Prenatal Nutritional Factors and Neurodevelopmental Disorders: A Narrative Review
Abstract
:1. Introduction
2. Preventive Nutrition
3. Neurodevelopmental Disorders
- Intellectual disability (intellectual development disorder);
- Communication disorders;
- Autism spectrum disorder;
- Attention deficit/hyperactivity disorder;
- Specific learning disorder;
- Movement disorders;
- Other neurodevelopmental disorders.
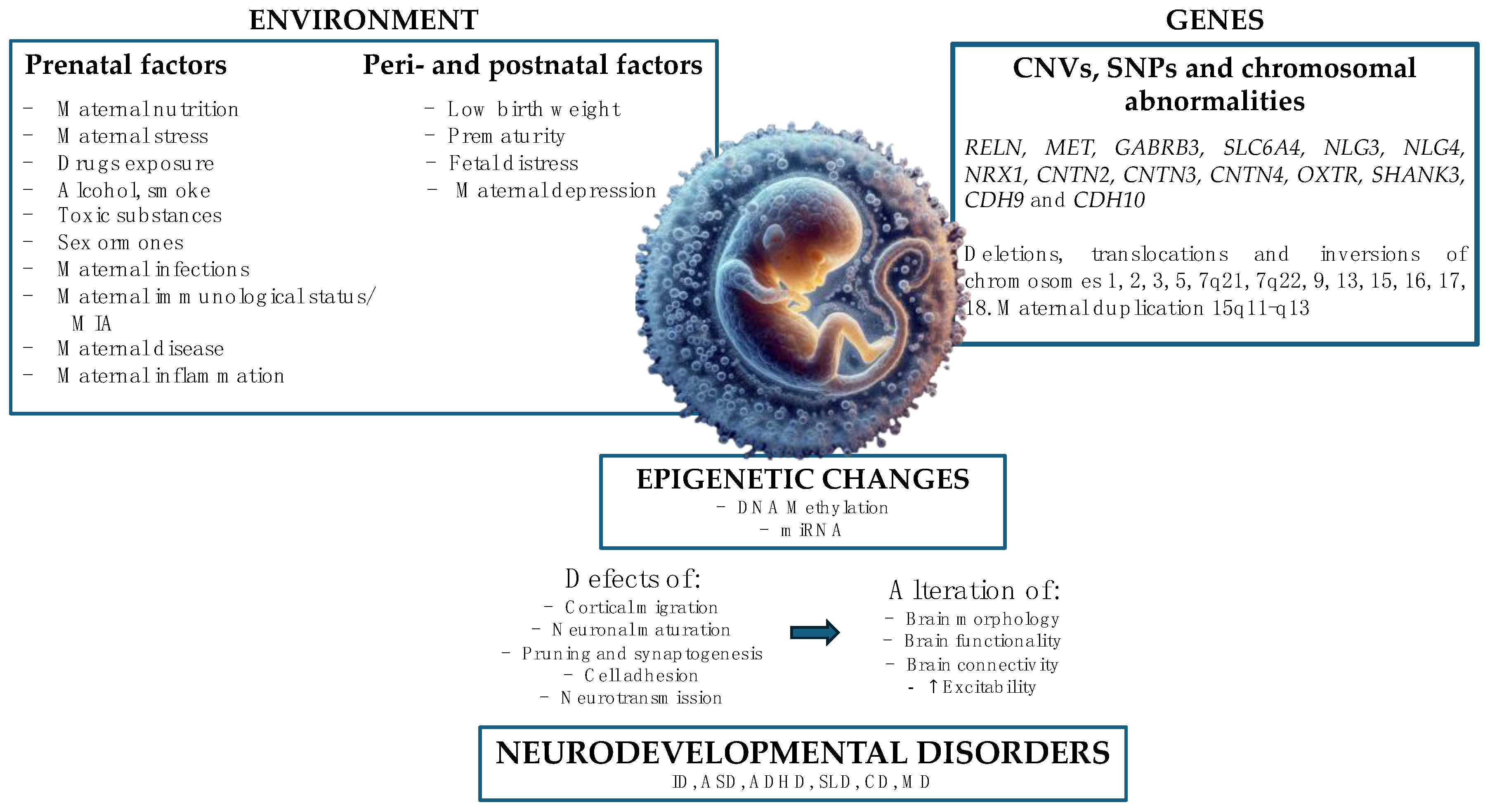
4. Etiology and Risk Factors
- Complications of pregnancy and childbirth;
- Prematurity;
- Low birth weight;
- Exposure to alcohol, drugs, or other toxic substances during pregnancy;
- Low socio-economic level of parents;
- Situation of early emotional deficiency;
- High parental age (>35 years).
4.1. Genetic and Epigenetic Factors
- DNA methylation.
- miRNAs or microRNAs, small sequences of 20–25 RNA nucleotides, organized as a single strand, not coding for any protein but having the function of regulating the translation of target genes through downregulation mechanisms. They induce gene silencing through binding to complementary sequences on target mRNA molecules, resulting in repression of translation or degradation of the target molecule.
4.2. Environmental Factors
- Heavy metals can alter many body functions, causing neurological and behavioral damage, which we will analyze later. A meta-analysis found a 60% increased risk of ASD due to exposure to high levels of inorganic mercury [40].
- Organophosphates are associated with a 60% increased risk of developing ASD [41]. This category includes non-persistent organic pollutants (phthalates and bisphenol A) and persistent organic pollutants (DDT, PCBs, and PBDEs). Exposure to these substances seems to alter calcium-related signaling pathways, leading to alterations in dendritic growth and anomalies in neuronal connectivity, both typical of ASD.
- Epigenetic/genetic mechanisms (discussed previously).
- Immune dysregulation and neuroinflammation—in fact, the interaction between the immune system and nerve cells is essential for neurodevelopment. For example, IL-6 and INF-γ regulate dendritic growth and synaptogenesis through signal transduction mechanisms and the MAPK pathway.
- Oxidative stress and mitochondrial dysfunction through an imbalance between free radicals and antioxidants leading to the alteration of ATP levels in nervous cells.
- Endocrine alterations, such as hormonal imbalances.
- Alterations of neurotransmitters (such as serotonin, glutamate, and GABA) and cell signaling pathways.
5. Prenatal Nutritional Factors
- The maternal diet before pregnancy allows to optimize nutritional status in order to maintain a healthy pregnancy and support fetal development [48].
- Maternal nutrition during the conception period is important for gametes’ function and health and for placental development [49]. For example, the first 2–3 weeks from conception represent a period of rapid development in which the embryo undergoes multiple processes (neuronal migration, synaptic formation, apoptosis) for fetal brain development, constituting a highly sensitive period to possible environmental disturbances that could predispose the fetus to develop neurodevelopmental disorders in the postnatal period.
- Behavioral and psychiatric disorders (ASD, ADHD, anxiety, depression, schizophrenia);
- Cognitive alterations (intellectual disability, language disorders, learning, and memory disorders);
- Neural tube defects;
- Visual alterations;
- Motor deficits;
- Neural molecular dysfunctions (synaptic alterations, neurotransmitters’ metabolism, myelination, cell differentiation, axonal growth, dendritic arborization, anti-inflammatory regulation, oxidative stress, death neuronal, vascular function, synaptic plasticity, neurogenesis, astrocytic alterations);
- Structural changes (reduction of brain volume, spina bifida, hydrocephalus, alteration of the hypothalamic and hippocampal pathways).
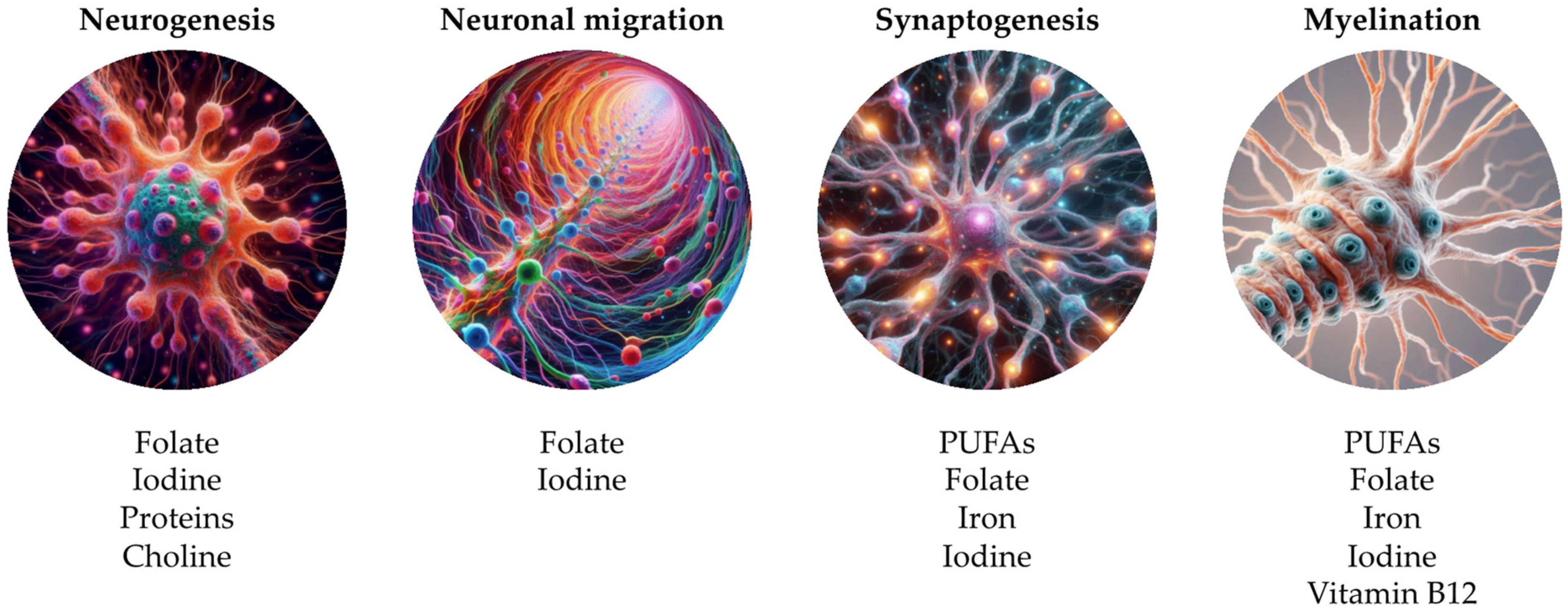
- Vitamins and minerals, such as vitamins B12, A, E, D, K, folic acid, iron, copper, creatine, choline, zinc, and iodine;
- Macronutrients such as polyunsaturated fatty acids (PUFAs) and proteins;
- General maternal nutritional status, such as obesity, overnutrition (high fat–HFD—and hypercaloric diets), and undernutrition;
- Other nutrients, such as gangliosides and caffeine.
5.1. Dietary Habits
5.1.1. Undernutrition
5.1.2. Overnutrition
5.2. Nutrients
5.2.1. Fatty Acids
5.2.2. Proteins
5.2.3. Folates
5.2.4. Iron
5.2.5. Iodine
5.2.6. Copper
5.2.7. Creatine
5.2.8. Choline
5.2.9. Zinc
5.2.10. Vitamin B12
5.2.11. Vitamin B6
5.2.12. Vitamin D
5.2.13. Vitamin A
5.2.14. Vitamin E and K
5.2.15. Gangliosides
5.2.16. Caffeine
5.3. Heavy Metals
5.3.1. Lead
5.3.2. Mercury
5.3.3. Cadmium
5.3.4. Manganese
5.3.5. Nickel
5.3.6. Arsenic
6. Postnatal Nutritional Factors
7. Conclusions
- -
- An increased protein requirement (different in the three trimesters of pregnancy);
- -
- A carbohydrate intake of at least 175 g/day, reducing monosaccharides and disaccharides (<10% of total carbohydrate);
- -
- An increased DHA requirement of 100–200 mg/day;
- -
- Supplementation of folic acid (400 g/day) from 30 days before conception;
- -
- Iodine requirement of 200–250 g/die.
Author Contributions
Funding
Conflicts of Interest
References
- Franzago, M.; Santurbano, D.; Vitacolonna, E.; Stuppia, L. Genes and Diet in the Prevention of Chronic Diseases in Future Generations. Int. J. Mol. Sci. 2020, 21, 2633. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, A.; Corsello, A.; Spolidoro, G.C.I.; Trovato, C.M.; Agostoni, C.; Orsini, A.; Milani, G.P.; Peroni, D.G. The Influence of Ketogenic Diet on Gut Microbiota: Potential Benefits, Risks and Indications. Nutrients 2023, 15, 3680. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The Impact of the Gut Microbiota on the Reproductive and Metabolic Endocrine System. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef]
- Strobel, K.M.; Juul, S.E.; Hendrixson, D.T. Maternal Nutritional Status and the Microbiome across the Pregnancy and the Post-Partum Period. Microorganisms 2023, 11, 1569. [Google Scholar] [CrossRef]
- Cömert, T.K.; Akpinar, F.; Erkaya, S.; Durmaz, B.; Durmaz, R. The Effect of Pre-Pregnancy Obesity on Gut and Meconium Microbiome and Relationship with Fetal Growth. J. Matern. Fetal Neonatal Med. 2022, 35, 10629–10637. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jia, F.; Differding, M.K.; Zhao, N.; Doyon, M.; Bouchard, L.; Perron, P.; Guérin, R.; Massé, E.; Hivert, M.F.; et al. Pre-Pregnancy Body Mass Index and Gut Microbiota of Mothers and Children 5 Years Postpartum. Int. J. Obes. 2023, 47, 807–816. [Google Scholar] [CrossRef]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, (DSM-5), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, (DSM-IV), 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Hu, V.W.; Devlin, C.A.; Debski, J.J. ASD Phenotype-Genotype Associations in Concordant and Discordant Monozygotic and Dizygotic Twins Stratified by Severity of Autistic Traits. Int. J. Mol. Sci. 2019, 20, 3804. [Google Scholar] [CrossRef]
- Boivin, M.J.; Kakooza, A.M.; Warf, B.C.; Davidson, L.L.; Grigorenko, E.L. Reducing Neurodevelopmental Disorders and Disability through Research and Interventions. Nature 2015, 527, S155–S160. [Google Scholar] [CrossRef]
- Grayson, D.R.; Guidotti, A. Merging Data from Genetic and Epigenetic Approaches to Better Understand Autistic Spectrum Disorder. Epigenomics 2016, 8, 85–104. [Google Scholar] [CrossRef]
- Masini, E.; Loi, E.; Vega-Benedetti, A.F.; Carta, M.; Doneddu, G.; Fadda, R.; Zavattari, P. An Overview of the Main Genetic, Epigenetic and Environmental Factors Involved in Autism Spectrum Disorder Focusing on Synaptic Activity. Int. J. Mol. Sci. 2020, 21, 8290. [Google Scholar] [CrossRef]
- Kaizuka, T.; Takumi, T. Postsynaptic Density Proteins and Their Involvement in Neurodevelopmental Disorders. J. Biochem. 2018, 163, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Vissers, L.E.L.M.; Gilissen, C.; Veltman, J.A. Genetic Studies in Intellectual Disability and Related Disorders. Nat. Rev. Genet. 2016, 17, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.; Halayem, S.; Mrad, R.; Bourgou, S.; Charfi, F.; Belhadj, A. Epigenetics’ Implication in Autism Spectrum Disorders: A Review. Encephale 2017, 43, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Janecka, M.; Mill, J.; Basson, M.A.; Goriely, A.; Spiers, H.; Reichenberg, A.; Schalkwyk, L.; Fernandes, C. Advanced Paternal Age Effects in Neurodevelopmental Disorders-Review of Potential Underlying Mechanisms. Transl. Psychiatry 2017, 7, e1019. [Google Scholar] [CrossRef]
- Gardener, H.; Spiegelman, D.; Buka, S.L. Perinatal and Neonatal Risk Factors for Autism: A Comprehensive Meta-Analysis. Pediatrics 2011, 128, 344–355. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Hodgson, N.W.; Trivedi, M.S.; Abdolmaleky, H.M.; Fournier, M.; Cuenod, M.; Do, K.Q.; Deth, R.C. Decreased Brain Levels of Vitamin B12 in Aging, Autism and Schizophrenia. PLoS ONE 2016, 11, e0146797. [Google Scholar] [CrossRef]
- Beghetti, I.; Barone, M.; Brigidi, P.; Sansavini, A.; Corvaglia, L.; Aceti, A.; Turroni, S. Early-Life Gut Microbiota and Neurodevelopment in Preterm Infants: A Narrative Review. Front. Nutr. 2023, 10, 1241303. [Google Scholar] [CrossRef]
- Rabe, H.; Lundell, A.C.; Sjöberg, F.; Ljung, A.; Strömbeck, A.; Gio-Batta, M.; Maglio, C.; Nordström, I.; Andersson, K.; Nookaew, I.; et al. Neonatal Gut Colonization by Bifidobacterium Is Associated with Higher Childhood Cytokine Responses. Gut Microbes 2020, 12, 1847628. [Google Scholar] [CrossRef]
- Dash, S.; Syed, Y.A.; Khan, M.R. Understanding the Role of the Gut Microbiome in Brain Development and Its Association with Neurodevelopmental Psychiatric Disorders. Front. Cell Dev. Biol. 2022, 10, 880544. [Google Scholar] [CrossRef] [PubMed]
- Foiadelli, T.; Santangelo, A.; Costagliola, G.; Costa, E.; Scacciati, M.; Riva, A.; Volpedo, G.; Smaldone, M.; Bonuccelli, A.; Clemente, A.M.; et al. Neuroinflammation and Status Epilepticus: A Narrative Review Unraveling a Complex Interplay. Front. Pediatr. 2023, 11, 1251914. [Google Scholar] [CrossRef]
- Werling, D.M. The Role of Sex-Differential Biology in Risk for Autism Spectrum Disorder. Biol. Sex Differ. 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Auyeung, B.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Abdallah, M.W.; Melgaard, L.; Cohen, A.S.; Chakrabarti, B.; Ruta, L.; Lombardo, M.V. Elevated Fetal Steroidogenic Activity in Autism. Mol. Psychiatry 2015, 20, 369–376. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Y.; Gong, X.; Wang, G. A Meta-Analysis of Maternal Smoking during Pregnancy and Autism Spectrum Disorder Risk in Offspring. Int. J. Environ. Res. Public Health 2015, 12, 10418–10431. [Google Scholar] [CrossRef]
- Gallagher, C.; McCarthy, F.P.; Ryan, R.M.; Khashan, A.S. Maternal Alcohol Consumption During Pregnancy and the Risk of Autism Spectrum Disorders in Offspring: A Retrospective Analysis of the Millennium Cohort Study. J. Autism Dev. Disord. 2018, 48, 3773–3782. [Google Scholar] [CrossRef]
- Meador, K.J. Effects of in Utero Antiepileptic Drug Exposure. Epilepsy Curr. 2008, 8, 143–147. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Cogo, E.; Rios, P.; Straus, S.E.; Finkelstein, Y.; Kealey, R.; Reynen, E.; Soobiah, C.; Thavorn, K.; Hutton, B.; et al. Comparative Safety of Anti-Epileptic Drugs during Pregnancy: A Systematic Review and Network Meta-Analysis of Congenital Malformations and Prenatal Outcomes. BMC Med. 2017, 15, 95. [Google Scholar] [CrossRef]
- Xu, G.; Jing, J.; Bowers, K.; Liu, B.; Bao, W. Maternal Diabetes and the Risk of Autism Spectrum Disorders in the Offspring: A Systematic Review and Meta-Analysis. J. Autism Dev. Disord. 2014, 44, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Xu, L.L.; Shao, L.; Xia, R.M.; Yu, Z.H.; Ling, Z.X.; Yang, F.; Deng, M.; Ruan, B. Maternal Infection during Pregnancy and Risk of Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2016, 58, 165–172. [Google Scholar] [CrossRef]
- Wu, S.; Ding, Y.; Wu, F.; Li, R.; Xie, G.; Hou, J.; Mao, P. Family History of Autoimmune Diseases Is Associated with an Increased Risk of Autism in Children: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2015, 55, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fallin, M.D.; Riley, A.; Landa, R.; Walker, S.O.; Silverstein, M.; Caruso, D.; Pearson, C.; Kiang, S.; Dahm, J.L.; et al. The Association of Maternal Obesity and Diabetes with Autism and Other Developmental Disabilities. Pediatrics 2016, 137, e20152206. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Ruiz, R.; Garza-Ocañas, L.; Camacho, A. Inflammatory Domains Modulate Autism Spectrum Disorder Susceptibility during Maternal Nutritional Programming. Neurochem. Int. 2019, 126, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Stevens, B. Microglia Function in Central Nervous System Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2015, 7, a020545. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal Immune Activation: Implications for Neuropsychiatric Disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Vohr, B.R.; Davis, E.P.; Wanke, C.A.; Krebs, N.F. Neurodevelopment: The Impact of Nutrition and Inflammation During Preconception and Pregnancy in Low-Resource Settings. Pediatrics 2017, 139, S38–S49. [Google Scholar] [CrossRef]
- Napoli, E.; Hung, C.; Wong, S.; Giulivi, C. Toxicity of the Flame-Retardant BDE-49 on Brain Mitochondria and Neuronal Progenitor Striatal Cells Enhanced by a PTEN-Deficient Background. Toxicol. Sci. 2013, 132, 196–210. [Google Scholar] [CrossRef]
- Yoshimasu, K.; Kiyohara, C.; Takemura, S.; Nakai, K. A Meta-Analysis of the Evidence on the Impact of Prenatal and Early Infancy Exposures to Mercury on Autism and Attention Deficit/Hyperactivity Disorder in the Childhood. Neurotoxicology 2014, 44, 121–131. [Google Scholar] [CrossRef]
- Shelton, J.F.; Geraghty, E.M.; Tancredi, D.J.; Delwiche, L.D.; Schmidt, R.J.; Ritz, B.; Hansen, R.L.; Hertz-Picciotto, I. Neurodevelopmental Disorders and Prenatal Residential Proximity to Agricultural Pesticides: The CHARGE Study. Environ. Health Perspect. 2014, 122, 1103–1109. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Olung, N.F.; Akingbade, G.T.; Okoh, C.O.A.; Aschner, M. Environmental Influence on Neurodevelopmental Disorders: Potential Association of Heavy Metal Exposure and Autism. J. Trace Elem. Med. Biol. 2020, 62, 126638. [Google Scholar] [CrossRef]
- MRC Vitamin Study Research Group. Prevention of Neural Tube Defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Morgan, E.H. Plasma-Iron and Haemoglobin Levels in Pregnancy. The Effect of Oral Iron. Lancet 1961, 1, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Francis, E.; Hinkle, S.N.; Ajjarapu, A.S.; Zhang, C. Preconception and Prenatal Nutrition and Neurodevelopmental Disorders: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A. Epigenetics, Nutrition, and the Brain: Improving Mental Health through Diet. Int. J. Mol. Sci. 2024, 25, 4036. [Google Scholar] [CrossRef] [PubMed]
- Gabbianelli, R.; Damiani, E. Epigenetics and Neurodegeneration: Role of Early-Life Nutrition. J. Nutr. Biochem. 2018, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. A Summary of Pathways or Mechanisms Linking Preconception Maternal Nutrition with Birth Outcomes. J. Nutr. 2016, 146, 1437S–1444S. [Google Scholar] [CrossRef]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the Beginning: Nutrition and Lifestyle in the Preconception Period and Its Importance for Future Health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-Van-meerbeke, A.; Talero-Gutiérrez, C. Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef]
- Lane, M.; Robker, R.L.; Robertson, S.A. Parenting from before Conception. Science 2014, 345, 756–760. [Google Scholar] [CrossRef]
- Debnath, M.; Venkatasubramanian, G.; Berk, M. Fetal Programming of Schizophrenia: Select Mechanisms. Neurosci. Biobehav. Rev. 2015, 49, 90–104. [Google Scholar] [CrossRef]
- Monk, C.; Georgieff, M.K.; Osterholm, E.A. Research Review: Maternal Prenatal Distress and Poor Nutrition—Mutually Influencing Risk Factors Affecting Infant Neurocognitive Development. J. Child. Psychol. Psychiatry 2013, 54, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Nousen, E.K.; Chamlou, K.A. Maternal High Fat Diet Consumption during the Perinatal Period Programs Offspring Behavior. Physiol. Behav. 2014, 123, 236–242. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of Maternal Obesity on the Long-Term Health of Offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal Pre-Pregnancy Obesity and Child Neurodevelopmental Outcomes: A Meta-Analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef] [PubMed]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose Metabolism and Metabolic Disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef] [PubMed]
- DeCapo, M.; Thompson, J.R.; Dunn, G.; Sullivan, E.L. Perinatal Nutrition and Programmed Risk for Neuropsychiatric Disorders: A Focus on Animal Models. Biol. Psychiatry 2019, 85, 122–134. [Google Scholar] [CrossRef]
- Martins, B.P.; Bandarra, N.M.; Figueiredo-Braga, M. The Role of Marine Omega-3 in Human Neurodevelopment, Including Autism Spectrum Disorders and Attention-Deficit/Hyperactivity Disorder—A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1431–1446. [Google Scholar] [CrossRef]
- van Elst, K.; Bruining, H.; Birtoli, B.; Terreaux, C.; Buitelaar, J.K.; Kas, M.J. Food for Thought: Dietary Changes in Essential Fatty Acid Ratios and the Increase in Autism Spectrum Disorders. Neurosci. Biobehav. Rev. 2014, 45, 369–378. [Google Scholar] [CrossRef]
- Kim, H.Y.; Spector, A.A.; Xiong, Z.M. A Synaptogenic Amide N-Docosahexaenoylethanolamide Promotes Hippocampal Development. Prostaglandins Other Lipid Mediat. 2011, 96, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.L.; Dewey, K.G. Nutrition and Brain Development in Early Life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef]
- Berti, C.; Biesalski, H.K.; Gärtner, R.; Lapillonne, A.; Pietrzik, K.; Poston, L.; Redman, C.; Koletzko, B.; Cetin, I. Micronutrients in Pregnancy: Current Knowledge and Unresolved Questions. Clin. Nutr. 2011, 30, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, E.; Pearce, E.N. Iodine Status and Supplementation before, during, and after Pregnancy. Best. Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101430. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, Oxidative Stress, and Human Health. Mol. Aspects Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Skogheim, T.S.; Weyde, K.V.F.; Engel, S.M.; Aase, H.; Surén, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Hornig, M.; et al. Metal and Essential Element Concentrations during Pregnancy and Associations with Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder in Children. Environ. Int. 2021, 152, 106468. [Google Scholar] [CrossRef]
- Li, Y.; Cha, C.; Lv, X.J.; Liu, J.; He, J.; Pang, Q.; Meng, L.; Kuang, H.; Fan, R. Association between 10 Urinary Heavy Metal Exposure and Attention Deficit Hyperactivity Disorder for Children. Environ. Sci. Pollut. Res. Int. 2020, 27, 31233–31242. [Google Scholar] [CrossRef]
- Richard, K.; Holland, O.; Landers, K.; Vanderlelie, J.J.; Hofstee, P.; Cuffe, J.S.M.; Perkins, A.V. Review: Effects of Maternal Micronutrient Supplementation on Placental Function. Placenta 2017, 54, 38–44. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional Influences on Brain Development. Acta Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.X.; Wang, B.H.; Zhang, Y.F. Relationship between Serum Zinc Levels and Attention Deficit Hyperactivity Disorder in Children. Zhongguo Dang Dai Er Ke Za Zhi 2015, 17, 980–983. [Google Scholar]
- Tous, M.; Villalobos, M.; Iglesias, L.; Fernández-Barrés, S.; Arija, V. Vitamin D Status during Pregnancy and Offspring Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Eur. J. Clin. Nutr. 2020, 74, 36–53. [Google Scholar] [CrossRef]
- Ryan, J.M.; Rice, G.E.; Mitchell, M.D. The Role of Gangliosides in Brain Development and the Potential Benefits of Perinatal Supplementation. Nutr. Res. 2013, 33, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, Q.; Ward, S.M.; Duan, E.; Zhang, Y. Impacts of Caffeine during Pregnancy. Trends Endocrinol. Metab. 2020, 31, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic Review of the Potential Adverse Effects of Caffeine Consumption in Healthy Adults, Pregnant Women, Adolescents, and Children. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar] [CrossRef] [PubMed]
- Linnet, K.M.; Wisborg, K.; Secher, N.J.; Hove Thomsen, P.; Obel, C.; Dalsgaard, S.; Henriksen, T.B. Coffee Consumption during Pregnancy and the Risk of Hyperkinetic Disorder and ADHD: A Prospective Cohort Study. Acta Paediatr. 2009, 98, 173–179. [Google Scholar] [CrossRef]
- Galéra, C.; Bernard, J.Y.; van der Waerden, J.; Bouvard, M.P.; Lioret, S.; Forhan, A.; De Agostini, M.; Melchior, M.; Heude, B. Prenatal Caffeine Exposure and Child IQ at Age 5.5 Years: The EDEN Mother-Child Cohort. Biol. Psychiatry 2016, 80, 720–726. [Google Scholar] [CrossRef]
- Roberts, A.L.; Lyall, K.; Hart, J.E.; Laden, F.; Just, A.C.; Bobb, J.F.; Koenen, K.C.; Ascherio, A.; Weisskopf, M.G. Perinatal Air Pollutant Exposures and Autism Spectrum Disorder in the Children of Nurses’ Health Study II Participants. Environ. Health Perspect. 2013, 121, 978–984. [Google Scholar] [CrossRef]
- El-Ansary, A.; Bjørklund, G.; Tinkov, A.A.; Skalny, A.V.; Al Dera, H. Relationship between Selenium, Lead, and Mercury in Red Blood Cells of Saudi Autistic Children. Metab. Brain Dis. 2017, 32, 1073–1080. [Google Scholar] [CrossRef]
- Nair, A.R.; Lee, W.K.; Smeets, K.; Swennen, Q.; Sanchez, A.; Thévenod, F.; Cuypers, A. Glutathione and Mitochondria Determine Acute Defense Responses and Adaptive Processes in Cadmium-Induced Oxidative Stress and Toxicity of the Kidney. Arch. Toxicol. 2015, 89, 2273–2289. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Miah, M.R.; Ijomone, O.M.; Tsatsakis, A.; Soares, F.A.A.; Tinkov, A.A.; Skalny, A.V.; Venkataramani, V.; Aschner, M. Lead (Pb) Exposure Induces Dopaminergic Neurotoxicity in Caenorhabditis Elegans: Involvement of the Dopamine Transporter. Toxicol. Rep. 2019, 6, 833–840. [Google Scholar] [CrossRef]
- Kim, K.N.; Kwon, H.J.; Hong, Y.C. Low-Level Lead Exposure and Autistic Behaviors in School-Age Children. Neurotoxicology 2016, 53, 193–200. [Google Scholar] [CrossRef]
- Fuentes-Albero, M.; Puig-Alcaraz, C.; Cauli, O. Lead Excretion in Spanish Children with Autism Spectrum Disorder. Brain Sci. 2015, 5, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Y.; Jian, B.; Wu, C.; Jiang, C.Z.; Kang, Y.; Zhou, J.X.; Yang, F.; Liang, Y. A Comparison of Blood Metal Levels in Autism Spectrum Disorder and Unaffected Children in Shenzhen of China and Factors Involved in Bioaccumulation of Metals. Environ. Sci. Pollut. Res. Int. 2018, 25, 17950–17956. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.M.; Walker, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental Mercury and Its Toxic Effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Jafari, T.; Rostampour, N.; Fallah, A.A.; Hesami, A. The Association between Mercury Levels and Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. J. Trace Elem. Med. Biol. 2017, 44, 289–297. [Google Scholar] [CrossRef]
- Golding, J.; Rai, D.; Gregory, S.; Ellis, G.; Emond, A.; Iles-Caven, Y.; Hibbeln, J.; Taylor, C. Prenatal Mercury Exposure and Features of Autism: A Prospective Population Study. Mol. Autism 2018, 9, 30. [Google Scholar] [CrossRef]
- Geier, D.A.; Kern, J.K.; King, P.G.; Sykes, L.K.; Geier, M.R. Hair Toxic Metal Concentrations and Autism Spectrum Disorder Severity in Young Children. Int. J. Environ. Res. Public Health 2012, 9, 4486–4497. [Google Scholar] [CrossRef]
- Kern, J.K.; Geier, D.A.; Audhya, T.; King, P.G.; Sykes, L.K.; Geier, M.R. Evidence of Parallels between Mercury Intoxication and the Brain Pathology in Autism. Acta Neurobiol. Exp. 2012, 72, 113–153. [Google Scholar] [CrossRef]
- Kern, J.K.; Grannemann, B.D.; Trivedi, M.H.; Adams, J.B. Sulfhydryl-Reactive Metals in Autism. J. Toxicol. Environ. Health A 2007, 70, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Yorbik, Ö.; Kurt, I.; Haşimi, A.; Öztürk, Ö. Chromium, Cadmium, and Lead Levels in Urine of Children with Autism and Typically Developing Controls. Biol. Trace Elem. Res. 2010, 135, 10–15. [Google Scholar] [CrossRef]
- Yasuda, H.; Kobayashi, M.; Yasuda, Y.; Tsutsui, T. Estimation of Autistic Children by Metallomics Analysis. Sci. Rep. 2013, 3, 1199. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Aluko, O.M.; Okoh, C.O.A.; Martins, A.C.; Aschner, M. Role for Calcium Signaling in Manganese Neurotoxicity. J. Trace Elem. Med. Biol. 2019, 56, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Morcillo, P.; Ijomone, O.M.; Venkataramani, V.; Harrison, F.E.; Lee, E.; Bowman, A.B.; Aschner, M. New Insights on the Role of Manganese in Alzheimer’s Disease and Parkinson’s Disease. Int. J. Environ. Res. Public Health 2019, 16, 3546. [Google Scholar] [CrossRef]
- Zota, A.R.; Riederer, A.M.; Ettinger, A.S.; Schaider, L.A.; Shine, J.P.; Amarasiriwardena, C.J.; Wright, R.O.; Spengler, J.D. Associations between Metals in Residential Environmental Media and Exposure Biomarkers over Time in Infants Living near a Mining-Impacted Site. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Henn, B.C.; Ettinger, A.S.; Schwartz, J.; Téllez-Rojo, M.M.; Lamadrid-Figueroa, H.; Hernández-Avila, M.; Schnaas, L.; Amarasiriwardena, C.; Bellinger, D.C.; Hu, H.; et al. Early Postnatal Blood Manganese Levels and Children’s Neurodevelopment. Epidemiology 2010, 21, 433–439. [Google Scholar] [CrossRef]
- Arora, M.; Reichenberg, A.; Willfors, C.; Austin, C.; Gennings, C.; Berggren, S.; Lichtenstein, P.; Anckarsäter, H.; Tammimies, K.; Bölte, S. Fetal and Postnatal Metal Dysregulation in Autism. Nat. Commun. 2017, 8, 15493. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Ly, A.R.; Goldberg, W.A.; Clarke-Stewart, K.A.; Dudgeon, J.V.; Mull, C.G.; Chan, T.J.; Kent, E.E.; Mason, A.Z.; Ericson, J.E. Heavy Metal in Children’s Tooth Enamel: Related to Autism and Disruptive Behaviors? J. Autism Dev. Disord. 2012, 42, 929–936. [Google Scholar] [CrossRef]
- Rahbar, M.H.; Samms-Vaughan, M.; Ma, J.; Bressler, J.; Dickerson, A.S.; Hessabi, M.; Loveland, K.A.; Grove, M.L.; Shakespeare-Pellington, S.; Beecher, C.; et al. Synergic Effect of GSTP1 and Blood Manganese Concentrations in Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2015, 18, 73–82. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Okori, S.O.; Ijomone, O.K.; Ebokaiwe, A.P. Sub-Acute Nickel Exposure Impairs Behavior, Alters Neuronal Microarchitecture, and Induces Oxidative Stress in Rats’ Brain. Drug Chem. Toxicol. 2018, 41, 377–384. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Miah, M.R.; Akingbade, G.T.; Bucinca, H.; Aschner, M. Nickel-Induced Developmental Neurotoxicity in C. elegans Includes Cholinergic, Dopaminergic and GABAergic Degeneration, Altered Behaviour, and Increased SKN-1 Activity. Neurotox. Res. 2020, 37, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, Y.M.; Waly, M.I.; Al-Sharbati, M.M.; Al-Shafaee, M.A.; Al-Farsi, O.A.; Al-Khaduri, M.M.; Gupta, I.; Ouhtit, A.; Al-Adawi, S.; Al-Said, M.F.; et al. Levels of Heavy Metals and Essential Minerals in Hair Samples of Children with Autism in Oman: A Case-Control Study. Biol. Trace Elem. Res. 2013, 151, 181–186. [Google Scholar] [CrossRef]
- Skalny, A.V.; Simashkova, N.V.; Klyushnik, T.P.; Grabeklis, A.R.; Radysh, I.V.; Skalnaya, M.G.; Tinkov, A.A. Analysis of Hair Trace Elements in Children with Autism Spectrum Disorders and Communication Disorders. Biol. Trace Elem. Res. 2017, 177, 215–223. [Google Scholar] [CrossRef]
- Bjørklund, G.; Skalny, A.V.; Rahman, M.M.; Dadar, M.; Yassa, H.A.; Aaseth, J.; Chirumbolo, S.; Skalnaya, M.G.; Tinkov, A.A. Toxic Metal(Loid)-Based Pollutants and Their Possible Role in Autism Spectrum Disorder. Environ. Res. 2018, 166, 234–250. [Google Scholar] [CrossRef]
- Forns, J.; Fort, M.; Casas, M.; Cáceres, A.; Guxens, M.; Gascon, M.; Garcia-Esteban, R.; Julvez, J.; Grimalt, J.O.; Sunyer, J. Exposure to Metals during Pregnancy and Neuropsychological Development at the Age of 4 Years. Neurotoxicology 2014, 40, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Ghisari, M.; Kjeldsen, L.; Wielsøe, M.; Nørgaard-Pedersen, B.; Mortensen, E.L.; Abdallah, M.W.; Bonefeld-Jørgensen, E.C. Autism Spectrum Disorders, Endocrine Disrupting Compounds, and Heavy Metals in Amniotic Fluid: A Case-Control Study. Mol. Autism 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Agency for Toxic Substances and Disease Registry (ATSDR) 2007. Available online: https://www.atsdr.cdc.gov/spl/previous/07list.html (accessed on 14 August 2024).
- Pinto, S.; Correia-de-Sá, T.; Sampaio-Maia, B.; Vasconcelos, C.; Moreira, P.; Ferreira-Gomes, J. Eating Patterns and Dietary Interventions in ADHD: A Narrative Review. Nutrients 2022, 14, 4332. [Google Scholar] [CrossRef] [PubMed]
- Del-Ponte, B.; Quinte, G.C.; Cruz, S.; Grellert, M.; Santos, I.S. Dietary Patterns and Attention Deficit/Hyperactivity Disorder (ADHD): A Systematic Review and Meta-Analysis. J. Affect. Disord. 2019, 252, 160–173. [Google Scholar] [CrossRef]
- Rios-Hernandez, A.; Alda, J.A.; Farran-Codina, A.; Ferreira-Garcia, E.; Izquierdo-Pulido, M. The Mediterranean Diet and ADHD in Children and Adolescents. Pediatrics 2017, 139, e20162027. [Google Scholar] [CrossRef]
- Klaus, W.; Lange, Y.N.A.R. Diet and Food in Attention-Deficit Hyperactivity Disorder. J. Future Foods 2022, 2, 112–118. [Google Scholar]
- Janssen, C.I.F.; Kiliaan, A.J. Long-Chain Polyunsaturated Fatty Acids (LCPUFA) from Genesis to Senescence: The Influence of LCPUFA on Neural Development, Aging, and Neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Belfort, M.B.; Rifas-Shiman, S.L.; Kleinman, K.P.; Bellinger, D.C.; Harris, M.H.; Taveras, E.M.; Gillman, M.W.; Oken, E. Infant Breastfeeding Duration and Mid-Childhood Executive Function, Behavior, and Social-Emotional Development. J. Dev. Behav. Pediatr. 2016, 37, 43–52. [Google Scholar] [CrossRef]
- Mendez, M.A.; Torrent, M.; Julvez, J.; Ribas-Fitó, N.; Kogevinas, M.; Sunyer, J. Maternal Fish and Other Seafood Intakes during Pregnancy and Child Neurodevelopment at Age 4 Years. Public Health Nutr. 2009, 12, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Hadders-Algra, M. Prenatal and Early Postnatal Supplementation with Long-Chain Polyunsaturated Fatty Acids: Neurodevelopmental Considerations. Am. J. Clin. Nutr. 2011, 94, S1874–S1879. [Google Scholar] [CrossRef] [PubMed]
| Nutrients | Alterations | Functions |
|---|---|---|
| PUFAs |
|
|
| Proteins |
|
|
| Folate |
|
|
| Iron |
|
|
| Iodine |
|
|
| Zinc |
|
|
| Vitamin D |
|
|
| Vitamin B12 |
|
|
| Vitamin B6 |
|
|
| Choline |
|
|
| Heavy Metals | Alterations | Pathogenetic Mechanisms |
|---|---|---|
| Lead |
|
|
| Mercury |
|
|
| Cadmium |
|
|
| Manganese |
|
|
| Nichel |
|
|
| Arsenic |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cernigliaro, F.; Santangelo, A.; Nardello, R.; Lo Cascio, S.; D’Agostino, S.; Correnti, E.; Marchese, F.; Pitino, R.; Valdese, S.; Rizzo, C.; et al. Prenatal Nutritional Factors and Neurodevelopmental Disorders: A Narrative Review. Life 2024, 14, 1084. https://doi.org/10.3390/life14091084
Cernigliaro F, Santangelo A, Nardello R, Lo Cascio S, D’Agostino S, Correnti E, Marchese F, Pitino R, Valdese S, Rizzo C, et al. Prenatal Nutritional Factors and Neurodevelopmental Disorders: A Narrative Review. Life. 2024; 14(9):1084. https://doi.org/10.3390/life14091084
Chicago/Turabian StyleCernigliaro, Federica, Andrea Santangelo, Rosaria Nardello, Salvatore Lo Cascio, Sofia D’Agostino, Edvige Correnti, Francesca Marchese, Renata Pitino, Silvia Valdese, Carmelo Rizzo, and et al. 2024. "Prenatal Nutritional Factors and Neurodevelopmental Disorders: A Narrative Review" Life 14, no. 9: 1084. https://doi.org/10.3390/life14091084








