COVID-19 in Brazilian Pediatric Patients: A Retrospective Cross-Sectional Study with a Predictive Model for Hospitalization
Abstract
1. Introduction
2. Materials and Methods
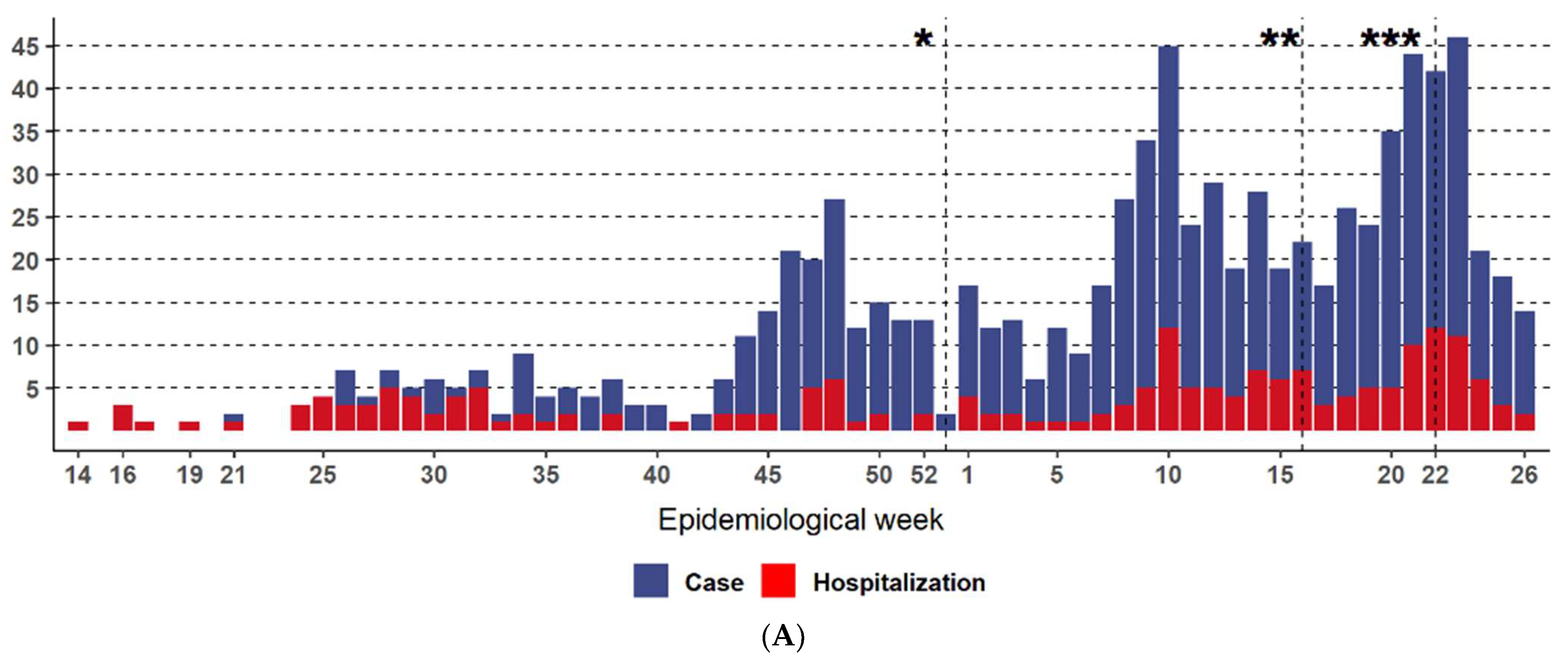
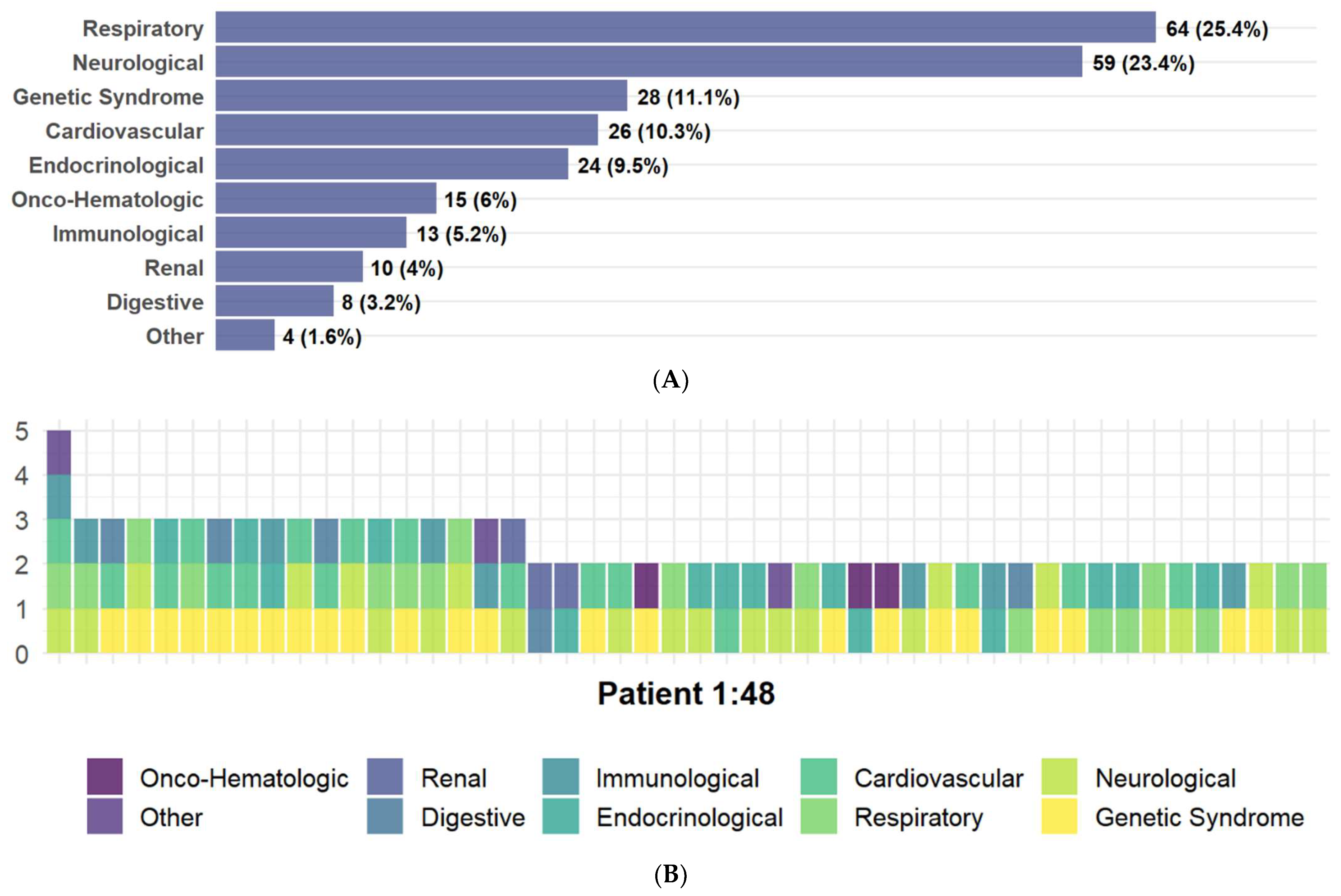
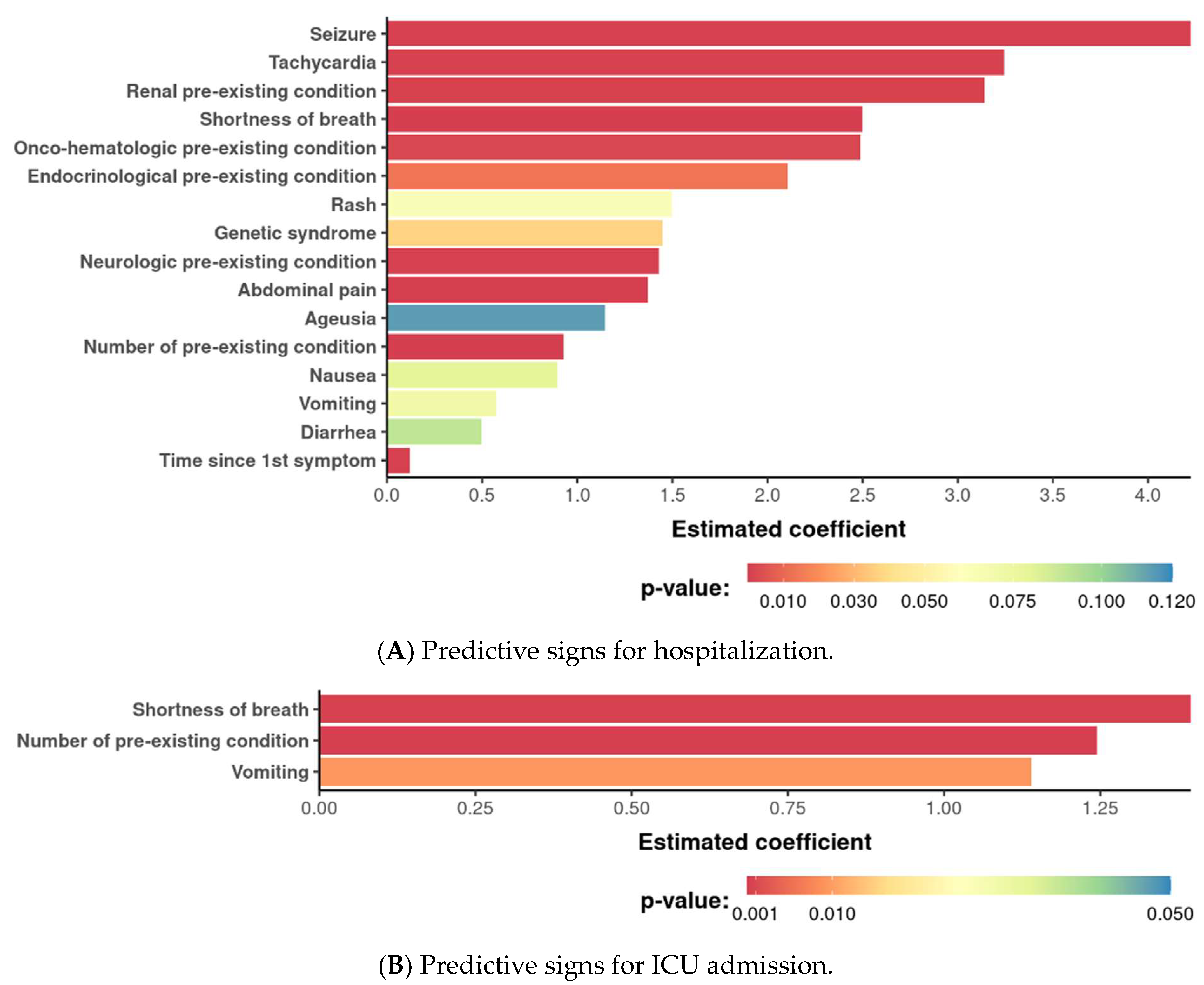
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Livingston, E.; Bucher, K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA 2020, 323, 1335. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins University & Medicine. Available online: https://coronavirus.jhu.edu/map.html (accessed on 26 September 2021).
- Ministério da Saúde do Brasil. Available online: https://www.gov.br/saude/pt-br/coronavirus/boletins-epidemiologicos/boletim-epidemiologico-covid-19-no-44.pdf (accessed on 26 September 2021).
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef]
- Oliveira, E.A.; Colosimo, E.A.; e Silva, A.C.; Mak, R.H.; Martelli, D.B.; Silva, L.R.; Martelli-Júnior, H.; Oliveira, M.C.L. Clinical characteristics and risk factors for death among hospitalized children and adolescents with COVID-19 in Brazil: An analysis of a nationwide database. Lancet Child. Adolesc. Health 2021, 5, 559–568. [Google Scholar] [CrossRef]
- Dufort, E.M.; Koumans, E.H.; Chow, E.J.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. Multisystem inflammatory syndrome in children in New York State. N. Engl. J. Med. 2020, 383, 347–358. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 15 April 2024).
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. R Package Version 1.0.9. 2022. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 15 April 2024).
- Wickham, H.; Girlich, M. tidyr: Tidy Messy Data. R Package Version 1.2.0. 2022. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 17 April 2024).
- Wickham, H. forcats: Tools for Working with Categorical Variables (Factors). R package Version 0.5.1. 2021. Available online: https://CRAN.R-project.org/package=forcats (accessed on 20 April 2024).
- Grolemund, G.; Wickham, H. Dates and Times Made Easy with lubridate. J. Stat. Softw. 2011, 40, 1–25. Available online: https://www.jstatsoft.org/v40/i03/ (accessed on 15 April 2024). [CrossRef]
- Wickham, H. stringr: Simple, Consistent Wrappers for Common String Operations. R Package Version 1.4.0. 2019. Available online: https://CRAN.R-project.org/package=stringr (accessed on 15 April 2024).
- Henry, L.; Wickham, H. purrr: Functional Programming Tools. R Package Version 0.3.4. 2020. Available online: https://CRAN.R-project.org/package=purrr (accessed on 16 April 2024).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Arel-Bundock, V. Marginal Effects: Marginal Effects, Marginal Means, Predictions, and Contrasts. R Package Version 0.5.0. 2022. Available online: https://CRAN.R-project.org/package=marginaleffects (accessed on 20 April 2024).
- Wickham, H. ggplot2, Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Pedersen, T.L. patchwork: The Composer of Plots. R Package Version 1.1.1. 2020. Available online: https://CRAN.R-project.org/package=patchwork (accessed on 15 April 2024).
- Howard, L.M.; Garguilo, K.; Gillon, J.; LeBlanc, K.; Seegmiller, A.C.; Schmitz, J.E.; Byrne, D.W.; Do menico, H.J.; Moore, R.P.; Webber, S.A.; et al. The first 1000 symptomatic pediatric SARS-CoV-2 infections in an integrated health care system: A prospective cohort study. BMC Pediatr. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Rabha, A.C.; Oliveira Junior, F.I.; Oliveira, T.A.; Cesar, R.G.; Fongaro, G.; Mariano, R.F.; Camargo, C.N.; Fernandes, F.R.; Wandalsen, G.F. Clinical manifestations of children and adolescents with COVID-19, report of the first 115 cases from sabará Hospital Infantil. Rev. Paul. Pediatr. 2020, 39, e2020305. [Google Scholar] [CrossRef]
- De Souza, T.H.; Nadal, J.A.; Nogueira, R.J.; Pereira, R.M.; Brandão, M.B. Clinical manifestations of children with COVID-19, a systematic review. Pediatr. Pulmonol. 2020, 55, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.M.; Neta, M.M.; Neto, A.R.; Carvalho, A.R.; Magalhães, R.L.; Valle, A.R.; Ferreira, J.; Aliaga, K.; Moura, M.; Freitas, D. Symptoms of COVID-19 in children. Braz. J. Med. Biol. Res. 2022, 55, e12038. [Google Scholar] [CrossRef]
- Sousa, B.L.; Silva, C.A.; Ferraro, A.A. An update on the epidemiology of pediatric COVID-19 in Brazil. Rev. Paul. Pediatr. 2022, 40, e2021367. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.Y.; Godfred-Cato, S.E.; Oster, M.E.; Chow, E.J.; Koumans, E.H.; Bryant, B.; Leung, J.W.; Belay, E.D. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2, a systematic review. J. Pediatr. 2020, 226, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lash, R.R.; Moonan, P.K.; Byers, B.L.; Bonacci, R.A.; Bonner, K.E.; Donahue, M.; Donovan, C.V.; Grome, H.N.; Janssen, J.M.; Magleby, R.; et al. COVID-19 case investigation and contact tracing in the US, 2020. JAMA Netw Open 2021, 4, e2115850. [Google Scholar] [CrossRef]
- Ustundag, G.; Yilmaz-Ciftdogan, D.; Kara-Aksay, A.; Sahin, A.; Ekemen-Keles, Y.; Orsdemir-Hortu, H.; Kanik, A.; Yuksel, N.C.; Arslan, F.D.; Yilmaz, N. Coronavirus disease 2019 in healthy children: What is the effect of household contact? Pediatr. Int. 2022, 64, e14890. [Google Scholar] [CrossRef]
- Iwamura, A.P.; Tavares da Silva, M.R.; Hümmelgen, A.L.; Soeiro Pereira, P.V.; Falcai, A.; Grumach, A.S.; Goudouris, E.; Neto, A.C.; Prando, C. Immunity and inflammatory biomarkers in COVID-19, a systematic review. Rev. Med. Virol. 2021, 31, e2199. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G.; Plebani, M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin. Chem. Lab. Med. 2020, 58, 1135–1138. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, F.; Wang, C.; Wu, J.; Liu, J.; Chen, X.; Xiao, H.; Liu, Z.; Wu, Z.; Lu, X.; et al. Children hospitalized with severe COVID-19 in Wuhan. Pediatr. Infect. Dis. J. 2020, 39, e91–e94. [Google Scholar] [CrossRef]
- Yonker, L.M.; Boucau, J.; Regan, J.; Choudhary, M.C.; Burns, M.D.; Young, N.; Farkas, E.J.; Davis, J.P.; Moschovis, P.P.; Bernard Kinane, T.; et al. Virologic features of severe acute respiratory syndrome coronavirus 2 infection in children. J. Infect. Dis. 2021, 224, 1821–1829. [Google Scholar] [CrossRef]
- Patel, N.A. Pediatric COVID-19, Systematic review of the literature. Am. J. Otolaryngol. 2020, 41, 102573. [Google Scholar] [CrossRef] [PubMed]

| Symptom | N Total | % | Outpatient | % | Inpatient | % |
|---|---|---|---|---|---|---|
| Respiratory | ||||||
| Cough | 412 | 40.08 | 326 | 31.71 | 86 | 8.37 |
| Runny nose | 304 | 29.57 | 253 | 24.61 | 51 | 4.96 |
| Odynophagia | 173 | 16.83 | 161 | 15.66 | 12 | 1.17 |
| Difficulty breathing | 123 | 11.96 | 42 | 4.09 | 81 | 7.88 |
| Sneezing | 64 | 6.23 | 55 | 5.35 | 9 | 0.88 |
| Nasal obstruction or congestion | 58 | 5.64 | 49 | 4.77 | 9 | 0.88 |
| Hoarseness | 14 | 1.36 | 13 | 1.26 | 1 | 0.10 |
| Tachypnea | 5 | 0.49 | 0 | 0.00 | 5 | 0.49 |
| Gastrointestinal | ||||||
| Diarrhea | 129 | 12.55 | 92 | 8.95 | 37 | 3.60 |
| Vomiting | 99 | 9.63 | 62 | 6.03 | 37 | 3.60 |
| Abdominal pain | 57 | 5.54 | 35 | 3.40 | 22 | 2.14 |
| Nausea | 48 | 4.67 | 34 | 3.31 | 14 | 1.36 |
| General | ||||||
| Fever | 659 | 64.11 | 520 | 50.58 | 139 | 13.52 |
| Headache | 200 | 19.46 | 179 | 17.41 | 21 | 2.04 |
| Lack of appetite | 98 | 9.53 | 76 | 7.39 | 22 | 2.14 |
| Myalgia | 77 | 7.49 | 64 | 6.23 | 13 | 1.26 |
| Ageusia | 21 | 2.04 | 16 | 1.56 | 5 | 0.49 |
| Anosmia | 20 | 1.95 | 16 | 1.56 | 4 | 0.39 |
| Seizures | 16 | 1.56 | 1 | 0.10 | 15 | 1.46 |
| Rash/exanthem | 12 | 1.17 | 4 | 0.39 | 8 | 0.78 |
| Tachycardia | 10 | 0.97 | 2 | 0.19 | 8 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, A.P.; Laureano, H.; Schidlowski, L.; Ciorcero, N.; Zanatto, T.; Borgmann, A.; Fragoso, G.; Giamberardino, A.L.; Dourado, R.; Anjos, K.d.; et al. COVID-19 in Brazilian Pediatric Patients: A Retrospective Cross-Sectional Study with a Predictive Model for Hospitalization. Life 2024, 14, 1083. https://doi.org/10.3390/life14091083
Pacheco AP, Laureano H, Schidlowski L, Ciorcero N, Zanatto T, Borgmann A, Fragoso G, Giamberardino AL, Dourado R, Anjos Kd, et al. COVID-19 in Brazilian Pediatric Patients: A Retrospective Cross-Sectional Study with a Predictive Model for Hospitalization. Life. 2024; 14(9):1083. https://doi.org/10.3390/life14091083
Chicago/Turabian StylePacheco, Ana Paula, Henrique Laureano, Laire Schidlowski, Natalia Ciorcero, Thalita Zanatto, Ariela Borgmann, Gabrielle Fragoso, Ana Luisa Giamberardino, Renata Dourado, Karine dos Anjos, and et al. 2024. "COVID-19 in Brazilian Pediatric Patients: A Retrospective Cross-Sectional Study with a Predictive Model for Hospitalization" Life 14, no. 9: 1083. https://doi.org/10.3390/life14091083
APA StylePacheco, A. P., Laureano, H., Schidlowski, L., Ciorcero, N., Zanatto, T., Borgmann, A., Fragoso, G., Giamberardino, A. L., Dourado, R., Anjos, K. d., João, P., Assahide, M., Silveira, M. C., Costa-Junior, V., Giamberardino, H., & Prando, C. (2024). COVID-19 in Brazilian Pediatric Patients: A Retrospective Cross-Sectional Study with a Predictive Model for Hospitalization. Life, 14(9), 1083. https://doi.org/10.3390/life14091083







