The Hepatoprotective Effects of Camellia sinensis on Cisplatin-Induced Acute Liver Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. The Experimental Animals and Study Groups
2.3. The Procurement and Preparation of White Tea
2.4. Blood and Liver Tissues Collection
2.5. Biochemical Analysis
2.5.1. Determination of Serum ALT and AST Levels
2.5.2. Determination of Tissue MDA and GSH Levels
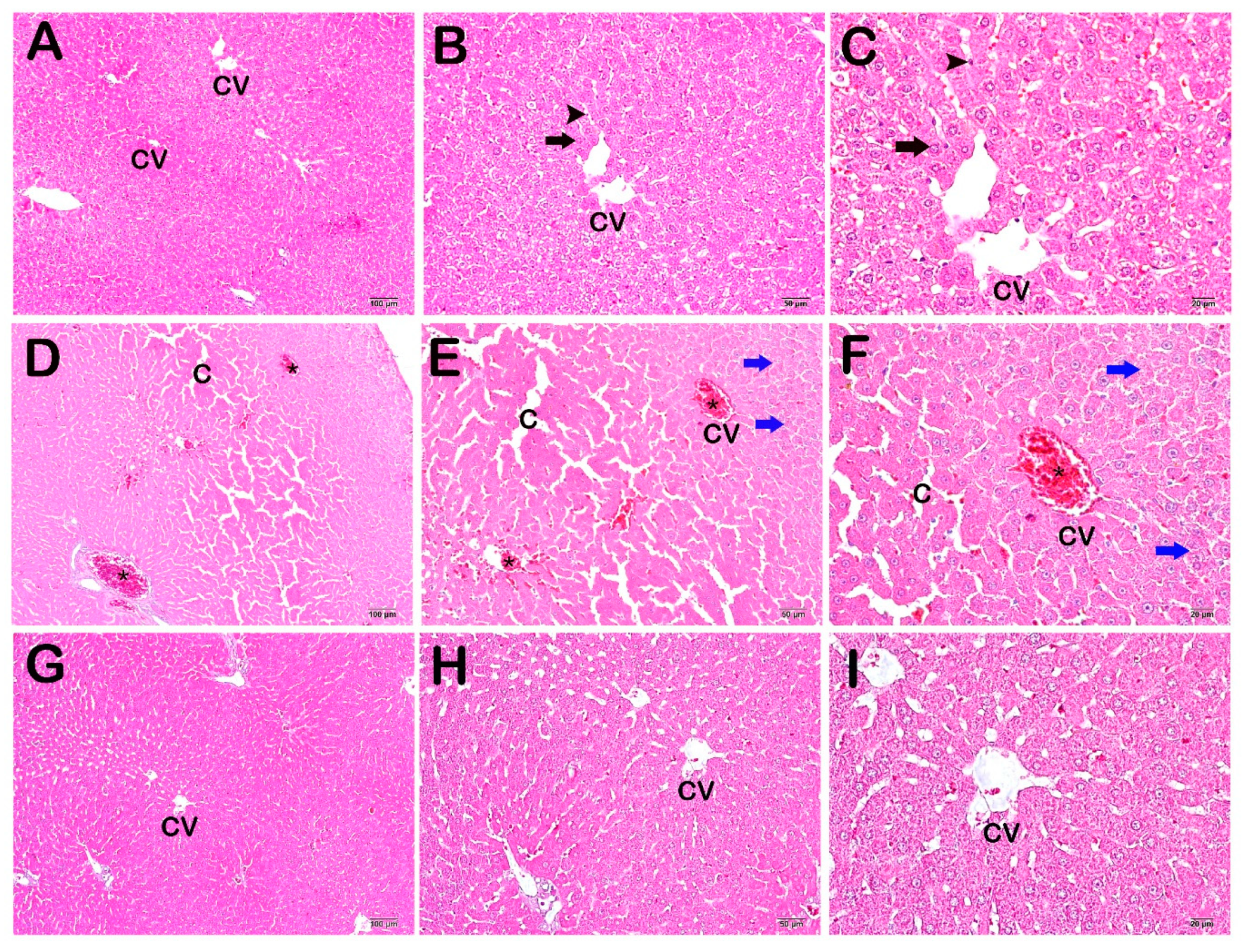
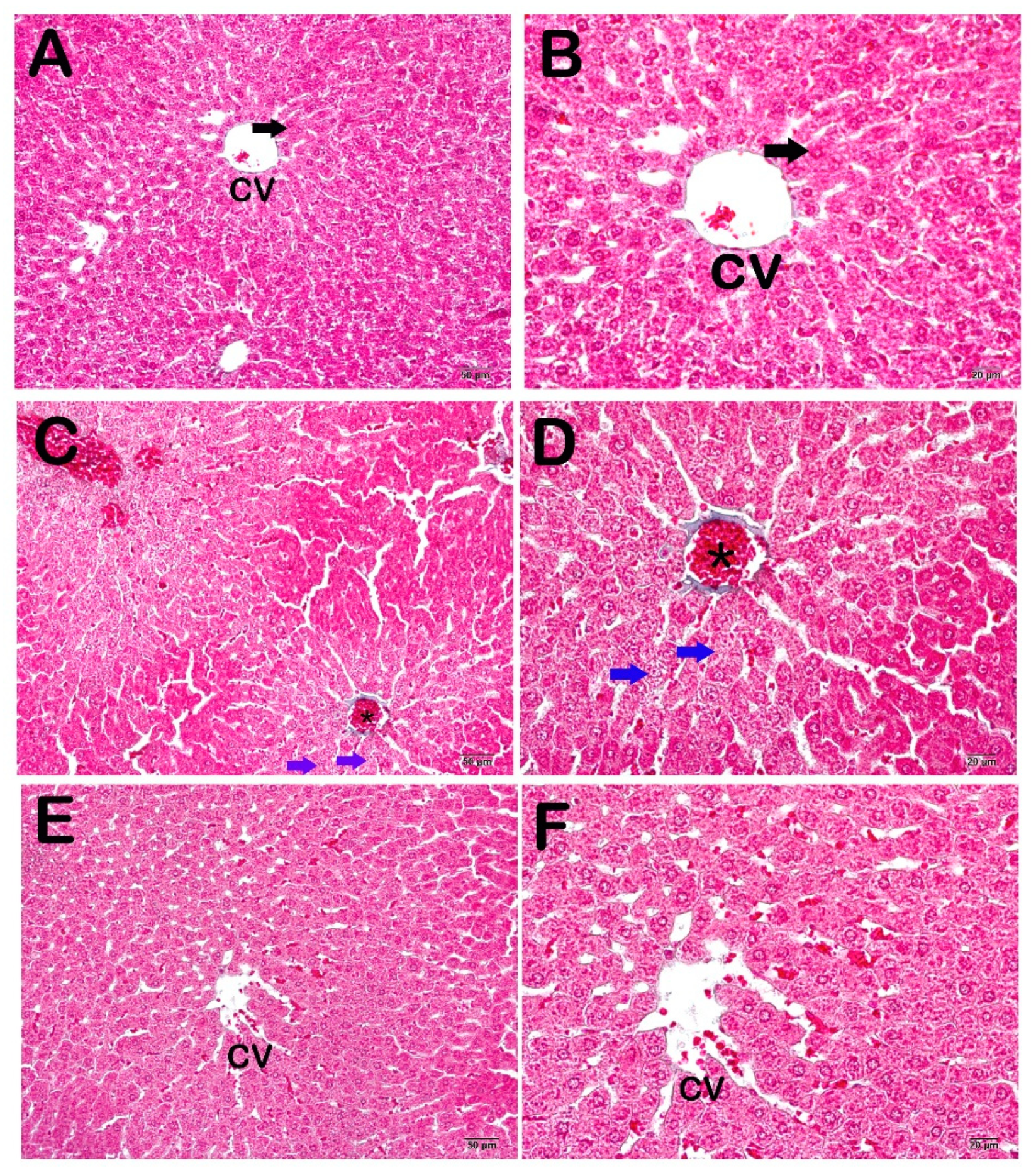
2.6. Histopathological Analysis
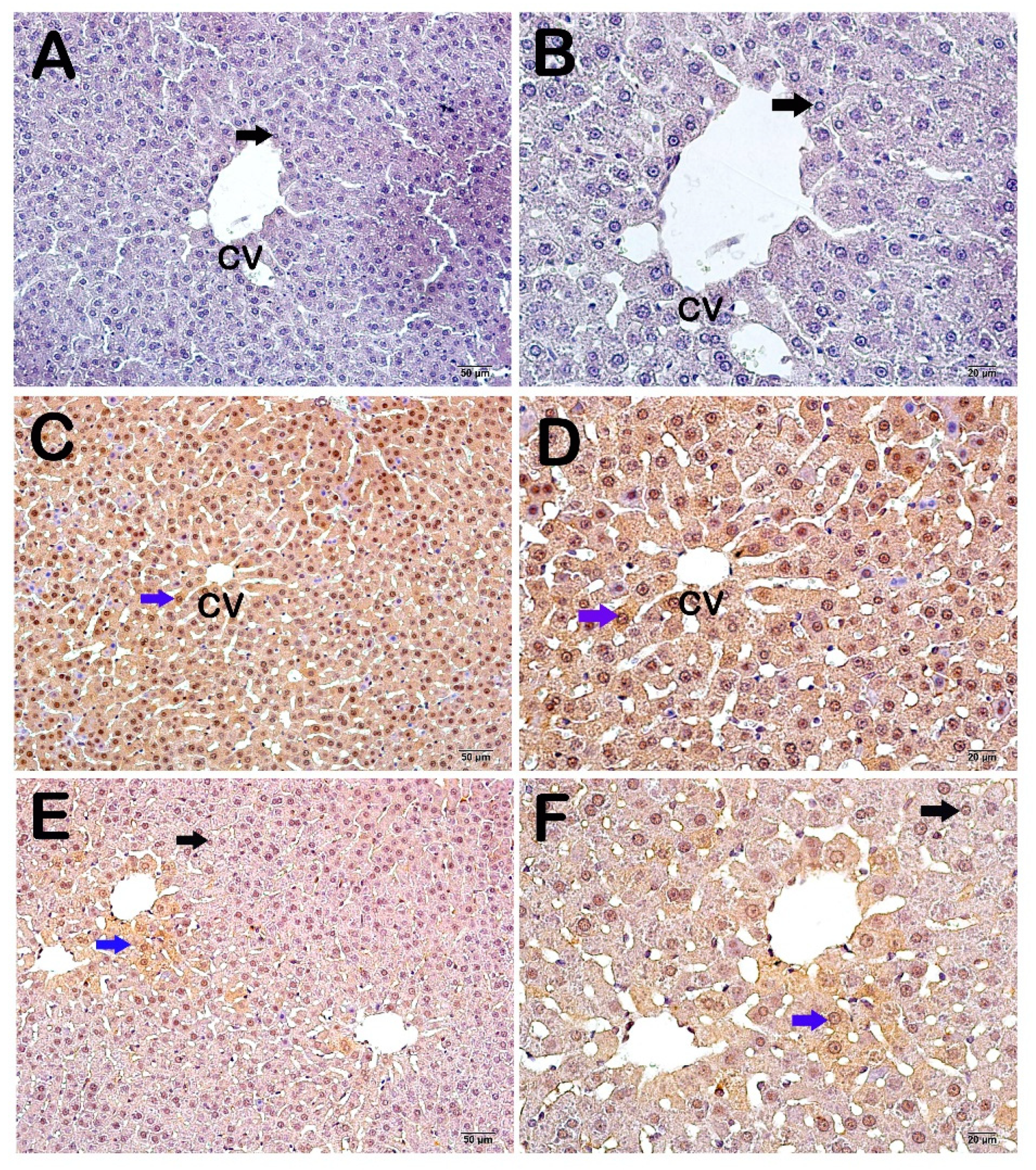
2.7. Immunohistochemical Analysis (IHC)
2.8. Statistical Analysis
3. Results
3.1. Biochemical Results
3.2. Histopathological and Immunohistochemical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Baykara, O. Current Modalities in Treatment of Cancer. Balikesir Health Sci. J. 2016, 5, 154–165. [Google Scholar] [CrossRef]
- Aynacıoglu, A.; Ozkur, M.; Nacak, M.; Saracaloglu, A. Farmabul, 1st ed.; Çukurova Nobel Tip Kitabevi: Adana, Turkey, 2014. [Google Scholar]
- Huang, X.; Gao, C.; Cai, W.; Tao, Y.; Zhong, X.; Liu, H.; Hong, X.; Ding, X.; Lu, H.; Lai, W.; et al. Effect of occupational exposure to antineoplastic drugs on DNA damage in nurses: A cross-sectional study. Occup. Environ. Med. 2022, 79, 253–258. [Google Scholar] [CrossRef]
- Saral, S.; Dokumacioglu, E.; Mercantepe, T.; Atak, M.; Cinar, S.; Saral, O.; Yildiz, L.; Iskender, H.; Tumkaya, L. The effect of white tea on serum TNF-α/NF-κB and immunohistochemical parameters in cisplatin-related renal dysfunction in female rats. Biomed. Pharmacother. 2019, 112, 108604. [Google Scholar] [CrossRef] [PubMed]
- Lebaschi, S.; Hekmati, M.; Veisi, H. Green synthesis of palladium nanoparticles mediated by black tea leaves (Camellia sinensis) extract: Catalytic activity in the reduction of 4-nitrophenol and Suzuki-Miyaura coupling reaction under ligand-free conditions. J. Colloid. Interface Sci. 2017, 485, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin nephrotoxicity: New insights and therapeutic implications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Mercantepe, T.; Unal, D.; Tümkaya, L.; Yazici, Z.A. Protective effects of amifostine, curcumin and caffeic acid phenethyl ester against cisplatin-induced testis tissue damage in rats. Exp. Ther. Med. 2018, 15, 3404–3412. [Google Scholar] [CrossRef]
- Mercantepe, F.; Mercantepe, T.; Topcu, A.; Yılmaz, A.; Tumkaya, L. Protective effects of amifostine, curcumin, and melatonin against cisplatin-induced acute kidney injury. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 915–931. [Google Scholar] [CrossRef]
- Alibakhshi, T.; Khodayar, M.J.; Khorsandi, L.; Rashno, M.; Zeidooni, L. Protective effects of zingerone on oxidative stress and inflammation in cisplatin-induced rat nephrotoxicity. Biomed. Pharmacother. 2018, 105, 225–232. [Google Scholar] [CrossRef]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, F.; Hosseinimehr, S.J.; Zargari, M.; Karimpour Malekshah, A.; Mirzaei, M.; Talebpour Amiri, F. Alleviation of cisplatin-induced hepatotoxicity by gliclazide: Involvement of oxidative stress and caspase-3 activity. Pharmacol. Res. Perspect. 2021, 9, e00788. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, A.; Kar, S.M.; Ozcan, A. Comparison of black, green and white teas in terms of quality criteria, mineral contents, antioxidant and antimicrobial activity. Acad. J. Agric. 2020, 9, 279–288. [Google Scholar]
- Wong, M.; Sirisena, S.; Ng, K. Phytochemical profile of differently processed tea: A review. J. Food Sci. 2022, 5, 1925–1942. [Google Scholar] [CrossRef]
- Gabr, G.A.; Hassan, H.M.M.; Seshadri, V.D.; Hassan, N.M.M. Comparative study of phenolic profile, antioxidant and antimicrobial activities of aqueous extract of white and green tea. Z. Naturforsch. C J. Biosci. 2022, 77, 483–492. [Google Scholar] [CrossRef]
- Haghparasti, Z.; Mahdavi Shahri, M. Green synthesis of water-soluble nontoxic inorganic polymer nanocomposites containing silver nanoparticles using white tea extract and assessment of their in vitro antioxidant and cytotoxicity activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 139–148. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, X.; Xu, X.; Lai, H.; Zeng, Z.; Shan, T.; Zhang, T.; Chen, M.; Huang, Y.; Huang, Z.; et al. Tumor-targeted hyaluronic acid-based oxidative stress nanoamplifier with ROS generation and GSH depletion for antitumor therapy. Int. J. Biol. Macromol. 2022, 207, 771–783. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Genzini, L.; Garella, D. Phytotherapeutics: An evaluation of the potential of 1000 plants. J. Clin. Pharm. Ther. 2010, 35, 11–48. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, R.; Reddy, S.; Smith, T.J. Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J. Clin. Oncol. 2006, 24, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, H.; Schellens, J.H.M.; Verheij, M. The combined use of radiotherapy and chemotherapy in the treatment of solid tumours. Eur. J. Cancer 2002, 38, 216–222. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar]
- Motwani, S.S.; Kaur, S.S.; Kitchlu, A. Cisplatin Nephrotoxicity: Novel Insights into Mechanisms and Preventative Strategies. Semin. Nephrol. 2022, 42, 151341. [Google Scholar] [CrossRef]
- Pezeshki, Z.; Khosravi, A.; Nekuei, M.; Khoshnood, S.; Zandi, E.; Eslamian, M.; Talebi, A.; Emami, S.N.; Nematbakhsh, M. Time course of cisplatin-induced nephrotoxicity and hepatotoxicity. J. Nephropathol. 2017, 6, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Tarladacalisir, Y.T.; Uygun, M.; Akpolat, M.; Uz, Y.H. Histological Examination of the Effects of Vitamins on Preventing Cisplatin Hepatotoxicity. Balkan Med. J. 2005, 22, 124–131. [Google Scholar]
- Martins, N.; Santos, N.; Curti, C.; Bianchi, L.; Santos, A. Thimerosal distribution and metabolism in neonatal mice. J. Appl. Toxicol. 2008, 28, 337–344. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Yürekli, V.A. Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: Focus on trace elements. Cell. Mol. Neurobiol. 2013, 33, 589–599. [Google Scholar] [CrossRef]
- Hockenberry, M.J.; Hooke, M.C.; Gregurich, M.; McCarthy, K. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J. Pediatr. Hematol. Oncol. 2009, 31, 664–669. [Google Scholar] [CrossRef]
- Karahan, I.; Yilmaz, S.; Atessahin, A. Effects of lycopene on cisplatin and gentamicin induced oxidative stres in blood and liver in rats. Fırat. Univ. Med. J. Health Sci. 2006, 20, 39–43. [Google Scholar]
- Blumberg, J. Use of biomarkers of oxidative stress in research studies. J. Nutr. Am. Soc. Nutr. 2004, 134, 3188S–3189S. [Google Scholar] [CrossRef] [PubMed]
- Tüközan, N.; Erdamar, H.; Seven, I. Measurement of Total Malondialdehyde in Plasma and Tissues by High-Performance Liquid Chromatography and Thiobarbituric Acid Assay. Firat Tip Derg. 2006, 11, 88–92. [Google Scholar]
- Ateşşahin, A.; Şanna, E.; Türk, G.; Çeribaşi, A.O.; Yilmaz, S.; Yüce, A.; Bulmuş, Ö. Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J. Pineal. Res. 2006, 41, 21–27. [Google Scholar] [CrossRef]
- Iraz, M.; Ozerol, E.; Gulec, M.; Tasdemir, S.; Idiz, N.; Fadillioglu, E.; Naziroglu, M.; Akyol, O. Protective effect of caffeic acid phenethyl ester (CAPE) administration on cisplatin-induced oxidative damage to liver in rat. Cell Biochem. Func. 2006, 24, 357–361. [Google Scholar] [CrossRef]
- Sugiyama, S.; Hayakawa, M.; Kato, T.; Hanaki, Y.; Shimizu, K.; Ozawa, T. Adverse effect of anti-tumor drug, cisplatin, on rat kidney mitochondria: Disturbances in glutathione peroxidase activity. Biochem. Biophys. Res. Commun. 1989, 159, 1121–1127. [Google Scholar] [CrossRef]
- Işeri, S.; Ercan, F.; Gedik, N.; Yüksel, M.; Alican, I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology 2007, 230, 256–264. [Google Scholar] [CrossRef]
- Greggi, L.M.; D’arc, C.; Darin, J.; Bianchi, M.D.L.P. Protective effects of vitamin C against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: A dose-dependent study. Pharmacol. Res. 2000, 41, 405–441. [Google Scholar] [CrossRef]
- Mora, L.; De, O.; Antunes, L.M.G.; Francescato, H.D.C.; Bianchi, M.L.P. The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats. Pharmacol. Res. 2003, 47, 517–522. [Google Scholar] [CrossRef]
- Naziroǧlu, M.; Karaoǧlu, A.; Aksoy, A.O. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 2004, 195, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Alshuweishi, Y.; Alfaifi, M.; Almoghrabi, Y.; Alfhili, M.A. AST and ALT APRI Scores and Dysglycemia in Saudi Arabia: A Retrospective Population Study. Life 2023, 13, 1881. [Google Scholar] [CrossRef]
- Tamber, S.S.; Bansal, P.; Sharma, S.; Singh, R.B.; Sharma, R. Biomarkers of liver diseases. Mol. Biol. Rep. 2023, 50, 7815–7823. [Google Scholar] [CrossRef]
- Zheng, X.N.; Wang, X.W.; Li, L.Y.; Xu, Z.W.; Huang, H.Y.; Zhao, J.S.; Zhang, D.; Yin, X.; Sheng, J.; Tang, J.T. Pu-erh tea powder preventive effects on cisplatin-induced liver oxidative damage in Wistar rats. Asian Pac. J. Cancer Prev. 2014, 15, 7389–7394. [Google Scholar] [CrossRef]
- Amidi, N.; Moradkhani, S.; Sedaghat, M.; Khiripour, N.; Larki-Harchegani, A.; Zadkhosh, N.; Ranjbar, A. Effect of green tea on inflammation and oxidative stress in cisplatin-induced experimental liver function. J. Herbmed Pharmacol. 2016, 5, 99–102. [Google Scholar]
- Ani, F.I.; Nabofa, E.E.W.; Omobowale, T.O.; Ajuzie, N.C.; Ajemigbitse, J.; Oyagbemi, A.A.; Attah, A.F.; Adeoye, B.K.; Azubuike-Osu, S.O.; Adedapo, A.A.; et al. Infused aqueous curry tea extracts ameliorate Nω -Nitro-L-Arginine Methyl Ester-induced liver dysfunction in male albino Wistar rats. J. Food Biochem. 2022, 46, e14198. [Google Scholar] [CrossRef]
- Tang, Z.; Zhan, L.; He, R.; Zhou, Y.; Tang, Q.; Liu, Z.; Zhang, S.; Liu, A. Hepatoprotective Effect of Tea Composite Solid Beverage on Alcohol-Caused Rat Liver Injury. Foods 2023, 12, 4126. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Z.; Zhang, J.; Mu, J.; Zhou, X.; Zhao, X. Liver Injury Induced by Carbon Tetrachloride in Mice Is Prevented by the Antioxidant Capacity of Anji White Tea Polyphenols. Antioxidants 2019, 8, 64. [Google Scholar] [CrossRef]
- Mostafa-Hedeab, G.; Ewaiss Hassan, M.; Halawa, T.F.; Ahmed Wani, F. Epigallocatechin gallate ameliorates tetrahydrochloride-induced liver toxicity in rats via inhibition of TGFβ/p-ERK/p-Smad1/2 signaling, antioxidant, anti-inflammatory activity. Saudi Pharm J. 2022, 30, 1293–1300. [Google Scholar] [CrossRef]
| Component | Amount in Dry Weight |
|---|---|
| Gallic acid | 0.13 (%) |
| Caffeine | 5.62 (%) |
| EGC (epigallocatechin) | 2.54 (%) |
| EC (epicatechin) | 0.91 (%) |
| EGCG (epigallocatechin gallate) | 10.39 (%) |
| ECG (epicatechin gallate) | 3.58 (%) |
| Copper | 0.08 (ppm) |
| Iron | 0.12 (ppm) |
| Zinc | 0.54 (ppm) |
| Sodium | 2.87 (ppm) |
| Potassium | 283.00 (ppm) |
| Calcium | 6.12 (ppm) |
| Magnesium | 26.88 (ppm) |
| Aluminum | 0.98 (ppm) |
| MDA (nmol/g Tissue) | GSH (µmol/g Tissue) | |
|---|---|---|
| Control | 1.17 ± 0.11 | 14.90 ± 2.04 |
| Cisplatin | 1.56 ± 0.26 a,* | 16.74 ± 2.00 a,** |
| Cisplatin + White Tea | 1.39 ± 0.39 | 15.08 ± 1.38 b,* |
| AST (U/L) | ALT (U/L) | |
|---|---|---|
| Control | 71.13 ± 4.49 | 35.25 ± 4.89 |
| Cisplatin | 97.88 ± 7.20 a,** | 43.75 ± 7.61 a,* |
| Cisplatin + White Tea | 77.00 ± 6.21 | 40.38 ± 3.89 |
| Score | Finding |
|---|---|
| 0 | Less than 5% caspase-3 positive hepatocytes |
| 1 | Less than 25% caspase-3 positive hepatocytes |
| 2 | Less than 50% caspase-3 positive hepatocytes |
| 3 | More than 50% caspase-3 positive hepatocytes |
| Group | Caspase-3 Positivity Score |
|---|---|
| Control | 0.00 ± 0.35 |
| Cisplatin | 3.00 ± 0.46 a |
| Cisplatin+ White tea | 1.00 ± 0.46 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, A.; Dizman, F.; Akyildiz, K.; Mataraci Karakas, S.; Mercantepe, T.; Uydu, H.A.; Tumkaya, L.; Ozturk, K. The Hepatoprotective Effects of Camellia sinensis on Cisplatin-Induced Acute Liver Injury. Life 2024, 14, 1077. https://doi.org/10.3390/life14091077
Yilmaz A, Dizman F, Akyildiz K, Mataraci Karakas S, Mercantepe T, Uydu HA, Tumkaya L, Ozturk K. The Hepatoprotective Effects of Camellia sinensis on Cisplatin-Induced Acute Liver Injury. Life. 2024; 14(9):1077. https://doi.org/10.3390/life14091077
Chicago/Turabian StyleYilmaz, Adnan, Fatih Dizman, Kerimali Akyildiz, Sibel Mataraci Karakas, Tolga Mercantepe, Huseyin Avni Uydu, Levent Tumkaya, and Koksal Ozturk. 2024. "The Hepatoprotective Effects of Camellia sinensis on Cisplatin-Induced Acute Liver Injury" Life 14, no. 9: 1077. https://doi.org/10.3390/life14091077
APA StyleYilmaz, A., Dizman, F., Akyildiz, K., Mataraci Karakas, S., Mercantepe, T., Uydu, H. A., Tumkaya, L., & Ozturk, K. (2024). The Hepatoprotective Effects of Camellia sinensis on Cisplatin-Induced Acute Liver Injury. Life, 14(9), 1077. https://doi.org/10.3390/life14091077







