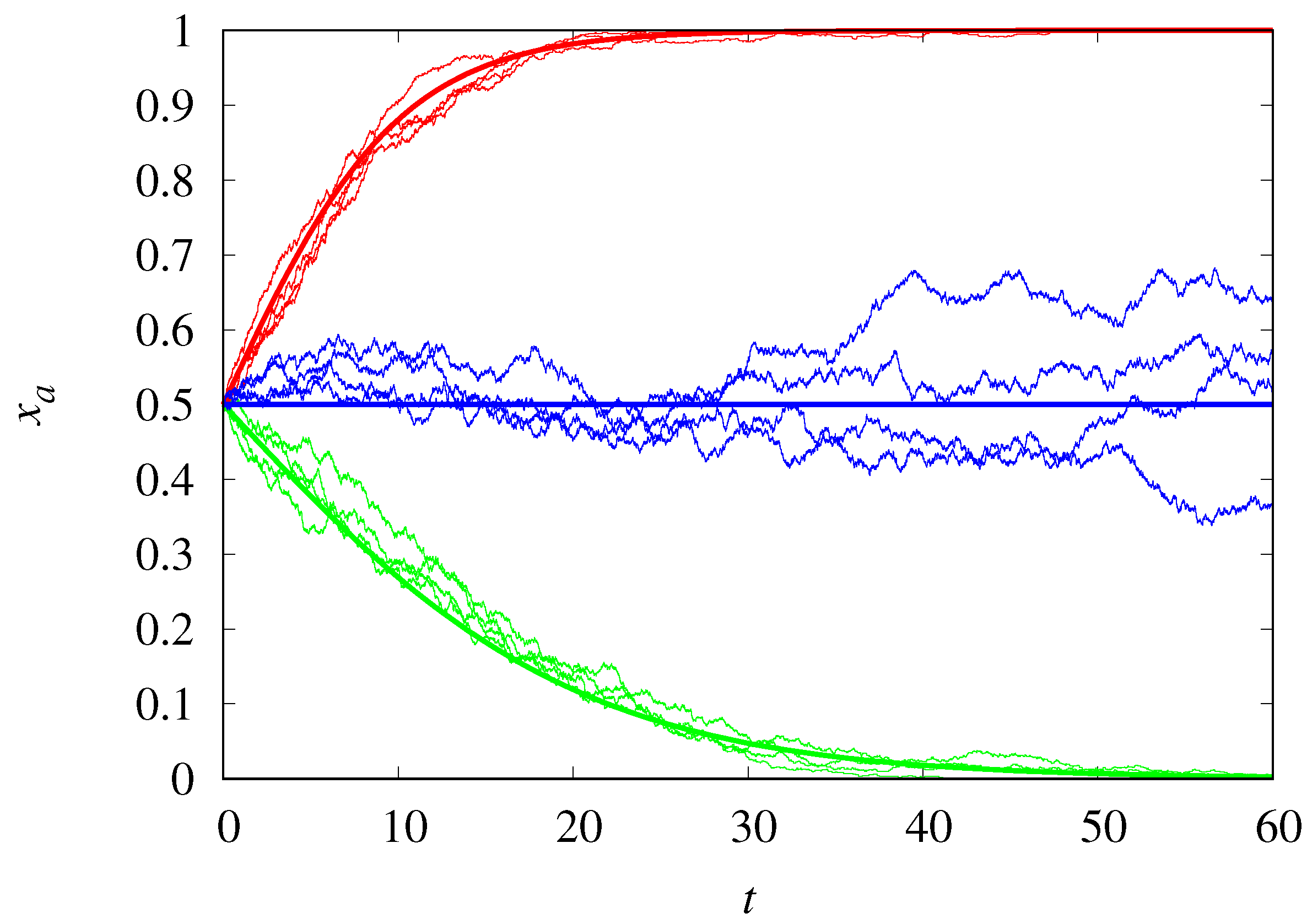
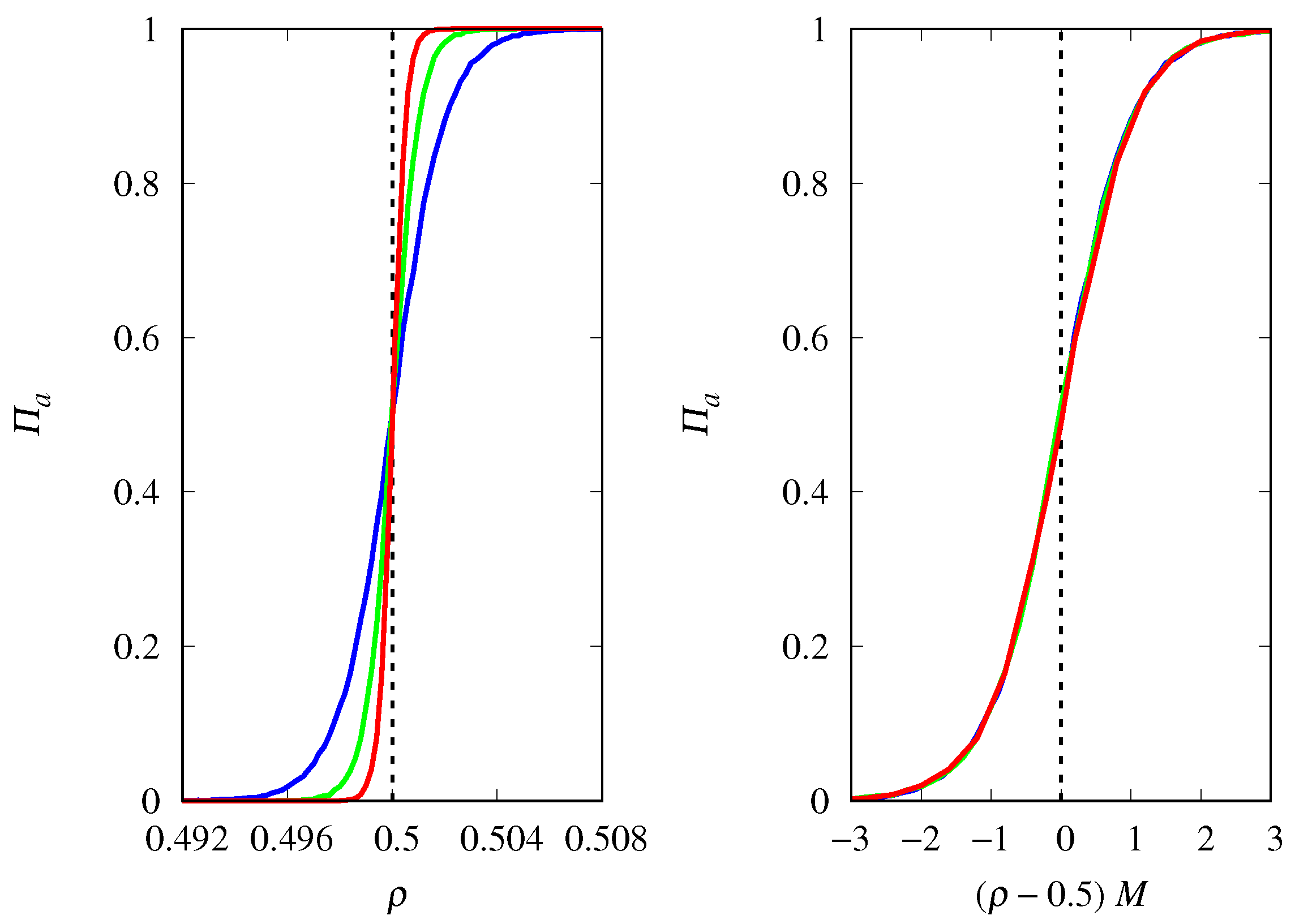
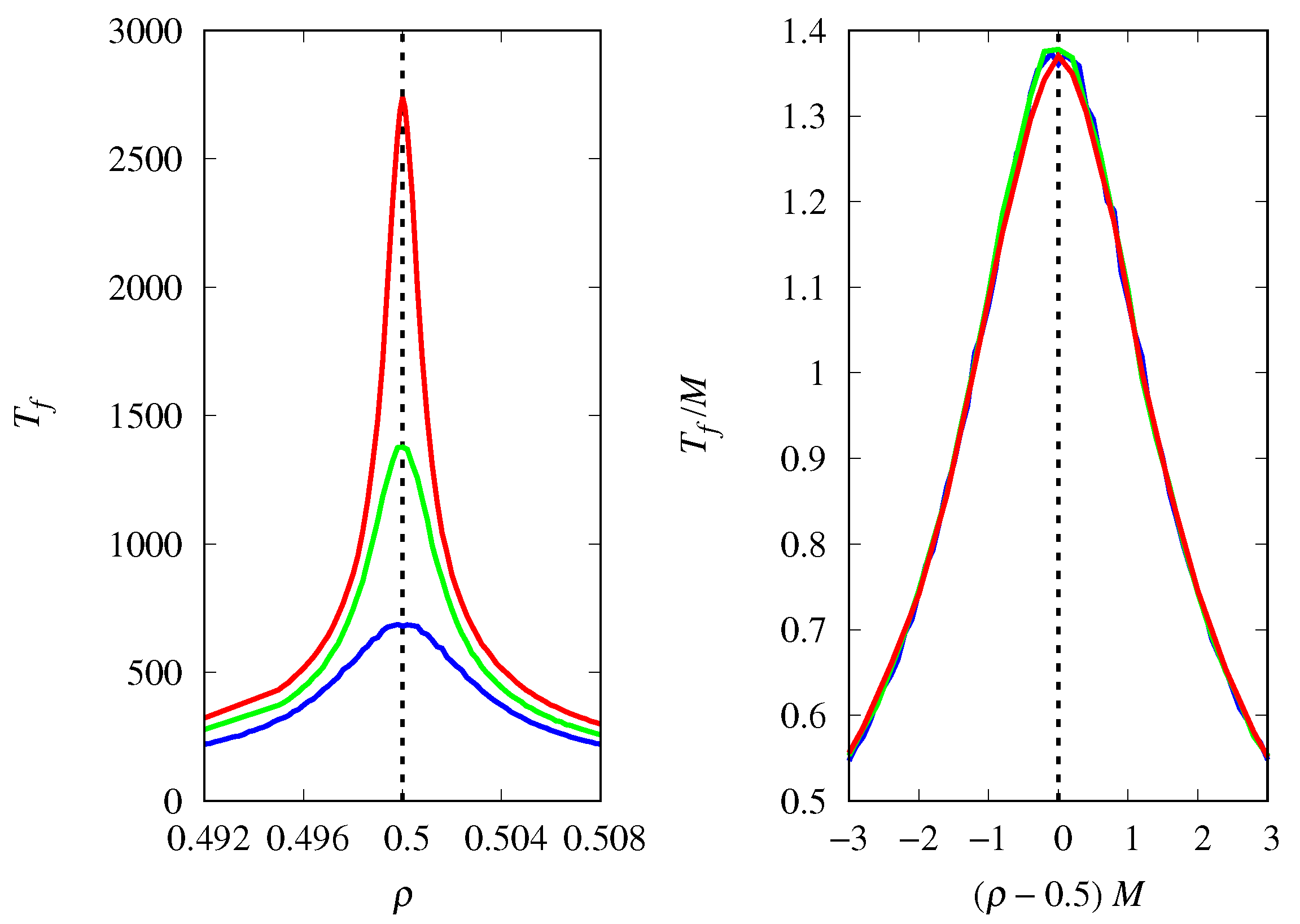
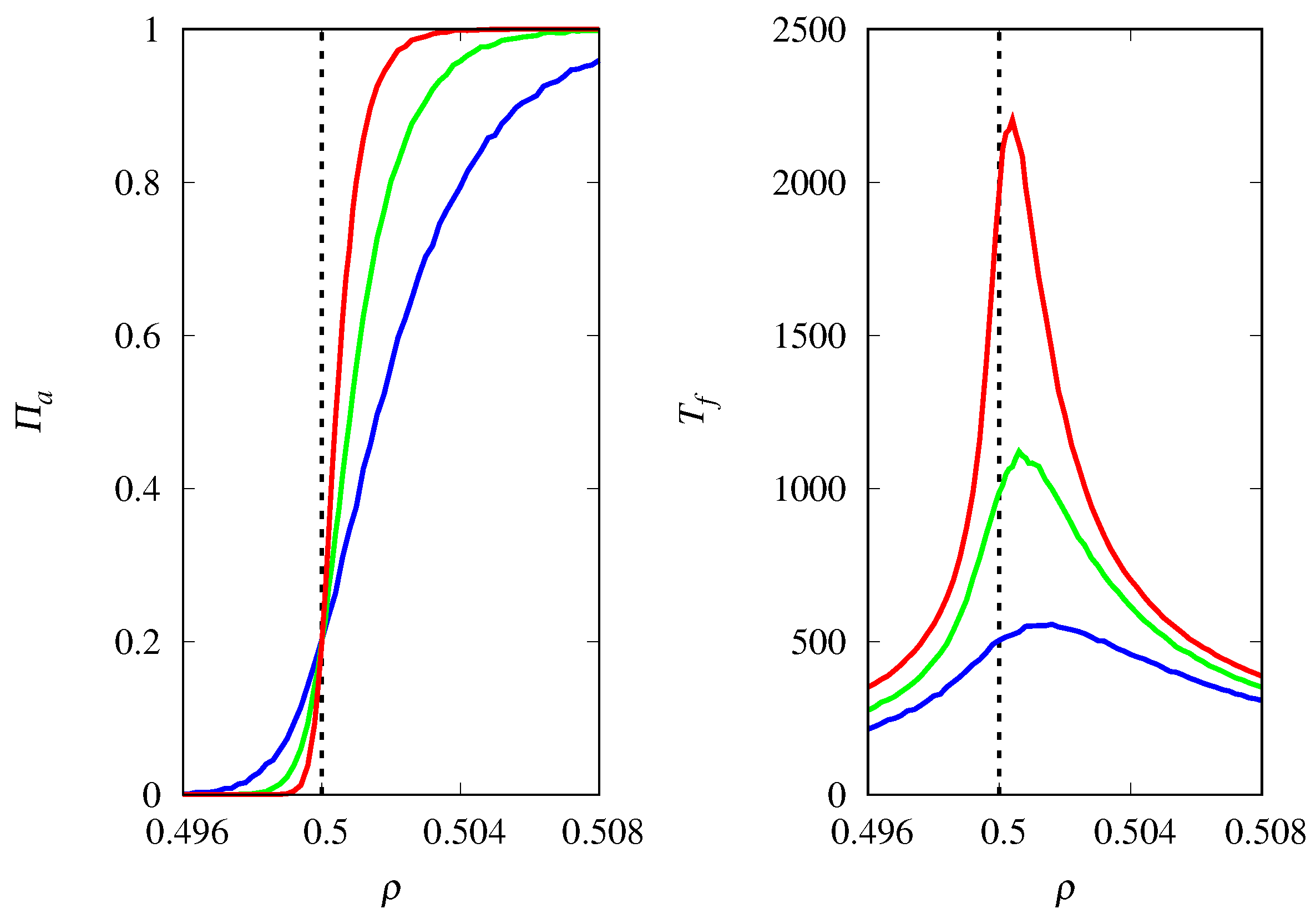
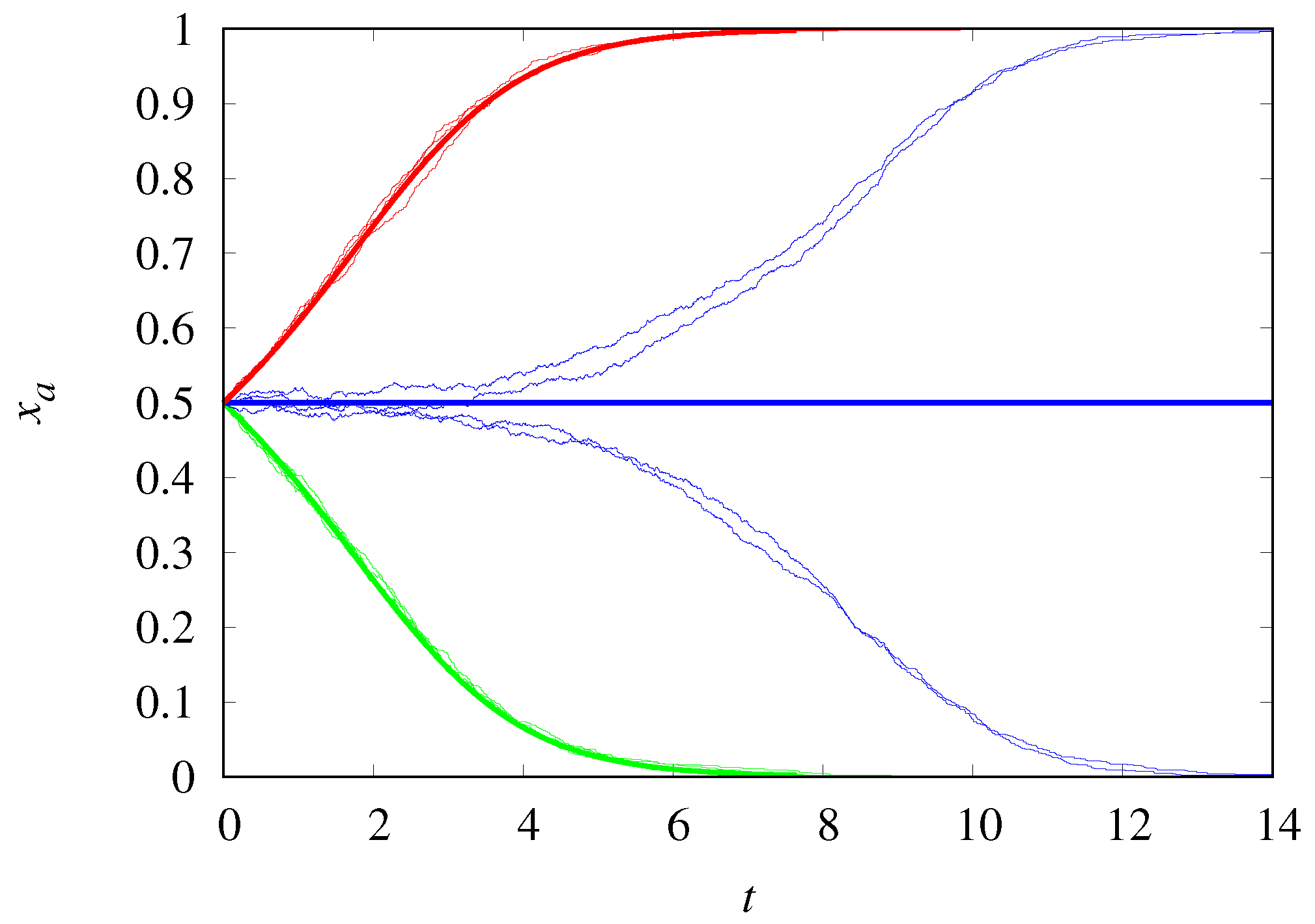
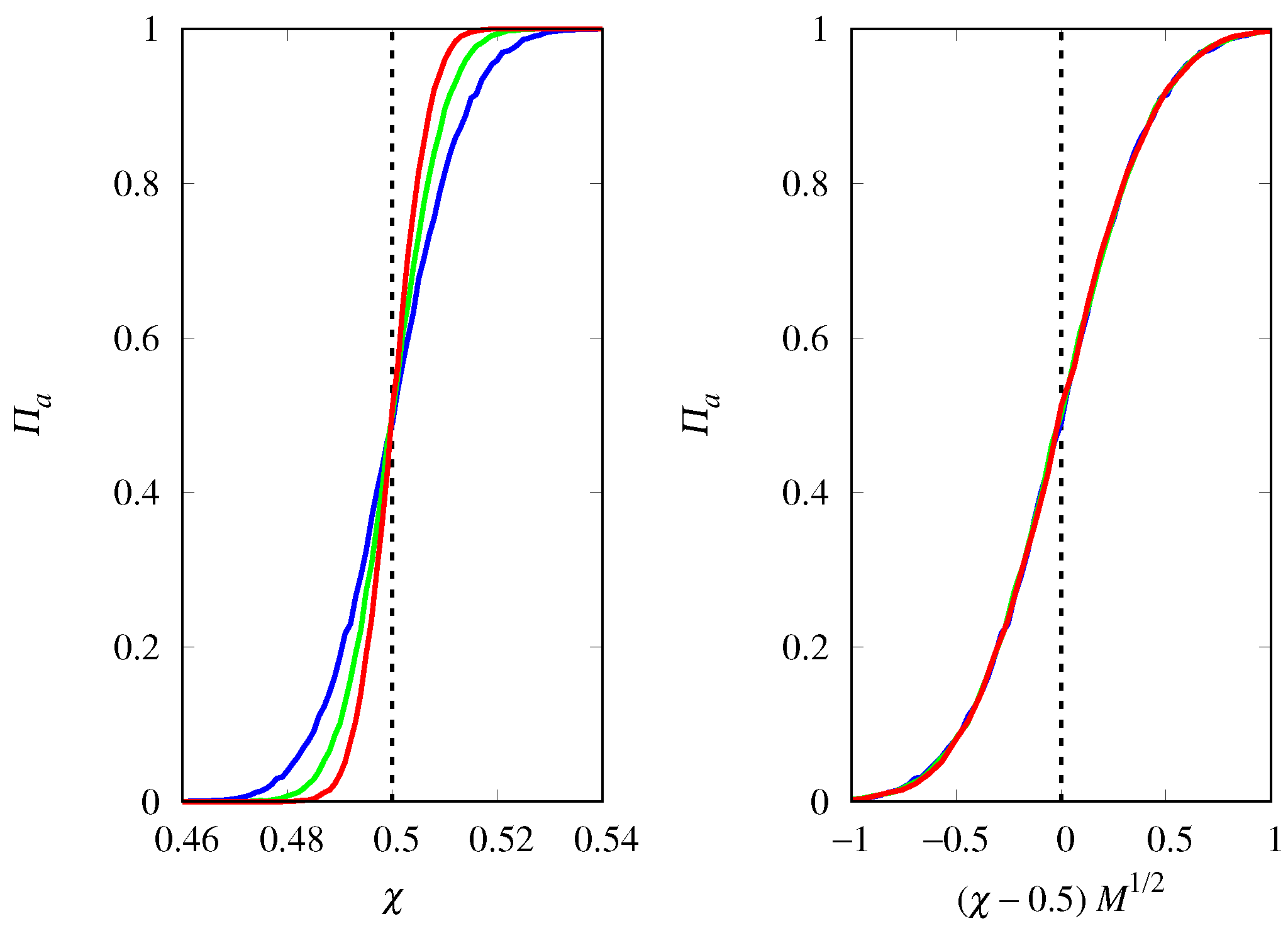
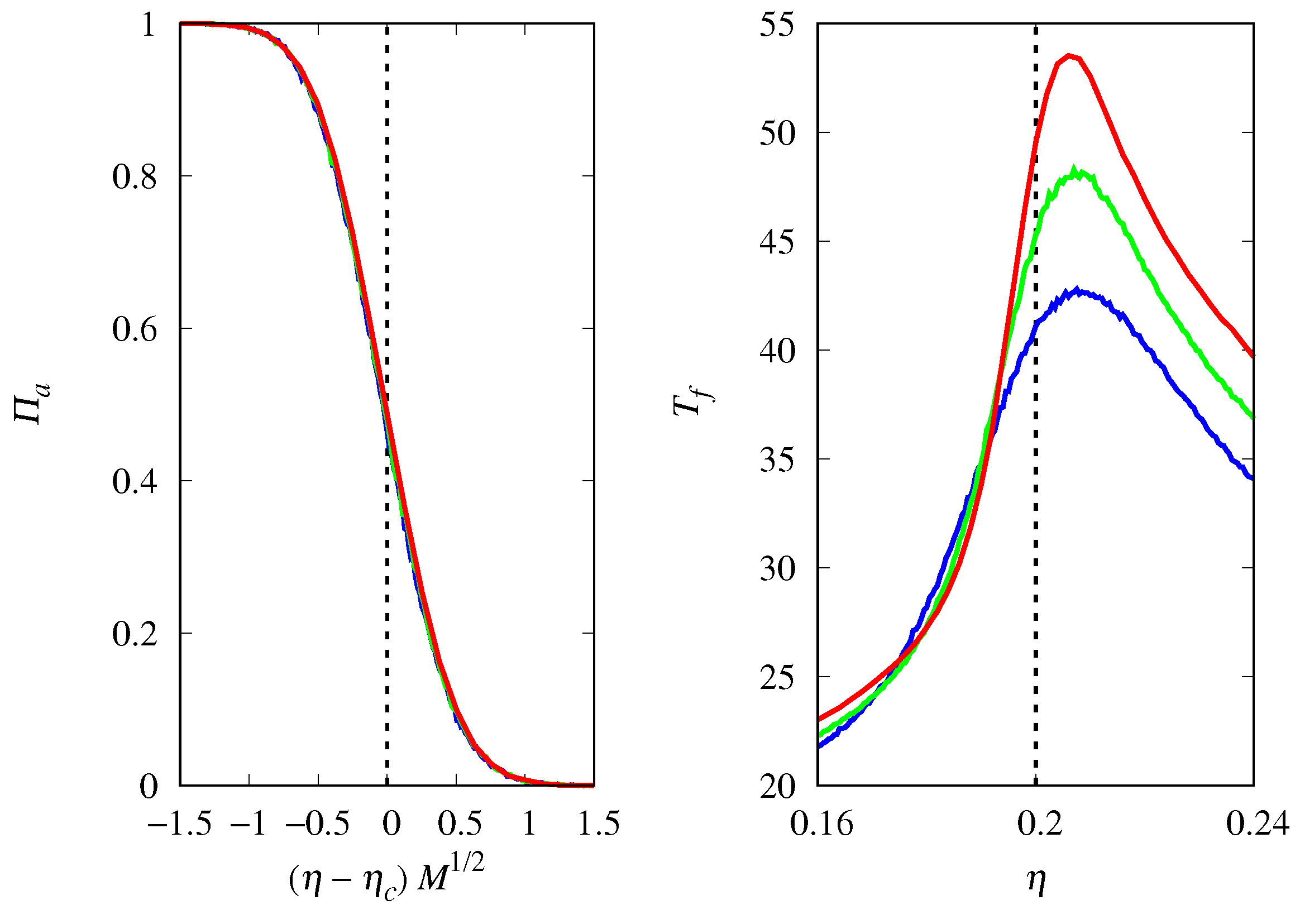
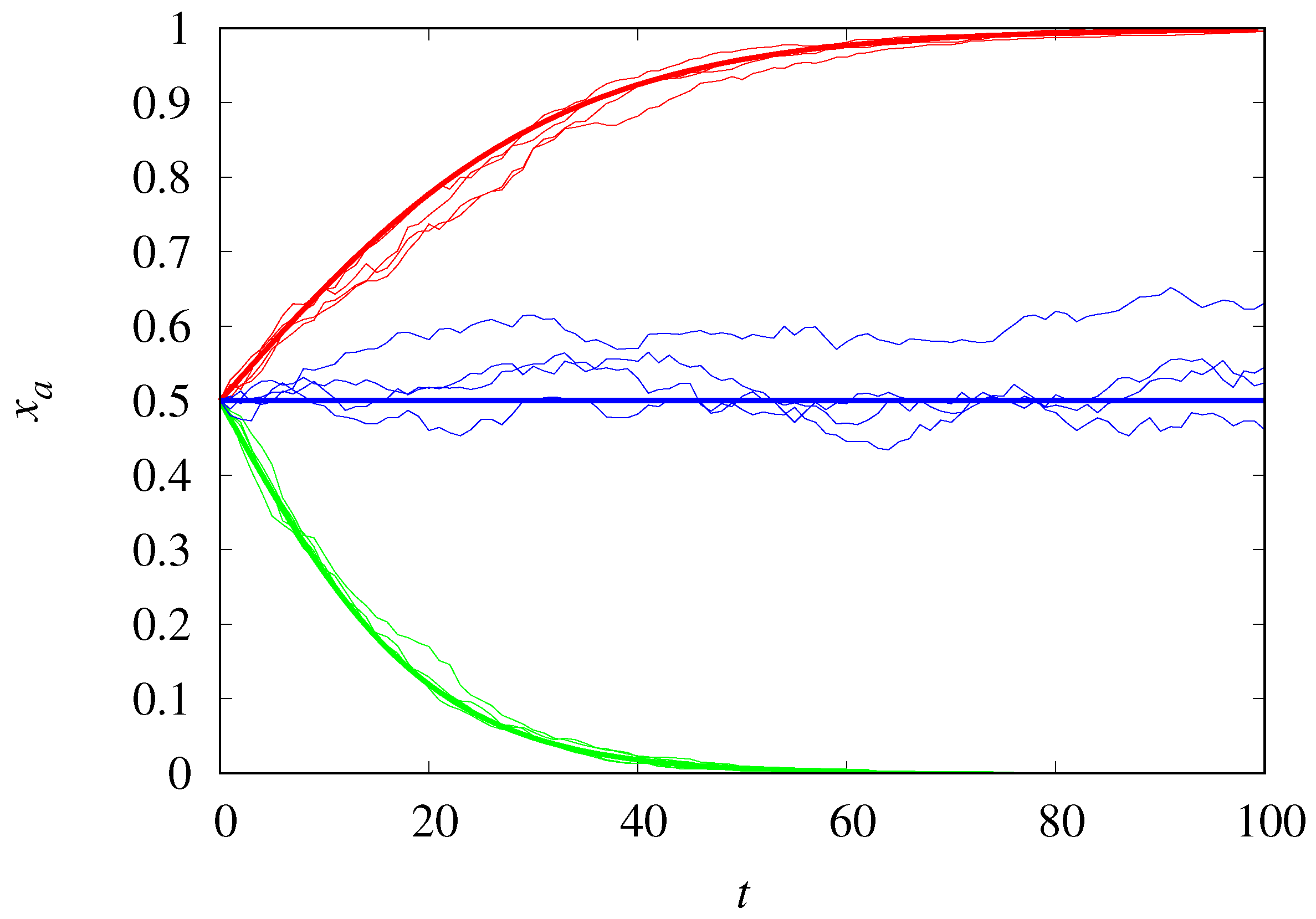
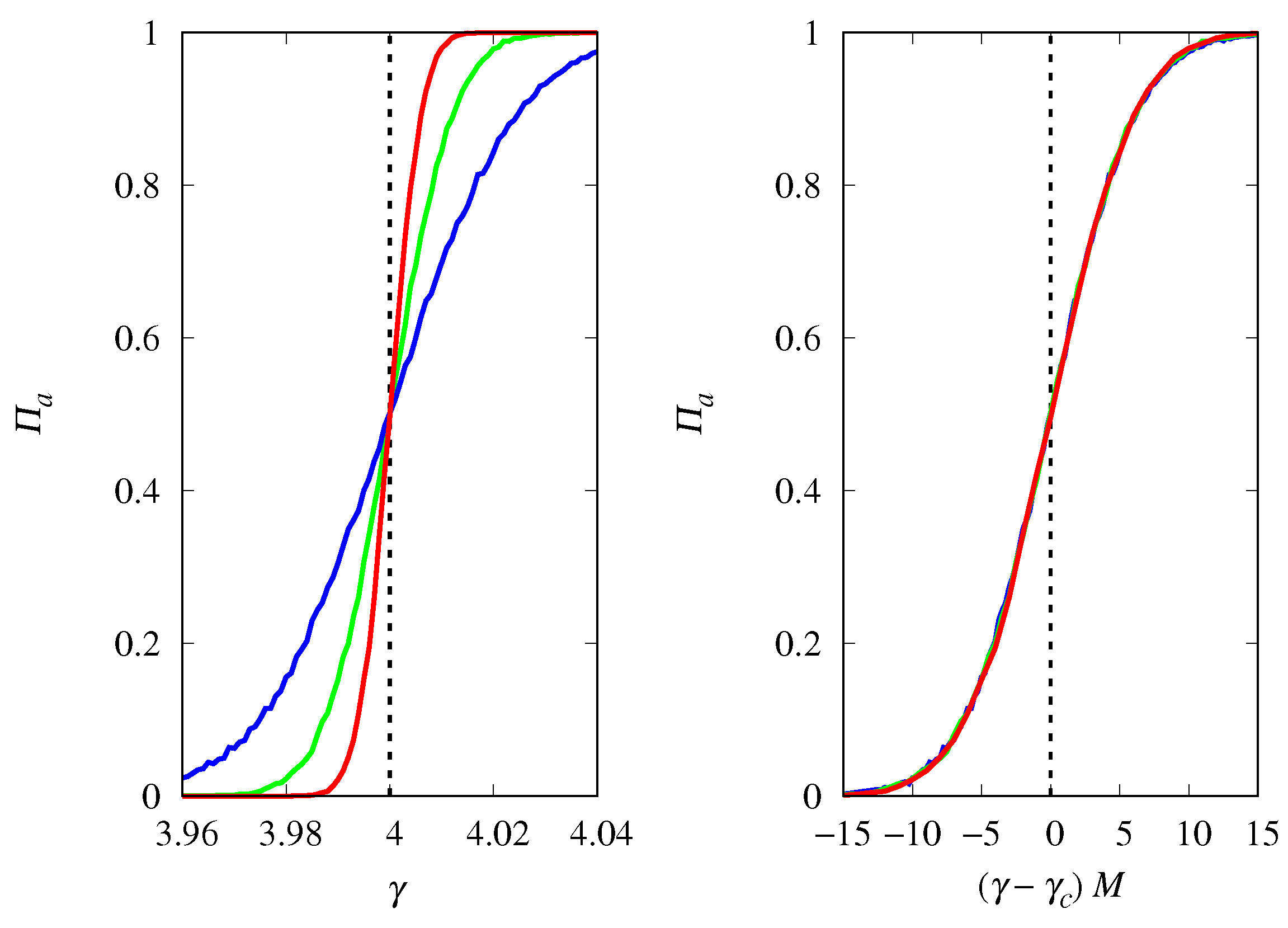
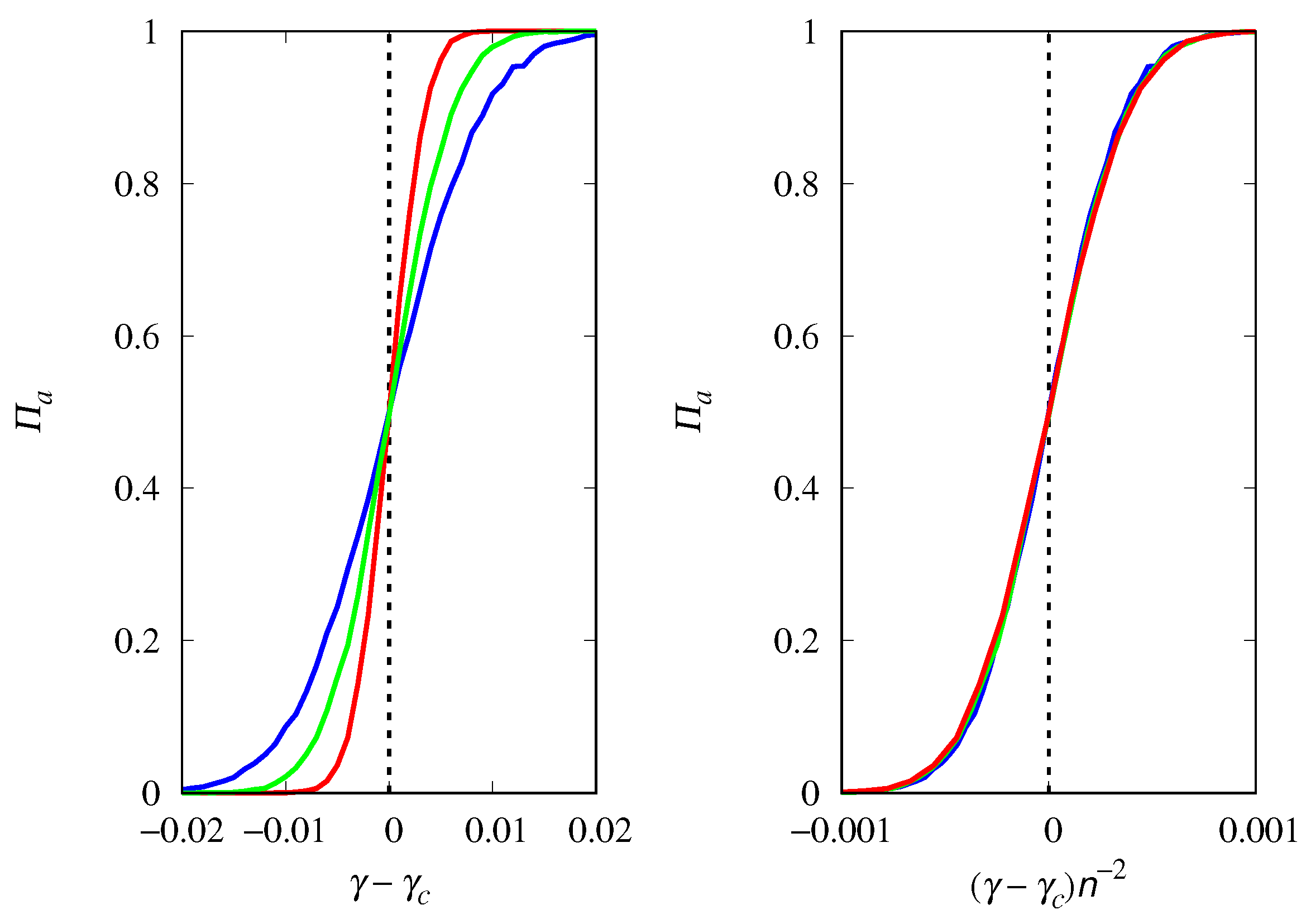
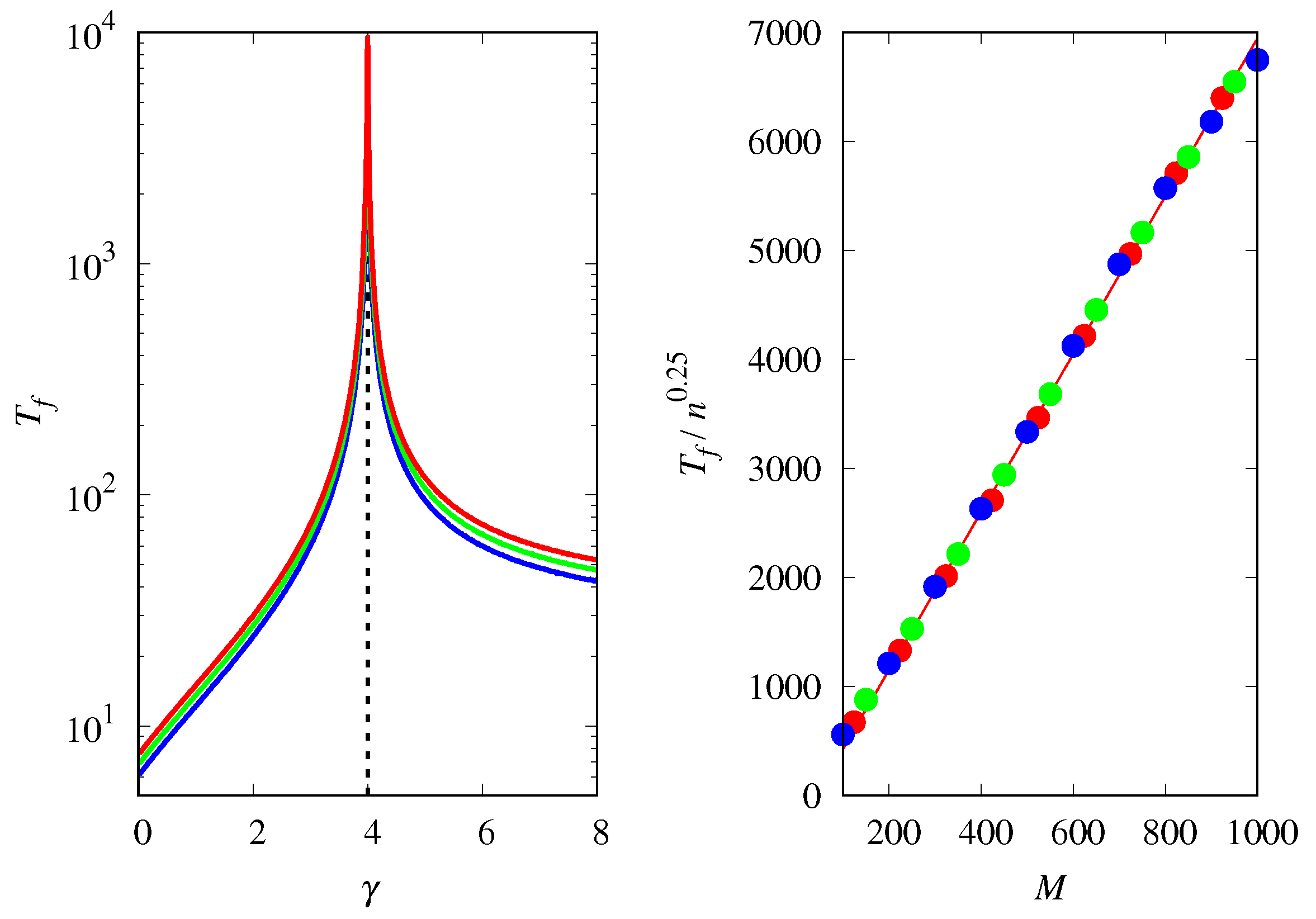
3.4. Finite Population Simulations
4. Discussion
It is beyond the scope of this paper to discuss the details of the reaction mechanisms …the properties of which resemble, in many ways, social behavior.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- (a)
- Replicator i is of type a at time t, and another replicator is selected to be challenged. The probability of this event is
- (b)
- Replicator i is of type a at time t and is selected to be challenged. The challenger also is of type a. The probability of this event is
- (c)
- Replicator i is of type a at time t and is selected to be challenged. The challenger is of type b, but fails to replace the challenged replicator. The probability of this event is
- (d)
- Replicator i is of type b at time t and is selected to be challenged. The challenger is of type a and succeeds to replace the challenged replicator. The probability of this event is
Appendix B
References
- Axelrod, R. The Evolution of Cooperation; Basic Books: New York, NY, USA, 1984. [Google Scholar]
- Maynard Smith, J. Hypercycles and the origin of life. Nature 1979, 280, 445–446. [Google Scholar] [CrossRef]
- Eigen, M. Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften 1971, 58, 465–526. [Google Scholar] [CrossRef]
- Eigen, M.; Schuster, P. The hypercycle. A principle of natural self-organization. B: The abstract hypercycle. Naturwissenschaften 1978, 65, 7–41. [Google Scholar] [CrossRef]
- Maynard Smith, J. Evolution and the Theory of Games; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Pfeiffer, T.; Schuster, S. Game-Theoretical Approaches to Studying the Evolution of Biochemical Systems. Trends Biochem. Sci. 2005, 30, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Kreft, J.U.; Schroeter, A.; Pfeiffer, T. Use of Game-Theoretical Methods in Biochemistry and Biophysics. J. Biol. Phys. 2008, 34, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Champagne-Ruel, A.; Charbonneau, P. A Mutation Threshold for Cooperative Takeover. Life 2022, 12, 254. [Google Scholar] [CrossRef]
- Elena, S.F.; Bernet, G.P.; Carrasco, J.L. The games plant viruses play. Curr. Opin. Virol. 2014, 8, 62–87. [Google Scholar] [CrossRef]
- Szathmáry, E. Natural selection and dynamical coexistence of defective and complementing virus segments. J. Theor. Biol. 1992, 157, 383–406. [Google Scholar] [CrossRef]
- Maynard Smith, J.; Szathmáry, E. The Origins of Life; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Blokhuis, A.; Nghe, P.; Peliti, L.; Lacoste, D. The generality of transient compartmentalization and its associated error thresholds. J. Theor. Biol. 2020, 487, 110110. [Google Scholar] [CrossRef]
- Swetina, J.; Schuster, P. Self-replication with errors: A model for polynucleotide replication. Biophys. Chem. 1982, 16, 329–345. [Google Scholar] [CrossRef]
- Michod, R.E. Population Biology of the First Replicators: On the Origin of the Genotype, Phenotype and Organism. Am. Zool. 1983, 23, 5–14. [Google Scholar] [CrossRef]
- Silvestre, D.A.M.M.; Fontanari, J.F. The information capacity of hypercycles. J. Theor. Biol. 2008, 254, 804–806. [Google Scholar] [CrossRef]
- Fontich, E.; Guillamon, A.; Perona, J.; Sardanyés, J. Functional Shift-Induced Degenerate Transcritical Neimark-Sacker Bifurcation in a Discrete Hypercycle. Int. J. Bifurc. Chaos 2024, 34, 2450045. [Google Scholar] [CrossRef]
- Bresch, C.; Niesert, U.; Harnasch, D. Hypercycles, parasites and packages. J. Theor. Biol. 1980, 85, 399–405. [Google Scholar] [CrossRef]
- Szathmáry, E.; Demeter, L. Group selection of early replicators and the origin of life. J. Theor. Biol. 1987, 128, 463–486. [Google Scholar] [CrossRef] [PubMed]
- Fontanari, J.F.; Santos, M.; Szathmáry, E. Coexistence and error propagation in pre-biotic vesicle models: A group selection approach. J. Theor. Biol. 2006, 239, 247–256. [Google Scholar] [CrossRef]
- Silvestre, D.A.M.M.; Fontanari, J.F. Package models and the information crisis of prebiotic evolution. J. Theor. Biol. 2008, 252, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Kun, A.; Ryckelynck, M.; Coldren, F.; Szilágyi, A.; Jossinet, F.; Rick, C.; Nghe, P.; Szathmáry, E.; Griffiths, A.D. Transient compartmentalization of RNA replicators prevents extinction due to parasites. Science 2016, 354, 1293–1296. [Google Scholar] [CrossRef]
- Blokhuis, A.; Lacoste, D.; Nghe, P.; Peliti, L. Selection dynamics in transient compartmentalization. Phys. Rev. Lett. 2018, 120, 158101. [Google Scholar] [CrossRef]
- Laurent, G.; Peliti, L.; Lacoste, D. Survival of Self-Replicating Molecules under Transient Compartmentalization with Natural Selection. Life 2019, 9, 78. [Google Scholar] [CrossRef]
- Arenzon, J.J.; Peliti, L. Emergent cooperative behavior in transient compartments. Phys. Rev. E 2023, 108, 034409. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.F.; Kimura, M. An Introduction to Population Genetics Theory; Harper and Row: New York, NY, USA, 1970. [Google Scholar]
- Traulsen, A.; Claussen, J.C.; Hauert, C. Coevolutionary Dynamics: From Finite to Infinite Populations. Phys. Rev. Lett. 2005, 95, 238701. [Google Scholar] [CrossRef]
- Fontanari, J.F. Imitation dynamics and the replicator equation. Europhys. Lett. 2024, 146, 47001. [Google Scholar] [CrossRef]
- Privman, V. Finite-Size Scaling and Numerical Simulations of Statistical Systems; World Scientific: Singapore, 1990. [Google Scholar]
- Fox, J.; Guyer, M. Public Choice and cooperation in N-person Prisoner’s Dilemma. J. Confl. Resolut. 1978, 22, 469–481. [Google Scholar] [CrossRef]
- Wilson, D.S. Weak altruism, strong group selection. Oikos 1990, 59, 135–140. [Google Scholar] [CrossRef]
- Hofbauer, J.; Sigmund, K. Evolutionary Games and Population Dynamics; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Gillespie, D.T. A General Method for Numerically Simulating the Stochastic Time Evolution of Coupled Chemical Reactions. J. Comput. Phys. 1976, 22, 403–434. [Google Scholar] [CrossRef]
- Ahmed, E.; El-Sayed, A.M.A.; El-Mesiry, A.E.M.; El-Saka, H.A.A. Numerical Solution for the Fractional Replicator Equation. Int. J. Mod. Phys. C 2005, 16, 1017–1025. [Google Scholar] [CrossRef]
- Goufo, E.F.D. An application of the Caputo-Fabrizio operator to replicator-mutator dynamics: Bifurcation, chaotic limit cycles and control. Eur. Phys. J. Plus 2018, 133, 80. [Google Scholar] [CrossRef]
- Gao, X.-L.; Li, Z.-Y.; Wang, Y.-L. Chaotic Dynamic Behavior of a Fractional-Order Financial System with Constant Inelastic Demand. Int. J. Bifurc. Chaos 2024, 34, 2450111. [Google Scholar] [CrossRef]
- Kirkpatrick, S.; Selman, B. Critical Behavior in the Satisfiability of Random Boolean Expressions. Science 1994, 264, 1297–1301. [Google Scholar] [CrossRef]
- Campos, P.R.A.; Fontanari, J.F. Finite-size scaling of the error threshold transition in finite populations. J. Phys. A Math. Gen. 1999, 32, L1–L7. [Google Scholar] [CrossRef]
- Towe, R.M. Environmental conditions surrounding the origin and early archean evolution of life: A hypothesis. Precambrian Res. 1981, 16, 1–10. [Google Scholar] [CrossRef]
- Hamilton, W.D. Innate social aptitudes of man: An approach from evolutionary genetics. In ASA Studies 4: Biological Anthropology; Fox, R., Ed.; Malaby Press: London, UK, 1975; pp. 133–153. [Google Scholar]
- Rosas, A.; Ferreira, C.P.; Fontanari, J.F. Evolution of protein synthesis in a lattice model of replicators. Phys. Rev. Lett. 2002, 89, 188101. [Google Scholar] [CrossRef] [PubMed]
- Perc, M.; Gómez-Gardeñes, J.; Szolnoki, A.; Floría, L.M.; Moreno, Y. Evolutionary dynamics of group interactions on structured populations: A review. J. R. Soc. Interface 2013, 10, 20120997. [Google Scholar] [CrossRef]
- Adami, C.; Schossau, J.; Hintze, A. Evolutionary game theory using agent-based methods. Phys. Life Rev. 2016, 19, 1–26. [Google Scholar] [CrossRef]
- Cooney, D.B.; Levin, S.A.; Mori, Y.; Plotkin, J.B. Evolutionary dynamics within and among competing groups. Proc. Natl. Acad. Sci. USA 2023, 120, e2216186120. [Google Scholar] [CrossRef]
- Oparin, A.I. The pathways of the primary development of metabolism and artificial modeling of this development in coacervate drops. In The Origins of Prebiological Systems; Fox, S.W., Ed.; Academic Press: New York, NY, USA, 1965; pp. 331–346. [Google Scholar]
- Wilson, D.S. A theory of group selection. Proc. Nat. Acad. Sci. USA 1975, 72, 143–146. [Google Scholar] [CrossRef]
- Fontanari, J.F.; Santos, M. The dynamics of casual groups can keep free-riders at bay. Math. Biosci. 2024, 372, 109188. [Google Scholar] [CrossRef]
- Archetti, M.; Scheuring, I. Review: Game theory of public goods in one-shot social dilemmas without assortment. J. Theor. Biol. 2012, 299, 9–20. [Google Scholar] [CrossRef]
- Xia, C.; Wang, J.; Perc, M.; Wang, Z. Reputation and reciprocity. Phys. Life Rev. 2023, 46, 8–45. [Google Scholar] [CrossRef]
- Boyd, R.; Richerson, R.J. Punishment allows the evolution of cooperation (and anything else), in sizable group. Ethol. Sociobiol. 1992, 13, 171–195. [Google Scholar] [CrossRef]
- Fontanari, J.F.; Santos, M. Solving the prisoner’s dilemma trap in Hamilton’s model of temporarily formed random groups. arXiv 2024, arXiv:2407.02440. [Google Scholar]
- Nowak, M.A. Evolutionary Dynamics: Exploring the Equations of Life; Belknap Press: New York, NY, USA, 2006. [Google Scholar]
- Donato, R.; Peliti, L.; Serva, M. The Selection of Altruistic Behaviour. Theory Biosci. 1997, 116, 309–320. [Google Scholar]
- Alves, D.; Campos, P.R.A.; Silva, A.T.C.; Fontanari, J.F. Group selection models in prebiotic evolution. Phys. Rev. E 2000, 63, 011911. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.M.; Santos, F.C.; Souza, M.O.; Skyrms, B. Evolutionary dynamics of collective action in N-person stag hunt dilemmas. Proc. R. Soc. B 2009, 276, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Feller, W. An Introduction to Probability Theory and Its Applications; Wiley: New York, NY, USA, 1968; Volume 1. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, M.S.; Fontanari, J.F. Evolutionary Game-Theoretic Approach to the Population Dynamics of Early Replicators. Life 2024, 14, 1064. https://doi.org/10.3390/life14091064
Mariano MS, Fontanari JF. Evolutionary Game-Theoretic Approach to the Population Dynamics of Early Replicators. Life. 2024; 14(9):1064. https://doi.org/10.3390/life14091064
Chicago/Turabian StyleMariano, Matheus S., and José F. Fontanari. 2024. "Evolutionary Game-Theoretic Approach to the Population Dynamics of Early Replicators" Life 14, no. 9: 1064. https://doi.org/10.3390/life14091064






