Alternative Pathways in Astrobiology: Reviewing and Synthesizing Contingency and Non-Biomolecular Origins of Terrestrial and Extraterrestrial Life
Abstract
1. Introduction
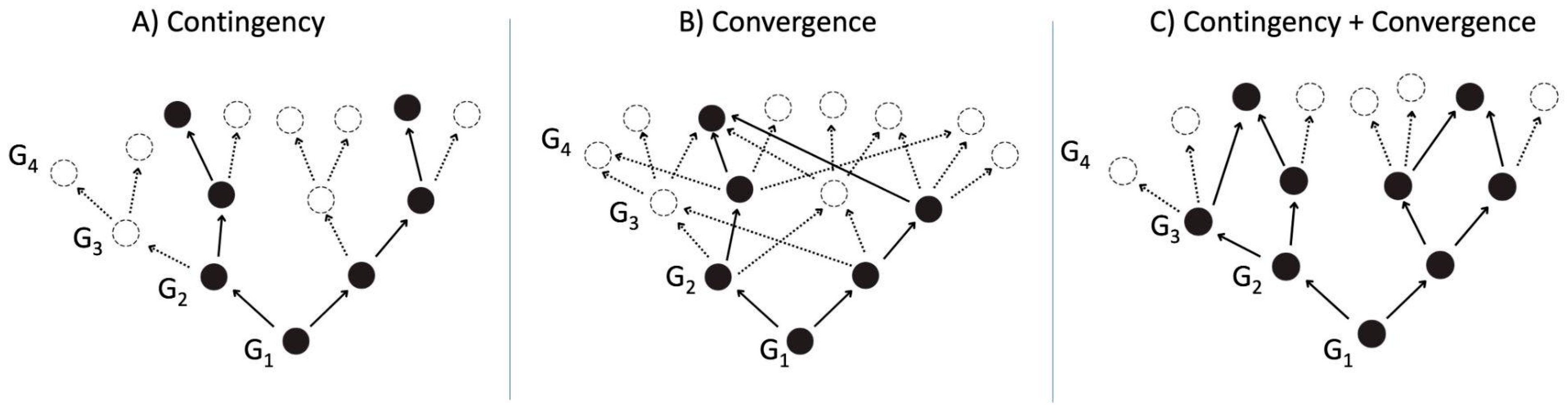
“…any replay of the tape (of life) would lead evolution down a pathway radically different from the road actually taken…”—Stephen Jay Gould, 1989
2. The N = 1 Problem and Its Relevance in the Search for Extraterrestrial Life
- (a)
- A “messy” chemical state is the default state of any prebiotic chemical space on a given planetary body of astrobiological interest (including primitive Earth). Such a messy prebiotic chemical space provides the starting material for prebiotic chemistry to happen, is likely different from planet to planet, is impossible to simulate completely precisely in the laboratory, and may not even be necessarily reproducible on the same planet.
- (b)
- Non-biological chemicals could have played a role during the OoL, which may have produced life with a molecular makeup different from life on Earth.
- (c)
- The role of contingency could shape life differently from life as we know it on Earth due to differing planetary conditions and “messy” prebiotic chemical spaces.
3. The “Messy” State of Prebiotic Chemistry
4. Non-Biological Chemicals and Their Prebiotic Chemistry During the OoL
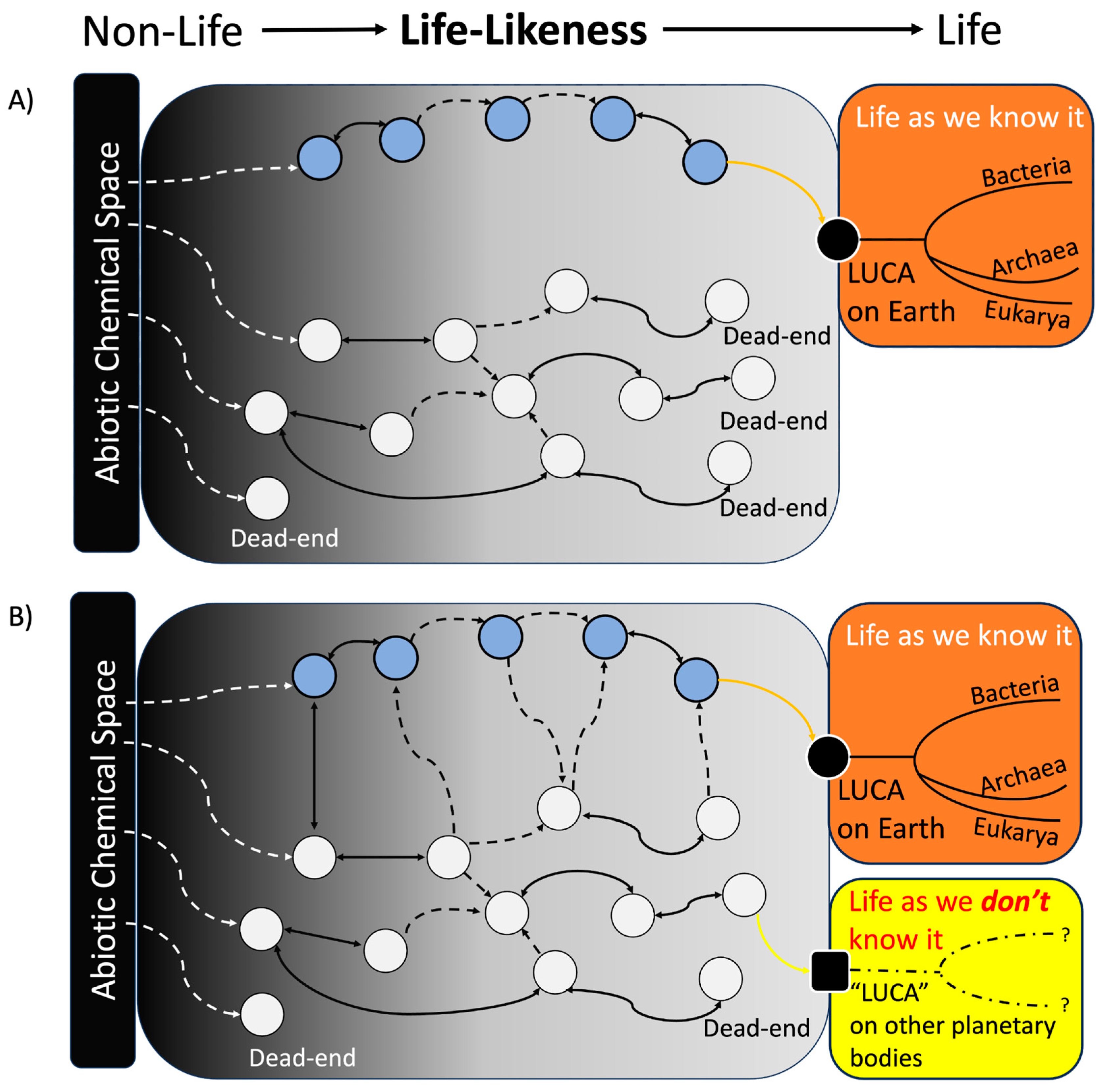
5. The Role of Contingencies during the OoL (on and off Earth) Linking Non-Biomolecular Compounds
- Black circle = arbitrary LUCA on Earth, eventually leading to the terrestrial life tree (orange box).
- Black square = arbitrary “LUCA” on another planetary body, which eventually follows its own unique “phylogenic” path towards ET life (yellow box).
- Blue circles = deterministic chemical states (CS) inspired by and leading to modern biochemistry, such as an RNA world, a metabolism first world, etc., which assumes a direct history between prebiotic chemistry and modern biology.
- White circles = the diverse potential CS that may not follow a deterministic path toward life. These CS are composed of a blend of biomolecules and non-biomolecules, likely dominated in abundance by non-biomolecules originating from the abiotic chemical space, encompassing plausible abiotic chemicals that existed on primitive Earth or other early-planetary bodies. Contingency (by geochemical “selection”) propels CS further towards different areas of the “life-likeness” spectrum (i.e., non-life, somewhat life-like, and life) towards a more “life-like” state (defined from a terrestrial perspective). More advanced “life-like” CS can move forward along this spectrum by manipulating their environment, potentially metabolizing and replicating through the use of novel molecules not found in the abiotic chemical space (i.e., autotrophy), all while still undergoing “selection”, advancing their status to become arbitrary “LUCA”. At any given point, any CS can reach a dead-end, meaning such a CS cannot progress to any form of life.
- White dashed arrows = the process for a CS to emerge, which is derived directly from a particular abiotic chemical space, i.e., all components within the CS are solely chemicals within the abiotic chemical space of any geological niches on early Earth or other planetary bodies.
- Double-headed solid black arrows = a transition from a CS to another CS. These black arrows are double-headed, which suggests that a CS can return to a former state through, for example, the degradation of certain components.
- Dashed black arrows = the mixing of two or more CS (the two or more circles at the origin of the arrows) to form a novel CS composed of a mixture of components of the initial CS (the one circle at the destination of the arrows). These arrows are single-headed, as de-mixing may not result in recovery of identical initial CS as before; however, de-mixing (not shown) could result in one CS splitting into two novel CS. The mixing can result from the mixing of two or more biomolecular CS (blue circles), some “non-biomolecular” CS (white circle) with some biomolecular CS, or two or more non-biomolecular CS. For example, mixing of a non-biomolecular CS with a biomolecular CS can result in a new CS where the components of the initial non-biomolecular CS drive some type of novel reaction amongst the components of the initial biomolecular CS, ultimately leading to the emergence of a more “life-like” biomolecular CS that is “scaffolded” by some non-biomolecular process or chemical(s).
- Orange and yellow arrows = the progression of a CS to a LUCA. These arrows are single-headed, indicating the fact that once LUCA emerges, which is defined as “life”, it cannot reversibly convert back to a “non-life” CS state (as “life” by definition must either be able to sustain its own life, or undergo extinction (see below for further explanation).
- Note 1: The arbitrary term LUCA was used as a point depicting the beginning of Darwinian-like evolution, but the concept of LUCA remains unclear in the field; for example, LUCA could either be a living entity that acquired the full ability of transcription-translation mechanisms [94] or one derived from progenotes [95,96]. The latter is not depicted in Figure 2 for simplicity. Similarly, the definition of terms we use to describe life in this paper, such as ‘life as we know it’ and ‘life as we don’t know it’, is not discussed here, as it is well covered elsewhere [97].
- Note 2: Though not explicitly shown in the model, the arrival of unique elements or organic compounds via by means of comets and meteorites [98] could have occurred during meteoritic bombardment periods, which were was common on Early Earth [99]. These additions might have influenced the available abiotic chemical space, potentially affecting the progression of “life”, and the existence of more “life-like” CSs at different times. This could also be applied to ET life cases. In this case, more emergent CSs could have emerged from the altered abiotic chemical space, while more “life-like” CSs could incorporate some aspects of the modified abiotic chemical space to access novel chemicals, chemistries, reactions, and functions. This concept simplifies to a larger abiotic chemical space, which can be seen as encompassing components from both the Earth’s abiotic chemical space and that introduced by comets and meteorites.
- Note 3: Although not mentioned explicitly, emergent polymers from the initial abiotic chemical space with early catalytic abilities could have altered the reaction topography within its own chemical space, leading to the formation of new CSs. These new CSs either enhance or hinder the life-like properties of a particular CS. Additionally, the formation of these new CSs could also be maintained by auto-catalytic reactions, potentially giving rise to a where new functional chemistries and reducing the dependence on input from other CSs for persistence.
- Note 4: Terrestrial life may be an outlier, and existence of ET life remains improbable (but not impossible), as we have not observed a single instance of ET life. Several factors contribute to the absence of signs of ET life, possibly because such life never developed. Firstly, chemistries that lead to the OoL (as well as the chemistries of life) must be viable for a long period of time from the onset of planetary formation due to the timescales of non-catalyzed chemical reactions [100]. Secondly, early OoL events (and life itself) occurring on a particular planet must remain persistent, i.e., keeping pace and changing to survive in the ever-changing environmental and geochemical settings by developing replication and fine-tuning metabolism and protection to avoid extinction [101]. Life on Earth has adapted and survived for ~3–3.5 billion years despite numerous extinction events. Hence, if any of the requirements for OoL off Earth are not met, we will never observe extant ET life, either due to early extinction of early life or failure to fend off its last planetary extinction threat. In either case, the planet would remain barren of life.
6. Caveats of the Contingency and Non-Biomolecular OoL Model
- (a)
- We did not specify the specific chemical, geological, and physical constraints necessary for conditions leading to life in the model because such constraints are not necessarily fixed and are bound to be updated with new data and modeling. Nevertheless, the emergence of any CS, like any prebiotic system, is driven and governed by constraints via its primitive environment parameters (pH, temperature, etc.) [51]. Early CSs are constrained by the rules of chemistry, which are governed by thermodynamics (before kinetic control) and also geology, i.e., geological niches present on early Earth (e.g., hydrothermal vents, evaporating ponds, etc.). Such constraints act as “selective” pressures on CSs and can fluctuate from time to time, either in short or longer durations. As CSs evolve to become more complex and functional, resembling life forms (as illustrated in Figure 2), the imperative to adapt to such constraints for survival intensifies. Sudden changing chemical and environmental constraints due to ambient geology, for example, temperature rising because of increasing atmospheric CO2 from volcanic events, may be too harsh on advanced CSs made up of macromolecules and could lead to thermal degradation. For example, RNA and polyesters within a particular CS in water are prone to accelerated hydrolysis when heat is added [105,106]. Alternatively, oceans getting cooler due to tectonic events, i.e., the movement of Earth’s primordial continental plates [107] allowing movement of water between them, could slow down the chemical reactions that make certain CSs. How these constraints act on a particular CS is of particular interest and helps evaluate their plausibility for survival and innovation (which may come when an (advanced) CS has the ability to create solutions for a problem likely via some replication system).
- (b)
- The presence and composition of the actual chemicals available within any abiotic chemical space is contingent upon the prevailing local and global geochemistry of a specific celestial body of astrobiological significance, e.g., early Earth or Noachian Mars. This likely will change from time to time (e.g., [108,109,110]) during the prebiotic epoch and across geological timescales, and hence primitive chemical systems will not be provided with continuous supplies of all organic chemicals all of the time; the supply of critical organic compounds would have implications on both the ability to generate emergent CS and on the identity of the emergent CS (it may be that no single emergent CS is identical to any other one). Global or local perturbating geological conditions provide further screening on the heterogeneous emergent CS [51], for example, through degradation or seclusion, that can eliminate some or all emergent CS from existence. This can lead to the preservation of the “fittest” CS, in terms of the persistence, while “functional” CS—those with abilities important for progressing towards a more “life-like” state, such as autocatalytic properties—may be expelled during such perturbations if they are not fit for persistence. A “functional” CS may be lost altogether at some point in time, although the function may be deemed useful towards achieving more “life-like” states. Hence, how would the system recover such a loss? Can the same lost “functional” CS be produced again if the chemical and geological conditions revert to the original state (e.g., the reversal of a snowball Earth-like event to a warm Earth-like one [111]) that produced the lost “functional” CS in the first place (it may never revert in the end)? Or can the system produce another CS that can perform the same (or a similar) function as the lost “functional” CS, simply with different components? This would, of course, depend on the function, and whether that function must necessarily be achieved in one way, or whether there are multiple ways to achieve that function. As the introduced model consists of a multitude of emergent CSs, which theoretically are constantly being produced (from the abiotic chemical space) and degraded, what are the probabilities of recovery of a lost “functional” CS if emergent CS may never be identical to any other one (and if more than one CS is required to lead to another resulting CS)? This is where we anticipate determinism coming into play, where the lost functionality can be possibly re-gained through a different pathway towards the formation and/or composition of a CS.
- (c)
- Without a replication system, Darwinian-like evolution is not possible, and the long-term existence of any life-like CSs edging closer to life in the spectrum of “life-likeness” is unsustainable. This is because the formation and continuity of CSs depend on the constant availability of the same chemical sources that originally produced them. As mentioned before, the constant changes in geological conditions on early Earth could have caused chemical fluctuations that led to the rapid disappearance of a multitude of CSs, both emergent and those that are more “life-like”. If any CS indeed had a hand in the initial development of a living system, it would likely have required the ability to replicate informationally at some point. This replication would allow the CS (or a similar CS produced later) to persist long enough to transfer its structures, functions, products, or reactions to the primitive living system, even amidst fluctuations and the loss of chemical availability (i.e., it must be able to be replicate, assuming that there is only one deterministic pathway to the emergence of life). This replication could have been through a genetic sequence system [112], a composomic informational system [113], a constant supply of the required materials (see above) through some type of auto-catalytic system [114], or some other type of not-yet-known replication system.
- (d)
- We have shown the possibility that sequences of prebiological chemical states that eventually led to the emergence of arbitrary LUCA may have been “scaffolded” by non-biological compounds throughout history’s existence (Figure 2B). If the conclusion from [69] is true—that the inclusion of biological choice from chemical space of biomolecules bootstraps a CS towards producing biological products—then understanding when and how the processes involving non-biomolecules (if they were essential for the emergence of life) were removed from primitive biology is crucial and requires further investigation. For example, it has been shown that alpha hydroxy acid-charged-tRNAs can undergo in vitro translation that results in the synthesis of polyesters with modern biological translation machinery [115,116]. Does this mean that if alpha hydroxy acids and/or polyesters participated in primitive biology, that they were removed only after the emergence of the central dogma? Or, is this simply a biochemical coincidence? Were there other non-biological systems that participated in primitive biology that are now absent in modern biology? How do we know if there are?
7. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Maar, A. Possible Uses of Counterfactual Thought Experiments in History. Principia Int. J. Epistemol. 2014, 18, 87–113. [Google Scholar] [CrossRef]
- Wenzlhuemer, R. Counterfactual Thinking as a Scientific Method. Hist. Soz. Forsch. 2009, 34, 27–54. [Google Scholar]
- Kray, L.J.; George, L.G.; Liljenquist, K.A.; Galinsky, A.D.; Tetlock, P.E.; Roese, N.J. From What Might Have Been to What Must Have Been: Counterfactual Thinking Creates Meaning. J. Pers. Soc. Psychol. 2010, 98, 106–118. [Google Scholar] [CrossRef]
- Gould, S.J. Wonderful Life: The Burgess Shale and the Nature of History; WW Norton & Company: New York, NY, USA, 1989; ISBN 9780393027051. [Google Scholar]
- Van Roy, P.; Orr, P.J.; Botting, J.P.; Muir, L.A.; Vinther, J.; Lefebvre, B.; el Hariri, K.; Briggs, D.E.G. Ordovician Faunas of Burgess Shale Type. Nature 2010, 465, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J. Replaying Life’s Tape. J. Philos. 2006, 103, 336–362. [Google Scholar] [CrossRef]
- Desjardins, E. Historicity and Experimental Evolution. Biol. Philos. 2011, 26, 339–364. [Google Scholar] [CrossRef]
- Turner, D.D. Gould’s Replay Revisited. Biol. Philos. 2011, 26, 65–79. [Google Scholar] [CrossRef]
- Powell, R.; Mariscal, C. Convergent Evolution as Natural Experiment: The Tape of Life Reconsidered. Interface Focus 2015, 5, 20150040. [Google Scholar] [CrossRef]
- Powell, R. Contingency and Convergence: Toward a Cosmic Biology of Body and Mind; MIT Press: Cambridge, MA, USA, 2020; ISBN 9780262356602. [Google Scholar]
- Gyenis, B. Determinism, Physical Possibility, and Laws of Nature. Found. Phys. 2020, 50, 568–581. [Google Scholar] [CrossRef]
- Morris, S.C. The Crucible of Creation: The Burgess Shale and the Rise of Animals; Oxford University Press: Oxford, UK, 1998; ISBN 9780198502562. [Google Scholar]
- Morris, S.C. Evolution: Like Any Other Science It Is Predictable. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 133–145. [Google Scholar] [CrossRef]
- Croco, E.; Marchionni, S.; Storci, G.; Bonafè, M.; Franceschi, C.; Stamato, T.D.; Sell, C.; Lorenzini, A. Convergent Adaptation of Cellular Machineries in the Evolution of Large Body Masses and Long Life Spans. Biogerontology 2017, 18, 485–497. [Google Scholar] [CrossRef]
- Bowler, P. Cambrian Conflict: Crucible an Assault on Gould’s Burgess Shale Interpretation. Am. Sci. 2017, 86, 472–475. [Google Scholar]
- Losos, J. Improbable Destinies: How Predictable Is Evolution? Penguin UK: New York, NY, USA, 2017; ISBN 9780241201947. [Google Scholar]
- Blount, Z.D.; Lenski, R.E.; Losos, J.B. Contingency and Determinism in Evolution: Replaying Life’s Tape. Science 2018, 362, eaam5979. [Google Scholar] [CrossRef] [PubMed]
- Mazel, F.; Wüest, R.O.; Gueguen, M.; Renaud, J.; Ficetola, G.F.; Lavergne, S.; Thuiller, W. The Geography of Ecological Niche Evolution in Mammals. Curr. Biol. 2017, 27, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.C. Life’s Solution: Inevitable Humans in a Lonely Universe; Cambridge University Press: Cambridge, UK, 2003; ISBN 9781139440806. [Google Scholar]
- Schreiber, U.; Locker-Grütjen, O.; Mayer, C. Hypothesis: Origin of Life in Deep-Reaching Tectonic Faults. Orig. Life Evol. Biosph. 2012, 42, 47–54. [Google Scholar] [CrossRef]
- Mayer, C.; Schreiber, U.; Dávila, M.J. Periodic Vesicle Formation in Tectonic Fault Zones--an Ideal Scenario for Molecular Evolution. Orig. Life Evol. Biosph. 2015, 45, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Schreiber, U.; Dávila, M.J. Selection of Prebiotic Molecules in Amphiphilic Environments. Life 2017, 7, 3. [Google Scholar] [CrossRef]
- Mayer, C.; Schreiber, U.; Dávila, M.J.; Schmitz, O.J.; Bronja, A.; Meyer, M.; Klein, J.; Meckelmann, S.W. Molecular Evolution in a Peptide-Vesicle System. Life 2018, 8, 16. [Google Scholar] [CrossRef]
- Cleaves, H.J. Prebiotic Chemistry: What We Know, What We Don’t. Evol. Educ. Outreach 2012, 5, 342–360. [Google Scholar] [CrossRef]
- Mariscal, C.; Barahona, A.; Aubert-Kato, N.; Aydinoglu, A.U.; Bartlett, S.; Cárdenas, M.L.; Chandru, K.; Cleland, C.; Cocanougher, B.T.; Comfort, N.; et al. Hidden Concepts in the History and Philosophy of Origins-of-Life Studies: A Workshop Report. Orig. Life Evol. Biosph. 2019, 49, 111–145. [Google Scholar] [CrossRef]
- Nelson, D.L.; Lehninger, A.L.; Cox, M.M. Lehninger Principles of Biochemistry, 2nd ed.; Macmillan: New York, NY, USA, 2008; ISBN 9780716771081. [Google Scholar]
- Pucino, V.; Bombardieri, M.; Pitzalis, C.; Mauro, C. Lactate at the Crossroads of Metabolism, Inflammation, and Autoimmunity. Eur. J. Immunol. 2017, 47, 14–21. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate as a Fulcrum of Metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef] [PubMed]
- Chandru, K.; Guttenberg, N.; Giri, C.; Hongo, Y.; Butch, C.; Mamajanov, I.; Cleaves, H.J. Simple Prebiotic Synthesis of High Diversity Dynamic Combinatorial Polyester Libraries. Commun. Chem. 2018, 1, 30. [Google Scholar] [CrossRef]
- Chandru, K.; Mamajanov, I.; Cleaves, H.J., 2nd; Jia, T.Z. Polyesters as a Model System for Building Primitive Biologies from Non-Biological Prebiotic Chemistry. Life 2020, 10, 6. [Google Scholar] [CrossRef]
- Kitadai, N.; Maruyama, S. Origins of Building Blocks of Life: A Review. Geosci. Front. 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Pace, N.R. The Universal Nature of Biochemistry. Proc. Natl. Acad. Sci. USA 2001, 98, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Budisa, N.; Kubyshkin, V.; Schmidt, M. Xenobiology: A Journey towards Parallel Life Forms. Chembiochem 2020, 21, 2228–2231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gladyshev, V.N. High Content of Proteins Containing 21st and 22nd Amino Acids, Selenocysteine and Pyrrolysine, in a Symbiotic Deltaproteobacterium of Gutless Worm Olavius Algarvensis. Nucleic Acids Res. 2007, 35, 4952–4963. [Google Scholar] [CrossRef]
- Ilardo, M.; Bose, R.; Meringer, M.; Rasulev, B.; Grefenstette, N.; Stephenson, J.; Freeland, S.; Gillams, R.J.; Butch, C.J.; James, C.H. Adaptive Properties of the Genetically Encoded Amino Acid Alphabet Are Inherited from Its Subsets. Sci. Rep. 2019, 9, 12468. [Google Scholar] [CrossRef]
- Chan, M.A.; Hinman, N.W.; Potter-McIntyre, S.L.; Schubert, K.E.; Gillams, R.J.; Awramik, S.M.; Boston, P.J.; Bower, D.M.; Des Marais, D.J.; Farmer, J.D.; et al. Deciphering Biosignatures in Planetary Contexts. Astrobiology 2019, 19, 1075–1102. [Google Scholar] [CrossRef]
- Hud, N.V.; Cafferty, B.J.; Krishnamurthy, R.; Williams, L.D. The Origin of RNA and “My Grandfather’s Axe”. Chem. Biol. 2013, 20, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R. Life’s Biological Chemistry: A Destiny or Destination Starting from Prebiotic Chemistry? Chemistry 2018, 24, 16708–16715. [Google Scholar] [CrossRef] [PubMed]
- Guttenberg, N.; Virgo, N.; Chandru, K.; Scharf, C.; Mamajanov, I. Bulk Measurements of Messy Chemistries Are Needed for a Theory of the Origins of Life. Philos. Trans. A Math. Phys. Eng. Sci. 2017, 375, 20160347. [Google Scholar] [CrossRef]
- Wołos, A.; Roszak, R.; Żądło-Dobrowolska, A.; Beker, W.; Mikulak-Klucznik, B.; Spólnik, G.; Dygas, M.; Szymkuć, S.; Grzybowski, B.A. Synthetic Connectivity, Emergence, and Self-Regeneration in the Network of Prebiotic Chemistry. Science 2020, 369, eaaw1955. [Google Scholar] [CrossRef]
- Surman, A.J.; Rodriguez-Garcia, M.; Abul-Haija, Y.M.; Cooper, G.J.T.; Gromski, P.S.; Turk-MacLeod, R.; Mullin, M.; Mathis, C.; Walker, S.I.; Cronin, L. Environmental Control Programs the Emergence of Distinct Functional Ensembles from Unconstrained Chemical Reactions. Proc. Natl. Acad. Sci. USA 2019, 116, 5387–5392. [Google Scholar] [CrossRef]
- Robinson, W.E.; Daines, E.; van Duppen, P.; de Jong, T.; Huck, W.T.S. Environmental Conditions Drive Self-Organization of Reaction Pathways in a Prebiotic Reaction Network. Nat. Chem. 2022, 14, 623–631. [Google Scholar] [CrossRef]
- Gesteland, R.F.; Atkins, J.F. The RNA World: The Nature of Modern RNA Suggests a Prebiotic RNA World; Cold Spring Harbor monograph series 24; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1993; ISBN 9780879693800. [Google Scholar]
- Cody, G.D. Geochemical Connections to Primitive Metabolism. Elements 2005, 1, 139–143. [Google Scholar] [CrossRef]
- Smith, E.; Morowitz, H.J. The Origin and Nature of Life on Earth: The Emergence of the Fourth Geosphere; Cambridge University Press: Cambridge, UK, 2016; ISBN 9781316489857. [Google Scholar]
- Lei, L.; Burton, Z.F. Evolution of the Genetic Code. Transcription 2021, 12, 28–53. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Burton, Z.F. Evolution of Life on Earth: tRNA, Aminoacyl-tRNA Synthetases and the Genetic Code. Life 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Opron, K.; Burton, Z.F. A tRNA- and Anticodon-Centric View of the Evolution of Aminoacyl-tRNA Synthetases, tRNAomes, and the Genetic Code. Life 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Escobar, L.; Xu, F.; Węgrzyn, E.; Nainytė, M.; Amatov, T.; Chan, C.-Y.; Pichler, A.; Carell, T. A Prebiotically Plausible Scenario of an RNA-Peptide World. Nature 2022, 605, 279–284. [Google Scholar] [CrossRef]
- Cleland, C.E. The Quest for a Universal Theory of Life: Searching for Life as We Don’t Know It; Cambridge University Press: Cambridge, UK, 2019; ISBN 9780521873246. [Google Scholar]
- Walton, C.R.; Rimmer, P.; Shorttle, O. Can Prebiotic Systems Survive in the Wild? An Interference Chemistry Approach. Front. Earth Sci. 2022, 10, 1011717. [Google Scholar] [CrossRef]
- Benner, S.A.; Bell, E.A.; Biondi, E.; Brasser, R.; Carell, T.; Kim, H.-J.; Mojzsis, S.J.; Omran, A.; Pasek, M.A.; Trail, D. When Did Life Likely Emerge on Earth in an RNA-first Process? ChemSystemsChem 2020, 2, e1900035. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Varma, S.J.; Moran, J. Synthesis and Breakdown of Universal Metabolic Precursors Promoted by Iron. Nature 2019, 569, 104–107. [Google Scholar] [CrossRef]
- Kaur, H.; Rauscher, S.A.; Werner, E.; Song, Y.; Yi, J.; Kazöne, W.; Martin, W.F.; Tüysüz, H.; Moran, J. A Prebiotic Krebs Cycle Analog Generates Amino Acids with H2 and NH3 over Nickel. Chem 2024, 10, 1528–1540. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P.; Hemmler, D.; Moritz, F.; Gougeon, R.D.; Lucio, M.; Meringer, M.; Müller, C.; Harir, M.; Hertkorn, N. Systems Chemical Analytics: Introduction to the Challenges of Chemical Complexity Analysis. Faraday Discuss. 2019, 218, 9–28. [Google Scholar] [CrossRef]
- Wollrab, E.; Scherer, S.; Aubriet, F.; Carré, V.; Carlomagno, T.; Codutti, L.; Ott, A. Chemical Analysis of a “Miller-Type” Complex Prebiotic Broth. Orig. Life Evol. Biosph. 2016, 46, 149–169. [Google Scholar] [CrossRef]
- Sandström, H.; Rahm, M. The Beginning of HCN Polymerization: Iminoacetonitrile Formation and Its Implications in Astrochemical Environments. ACS Earth Space Chem 2021, 5, 2152–2159. [Google Scholar] [CrossRef]
- Omran, A.; Menor-Salvan, C.; Springsteen, G.; Pasek, M. The Messy Alkaline Formose Reaction and Its Link to Metabolism. Life 2020, 10, 125. [Google Scholar] [CrossRef]
- Novikov, Y.; Copley, S.D. Reactivity Landscape of Pyruvate under Simulated Hydrothermal Vent Conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 13283–13288. [Google Scholar] [CrossRef]
- Schwartz, A.W. Intractable Mixtures and the Origin of Life. Chem. Biodivers. 2007, 4, 656–664. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeon, R.D.; Fekete, A.; Kanawati, B.; Harir, M.; Gebefuegi, I.; Eckel, G.; Hertkorn, N. High Molecular Diversity of Extraterrestrial Organic Matter in Murchison Meteorite Revealed 40 Years after Its Fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef]
- Potiszil, C.; Yamanaka, M.; Sakaguchi, C.; Ota, T.; Kitagawa, H.; Kunihiro, T.; Tanaka, R.; Kobayashi, K.; Nakamura, E. Organic Matter in the Asteroid Ryugu: What We Know So Far. Life 2023, 13, 1448. [Google Scholar] [CrossRef]
- Potiszil, C.; Ota, T.; Yamanaka, M.; Sakaguchi, C.; Kobayashi, K.; Tanaka, R.; Kunihiro, T.; Kitagawa, H.; Abe, M.; Miyazaki, A.; et al. Insights into the Formation and Evolution of Extraterrestrial Amino Acids from the Asteroid Ryugu. Nat. Commun. 2023, 14, 1482. [Google Scholar] [CrossRef]
- Forsythe, J.G.; Yu, S.-S.; Mamajanov, I.; Grover, M.A.; Krishnamurthy, R.; Fernández, F.M.; Hud, N.V. Ester-Mediated Amide Bond Formation Driven by Wet–Dry Cycles: A Possible Path to Polypeptides on the Prebiotic Earth. Angew. Chem. Int. Ed. Engl. 2015, 54, 9871–9875. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.M.; Despotović, D.; Weil-Ktorza, O.; Walker, M.J.; Jabłońska, J.; Fridmann-Sirkis, Y.; Varani, G.; Metanis, N.; Tawfik, D.S. Primordial Emergence of a Nucleic Acid-Binding Protein via Phase Separation and Statistical Ornithine-to-Arginine Conversion. Proc. Natl. Acad. Sci. USA 2020, 117, 15731–15739. [Google Scholar] [CrossRef] [PubMed]
- Frenkel-Pinter, M.; Haynes, J.W.; C, M.; Petrov, A.S.; Burcar, B.T.; Krishnamurthy, R.; Hud, N.V.; Leman, L.J.; Williams, L.D. Selective Incorporation of Proteinaceous over Nonproteinaceous Cationic Amino Acids in Model Prebiotic Oligomerization Reactions. Proc. Natl. Acad. Sci. USA 2019, 116, 16338–16346. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.T. A Co-Evolution Theory of the Genetic Code. Proc. Natl. Acad. Sci. USA 1975, 72, 1909–1912. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, E.N. Consensus Temporal Order of Amino Acids and Evolution of the Triplet Code. Gene 2000, 261, 139–151. [Google Scholar] [CrossRef]
- Ilardo, M.; Meringer, M.; Freeland, S.; Rasulev, B.; Cleaves, H.J. Extraordinarily Adaptive Properties of the Genetically Encoded Amino Acids. Sci. Rep. 2015, 5, 9414. [Google Scholar] [CrossRef]
- Pross, A.; Pascal, R. The Origin of Life: What We Know, What We Can Know and What We Will Never Know. Open Biol. 2013, 3, 120190. [Google Scholar] [CrossRef] [PubMed]
- Lancet, D.; Zidovetzki, R.; Markovitch, O. Systems Protobiology: Origin of Life in Lipid Catalytic Networks. J. R. Soc. Interface 2018, 15, 20180159. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.T.; Cleaves, H.J.; Bada, J.L. Quantitation of α-hydroxy Acids in Complex Prebiotic Mixtures via Liquid Chromatography/tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2016, 20, 2043–2051. [Google Scholar] [CrossRef]
- Cooper, G.W.; Cronin, J.R. Linear and Cyclic Aliphatic Carboxamides of the Murchison Meteorite: Hydrolyzable Derivatives of Amino Acids and Other Carboxylic Acids. Geochim. Cosmochim. Acta 1995, 59, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Barge, L.M.; Flores, E.; Baum, M.M.; VanderVelde, D.G.; Russell, M.J. Redox and pH Gradients Drive Amino Acid Synthesis in Iron Oxyhydroxide Mineral Systems. Proc. Natl. Acad. Sci. USA 2019, 116, 4828–4833. [Google Scholar] [CrossRef]
- Jia, T.Z.; Chandru, K. Recent Progress in Primitive Polyester Synthesis and Membraneless Microdroplet Assembly. Biophys. Physicobiology 2023, 20, e200012. [Google Scholar]
- Jia, T.Z.; Chandru, K.; Hongo, Y.; Afrin, R.; Usui, T.; Myojo, K.; Cleaves, H.J. Membraneless Polyester Microdroplets as Primordial Compartments at the Origins of Life. Proc. Natl. Acad. Sci. USA 2019, 116, 15830–15835. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Bose, R.; Tang, T.-Y.D. Can Coacervation Unify Disparate Hypotheses in the Origin of Cellular Life? Curr. Opin. Colloid Interface Sci. 2021, 52, 101415. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Nozawa, R.-S.; Jia, T.Z.; Saio, T.; Mori, E. Biological Phase Separation: Cell Biology Meets Biophysics. Biophys. Rev. 2020, 12, 519–539. [Google Scholar] [CrossRef]
- Chen, I.A.; Walde, P. From Self-Assembled Vesicles to Protocells. Cold Spring Harb. Perspect. Biol. 2010, 2, a002170. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yi, R.; Igisu, M.; Sakaguchi, C.; Afrin, R.; Potiszil, C.; Kunihiro, T.; Kobayashi, K.; Nakamura, E.; Ueno, Y.; et al. Spectroscopic and Biophysical Methods to Determine Differential Salt-Uptake by Primitive Membraneless Polyester Microdroplets. Small Methods 2023, 7, e2300119. [Google Scholar] [CrossRef]
- Monnard, P.-A.; Walde, P. Current Ideas about Prebiological Compartmentalization. Life 2015, 5, 1239–1263. [Google Scholar] [CrossRef]
- Joyce, G.F.; Szostak, J.W. Protocells and RNA Self-Replication. Cold Spring Harb. Perspect. Biol. 2018, 10, a034801. [Google Scholar] [CrossRef]
- Sharma, C.; Awasthi, S.K. Versatility of Peptide Nucleic Acids (PNAs): Role in Chemical Biology, Drug Discovery, and Origins of Life. Chem. Biol. Drug Des. 2017, 89, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, S.; Krishnamurthy, R. The Role of Sugar-Backbone Heterogeneity and Chimeras in the Simultaneous Emergence of RNA and DNA. Nat. Chem. 2019, 11, 1009–1018. [Google Scholar] [CrossRef]
- McKay, C.P. What Is Life—and How Do We Search for It in Other Worlds? PLoS Biol. 2004, 2, E302. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Hystad, G.; Prabhu, A.; Wong, M.L.; Cody, G.D.; Economon, S.; Hazen, R.M. A Robust, Agnostic Molecular Biosignature Based on Machine Learning. Proc. Natl. Acad. Sci. USA 2023, 120, e2307149120. [Google Scholar] [CrossRef] [PubMed]
- Lehmer, O.R.; Catling, D.C.; Krissansen-Totton, J. Carbonate-Silicate Cycle Predictions of Earth-like Planetary Climates and Testing the Habitable Zone Concept. Nat. Commun. 2020, 11, 6153. [Google Scholar] [CrossRef] [PubMed]
- Sephton, M.A. Organic Compounds in Carbonaceous Meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Davidowski, S.K.; Holland, G.P.; Williams, L.B. Processing of Meteoritic Organic Materials as a Possible Analog of Early Molecular Evolution in Planetary Environments. Proc. Natl. Acad. Sci. USA 2013, 110, 15614–15619. [Google Scholar] [CrossRef]
- Ross, D.S.; Deamer, D. Dry/Wet Cycling and the Thermodynamics and Kinetics of Prebiotic Polymer Synthesis. Life 2016, 6, 28. [Google Scholar] [CrossRef]
- Maurette, M. Carbonaceous Micrometeorites and the Origin of Life. Orig. Life Evol. Biosph. 1998, 28, 385–412. [Google Scholar] [CrossRef]
- Jenkins, J.M.; Twicken, J.D.; Batalha, N.M.; Caldwell, D.A.; Cochran, W.D.; Endl, M.; Latham, D.W.; Esquerdo, G.A.; Seader, S.; Bieryla, A.; et al. Discovery and Validation of Kepler-452b: A 1.6 R⨁ Super Earth Exoplanet in the Habitable Zone of a G2 Star. AJS 2015, 150, 56. [Google Scholar] [CrossRef]
- Georgiou, C.D.; McKay, C.; Reymond, J.-L. Organic Catalytic Activity as a Method for Agnostic Life Detection. Astrobiology 2023, 23, 1118–1127. [Google Scholar] [CrossRef]
- Gogarten, J.P.; Deamer, D. Is LUCA a Thermophilic Progenote? Nat. Microbiol. 2016, 1, 16229. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V. Carl Woese’s Vision of Cellular Evolution and the Domains of Life. RNA Biol. 2014, 11, 197–204. [Google Scholar] [CrossRef]
- Di Giulio, M. The Origins of the Cell Membrane, the Progenote, and the Universal Ancestor (LUCA). Biosystems 2022, 222, 104799. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.; Wong, M.L. Defining Lyfe in the Universe: From Three Privileged Functions to Four Pillars. Life 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Sithamparam, M.; Satthiyasilan, N.; Chen, C.; Jia, T.Z.; Chandru, K. A Material-Based Panspermia Hypothesis: The Potential of Polymer Gels and Membraneless Droplets. Biopolymers 2022, 113, e23486. [Google Scholar] [CrossRef] [PubMed]
- Claeys, P.; Morbidelli, A. Late Heavy Bombardment. In Encyclopedia of Astrobiology; Gargaud, M., Amils, R., Quintanilla, J.C., Cleaves, H.J., Irvine, W.M., Pinti, D.L., Viso, M., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2011; pp. 909–912. ISBN 9783642112744. [Google Scholar]
- Wolfenden, R. Benchmark Reaction Rates, the Stability of Biological Molecules in Water, and the Evolution of Catalytic Power in Enzymes. Annu. Rev. Biochem. 2011, 80, 645–667. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Lineweaver, C.H. The Case for a Gaian Bottleneck: The Biology of Habitability. Astrobiology 2016, 16, 7–22. [Google Scholar] [CrossRef]
- Michaelian, K. Non-Equilibrium Thermodynamic Foundations of the Origin of Life. Foundations 2022, 2, 308–337. [Google Scholar] [CrossRef]
- Rastogi, A. Network Science to Study the Origins of Life. Nat. Comput. Sci. 2022, 2, 470. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic Systems Chemistry: New Perspectives for the Origins of Life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef]
- Kawamura, K. Measurement of the Rate of RNA Hydrolysis in Aqueous Solution at Elevated Temperatures Using a New Monitoring Method for Hydrothermal Reactions. Nucleic Acids Symp. Ser. 1999, 42, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, M.; Murase, S. Hydrolysis Kinetics of Poly(lactic Acid) Fiber. Sen Gakkaishi 2003, 59, 371–374. [Google Scholar] [CrossRef]
- Azuma, S.; Yamamoto, S.; Ichikawa, H.; Maruyama, S. Why Primordial Continents Were Recycled to the Deep: Role of Subduction Erosion. Geosci. Front. 2017, 8, 337–346. [Google Scholar] [CrossRef]
- Tivey, M.K. Generation of Seafloor Hydrothermal Vent Fluids and Associated Mineral Deposits. Oceanography 2007, 20, 50–65. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of First Cells at Terrestrial, Anoxic Geothermal Fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef] [PubMed]
- Rickaby, R.E.M. Goldilocks and the Three Inorganic Equilibria: How Earth’s Chemistry and Life Coevolve to Be Nearly in Tune. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140188. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.F.; Schrag, D.P. The Snowball Earth Hypothesis: Testing the Limits of Global Change. Terra Nova 2002, 14, 129–155. [Google Scholar] [CrossRef]
- Joyce, G.F. Evolution in an RNA World. Cold Spring Harb. Symp. Quant. Biol. 2009, 74, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Markovitch, O.; Lancet, D. Multispecies Population Dynamics of Prebiotic Compositional Assemblies. J. Theor. Biol. 2014, 357, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Hordijk, W.; Steel, M. Autocatalytic Networks at the Basis of Life’s Origin and Organization. Life 2018, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Murakami, H.; Higashimura, E.; Suga, H. Synthesis of Polyester by Means of Genetic Code Reprogramming. Chem. Biol. 2007, 14, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Murakami, H.; Suga, H. Polymerization of Alpha-Hydroxy Acids by Ribosomes. Chembiochem 2008, 9, 2773–2778. [Google Scholar] [CrossRef]

| Term | Definition | Reference |
|---|---|---|
| Abiotic Chemistry | Any chemistry involving non-living or inanimate matter. | [24] |
| Prebiotic Chemistry | A subset of abiotic chemistry that has led to life or chemistry that happened during the OoL. | [24,25] |
| Biomolecules | Molecules that make up the structure and function of living organisms on Earth. | [26] |
| Non-Biomolecules | Refers to prebiotic organic molecules, which are not directly employed in biochemistry or serve different roles to their biochemical functions. An example of non-biomolecules are alpha hydroxy acids. These compounds can polymerize prebiotically to become prebiotic polyesters. While some alpha hydroxy acids (e.g., lactic acid, malic acid, etc.) are considered to be biomolecular compounds in the broad sense, with roles in respiration, inflammation regulation, and molecular signaling [27,28], they are considered non-biomolecules in the prebiotic context. This is because, in prebiotic chemistry, these compounds are involved in polymerization [29], a function not used by living organisms for these compounds. Additionally, within the paper, it is important to distinguish between the terms ‘biomolecules’ and ‘non-biomolecules’ for clarity. Even if some molecules transition from prebiotic to biological roles, the term ‘non-biomolecules’ are not reclassified to ‘biomolecules’. However, outside this paper’s scope, biomolecules could include those that originally were non-biomolecules but became integral to life on Earth or elsewhere. | [30] and further updated in this paper |
| Building Blocks | Refers to the fundamental chemical compounds, biomolecular and/or non-biomolecular, essential for the formation and function of life. This concept applies to terrestial life forms [31] and hypothetical or theoretical forms of extraterrestrial (ET) life. | [31] and this paper |
| Terrestrial-biochemistries | Refers to the biochemical processes and molecular compositions associated with life as we know it on Earth. | [32] |
| Xeno-biochemistries | Refers to “bio”chemical processes and ”bio”molecular compositions associated with ET life forms that could exist in environments different from those on Earth. In this paper, Xeno-biochemistry could, in principle, accommodate both biomolecules and/or non-biomolecules within its functionality and form. The word ‘Xeno-biochemistries’ carries the connotation of ‘xeno-biology’. However, other definitions of xeno-biology include the design and engineering of new life forms by humans using technology [33]. For the sake of simplicity, we will equate the ET definition mentioned above with xeno-biology. | This paper |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandru, K.; Potiszil, C.; Jia, T.Z. Alternative Pathways in Astrobiology: Reviewing and Synthesizing Contingency and Non-Biomolecular Origins of Terrestrial and Extraterrestrial Life. Life 2024, 14, 1069. https://doi.org/10.3390/life14091069
Chandru K, Potiszil C, Jia TZ. Alternative Pathways in Astrobiology: Reviewing and Synthesizing Contingency and Non-Biomolecular Origins of Terrestrial and Extraterrestrial Life. Life. 2024; 14(9):1069. https://doi.org/10.3390/life14091069
Chicago/Turabian StyleChandru, Kuhan, Christian Potiszil, and Tony Z. Jia. 2024. "Alternative Pathways in Astrobiology: Reviewing and Synthesizing Contingency and Non-Biomolecular Origins of Terrestrial and Extraterrestrial Life" Life 14, no. 9: 1069. https://doi.org/10.3390/life14091069
APA StyleChandru, K., Potiszil, C., & Jia, T. Z. (2024). Alternative Pathways in Astrobiology: Reviewing and Synthesizing Contingency and Non-Biomolecular Origins of Terrestrial and Extraterrestrial Life. Life, 14(9), 1069. https://doi.org/10.3390/life14091069








