The Potential of Enamel Matrix Derivative in Countering Bisphosphonate-Induced Effects in Osteoblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Treatments
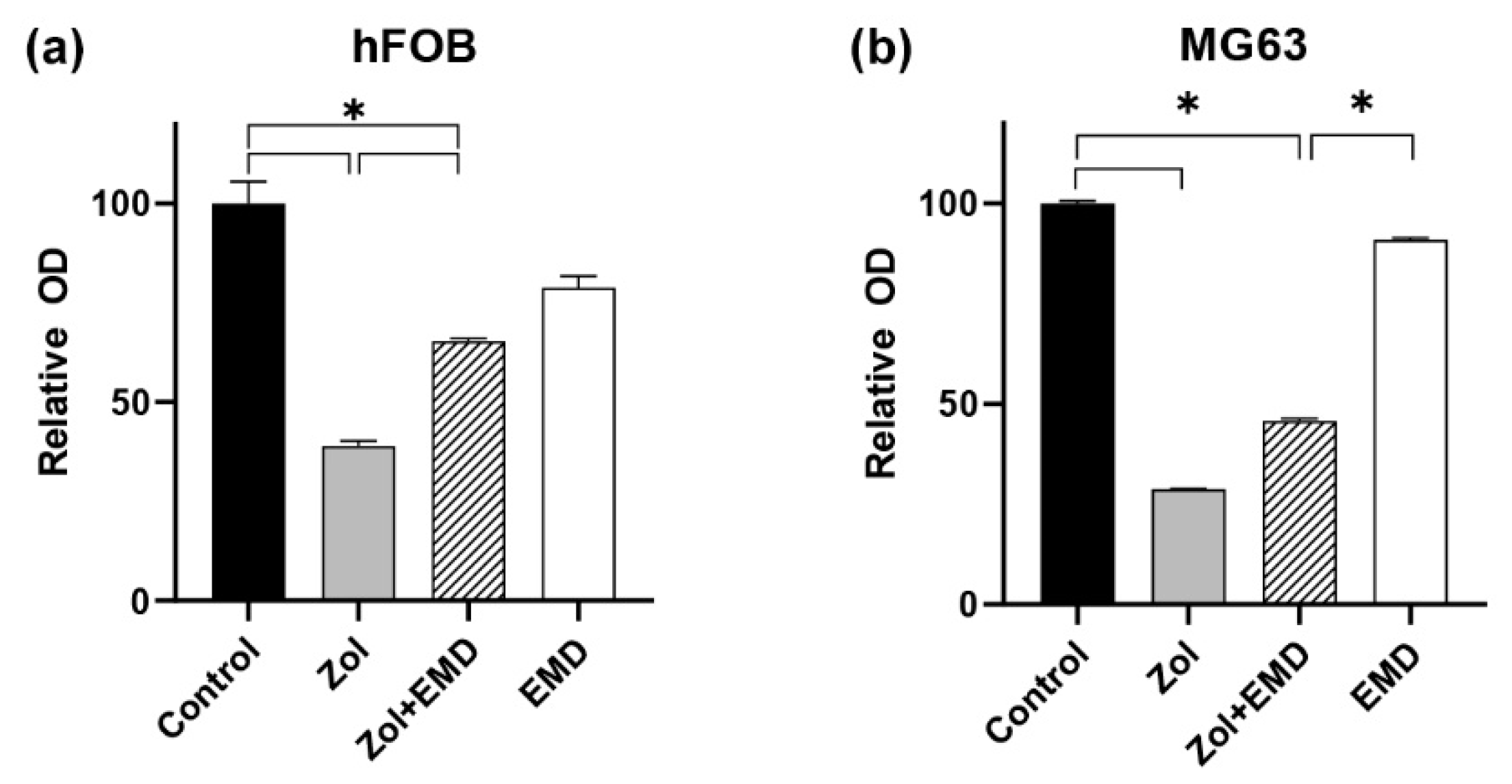
- Group I (control), cells without Zol and EMD;
- Group II (Zol), cells treated with Zol only;
- Group III (Zol + EMD), cells treated with Zol and EMD;
- Group IV (EMD), cells treated with EMD only.
2.2. Cell Viability
2.3. Cell Apoptosis Analysis
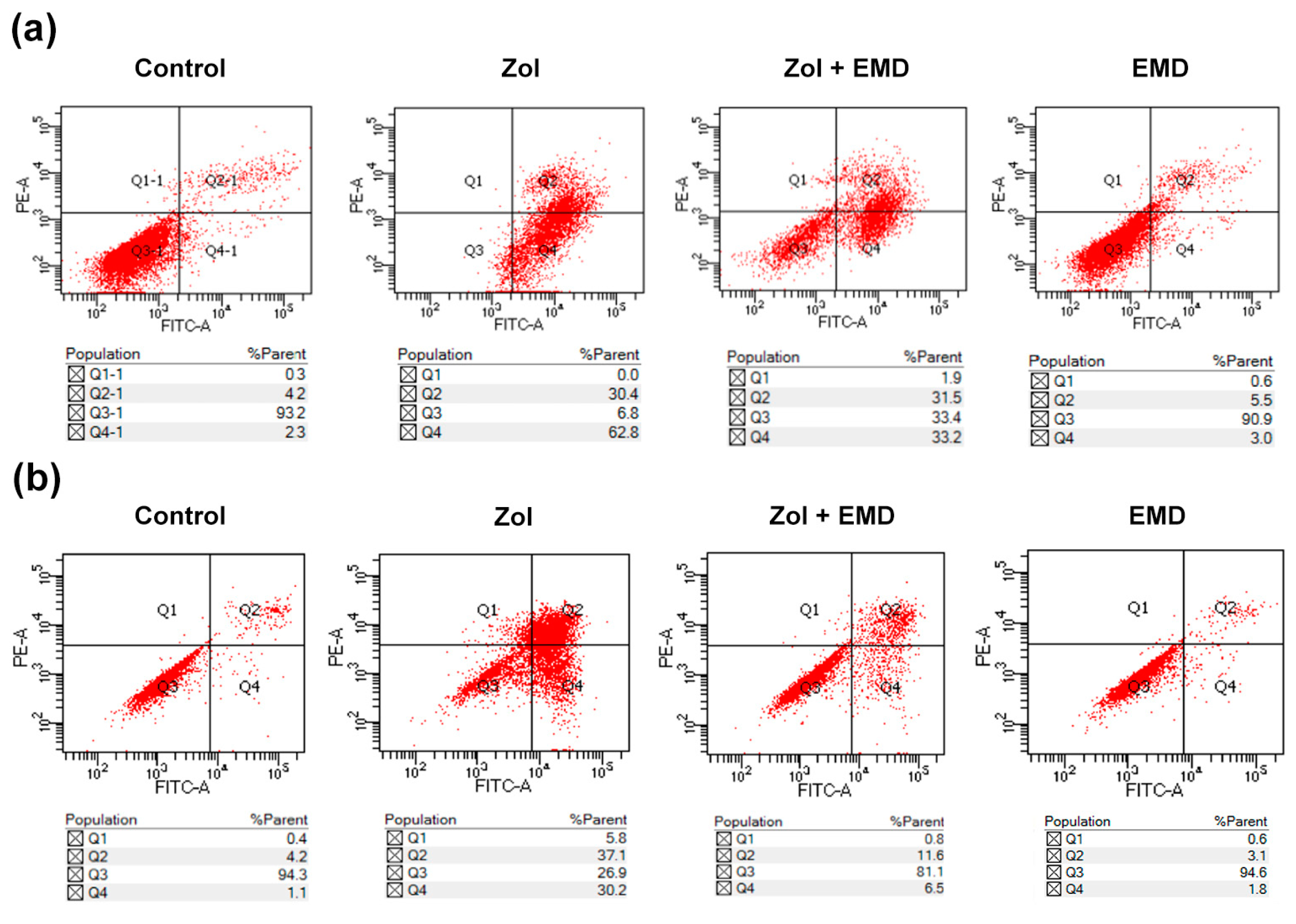
- Q1: necrotic cells (annexin V-FITC-negative/PI-positive)
- Q2: late apoptotic cells (annexin V-FITC-positive/PI-positive)
- Q3: viable cells (annexin V-FITC-negative/PI-negative)
- Q4: early apoptotic cells (annexin V-FITC-positive/PI-negative)
2.4. Cell Migration
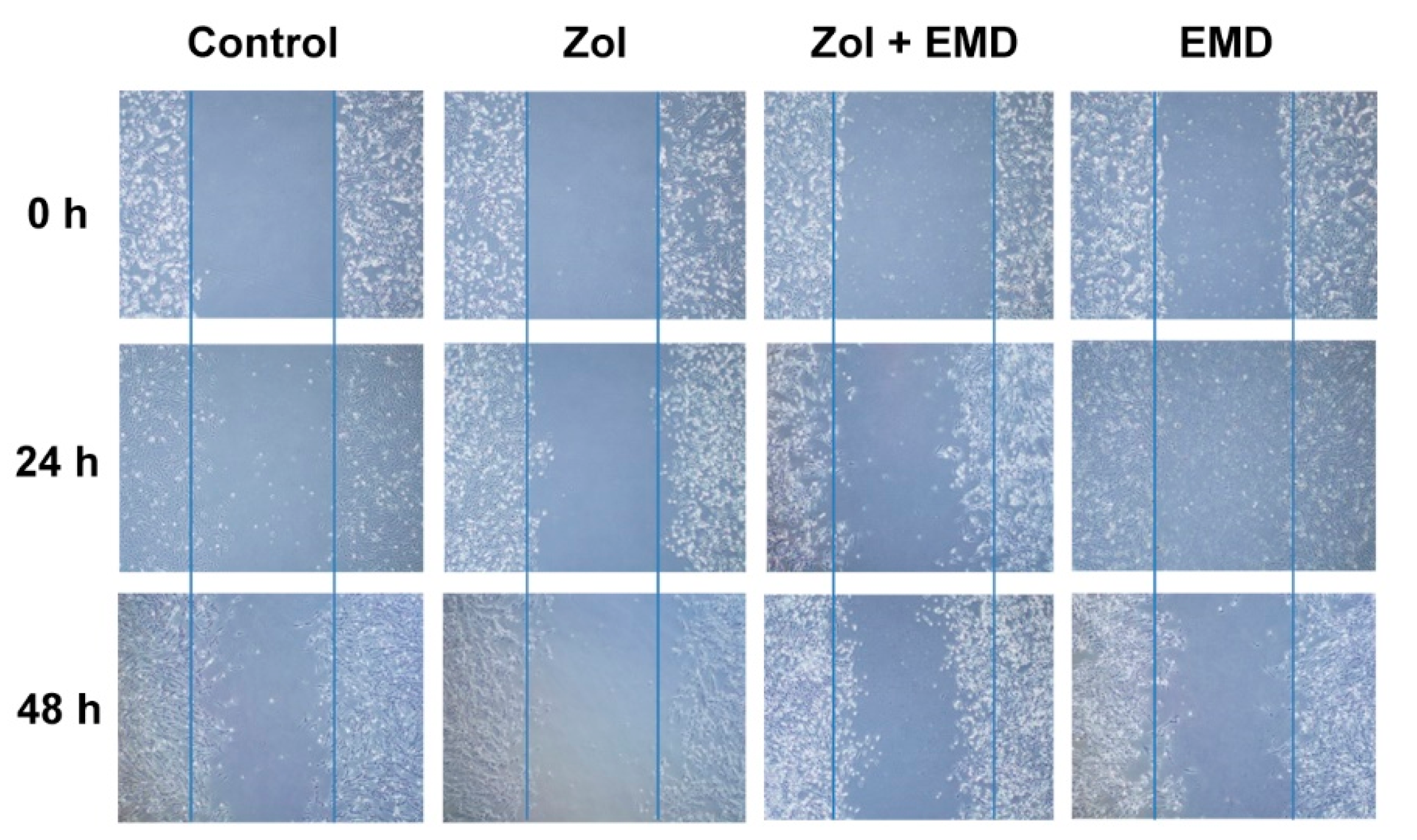
2.4.1. Scratch Wound Healing Assay
2.4.2. Boyden Chamber Assay
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Cell Viability
3.2. Cell Apoptosis
3.3. Cell Migration
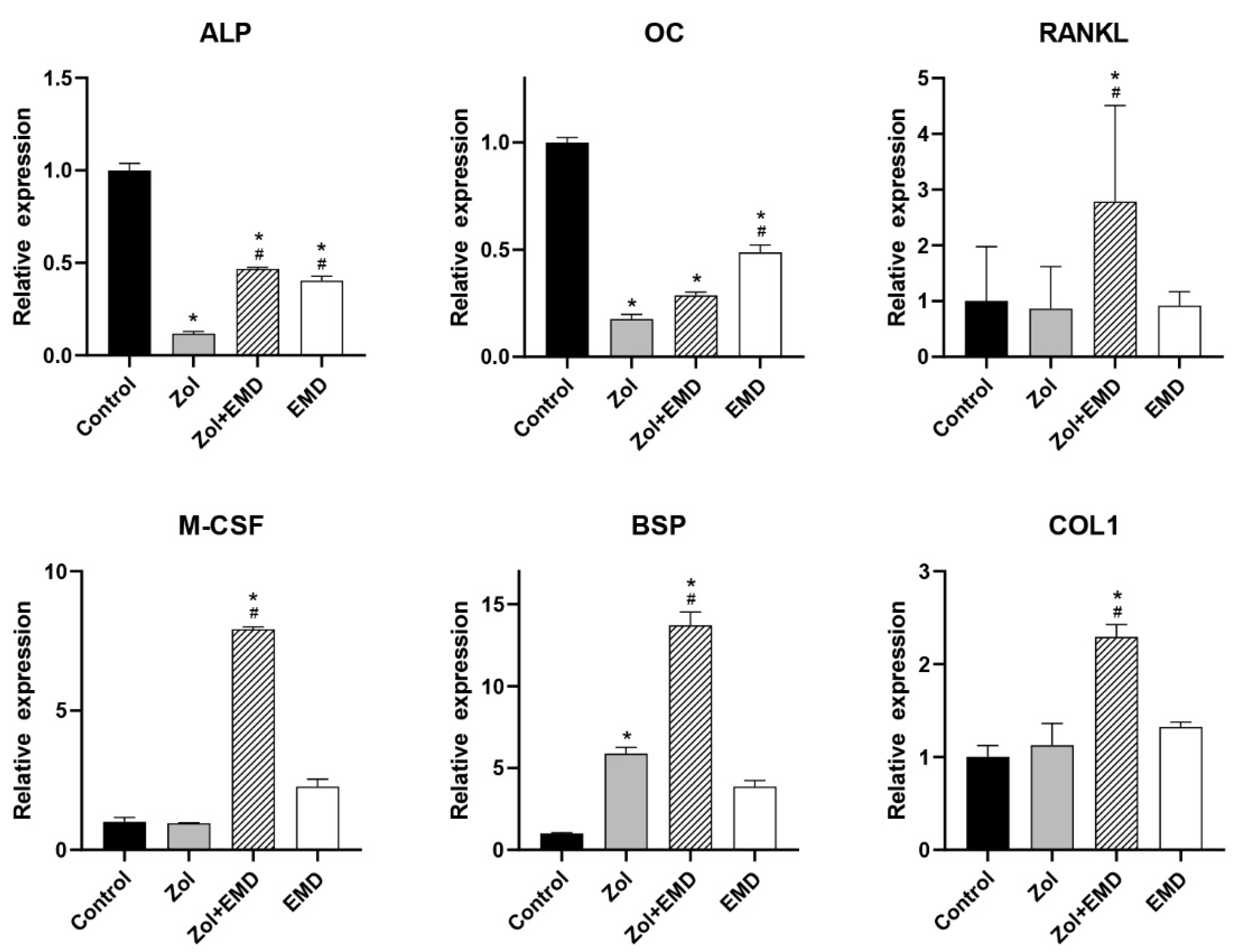
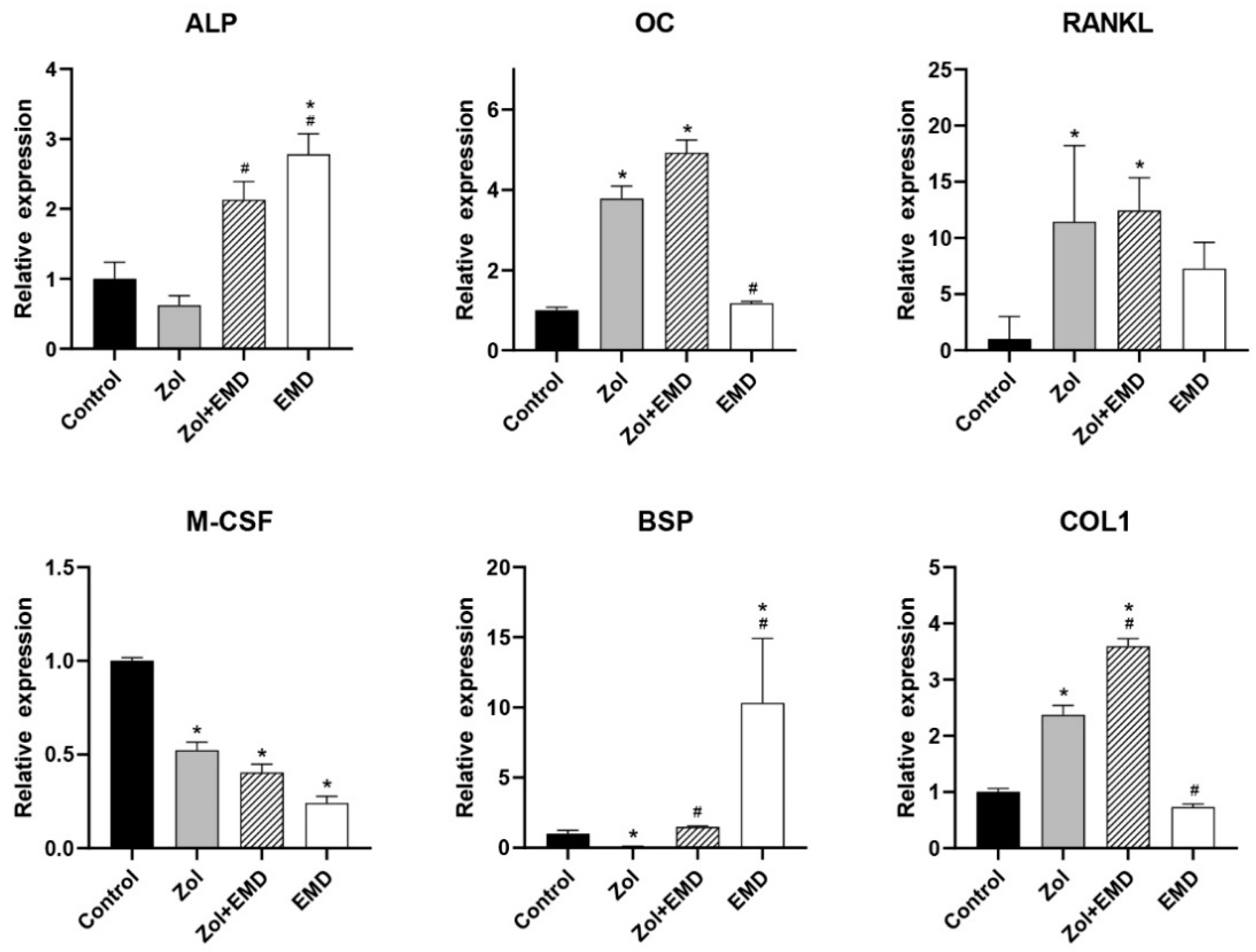
3.4. Gene Expression
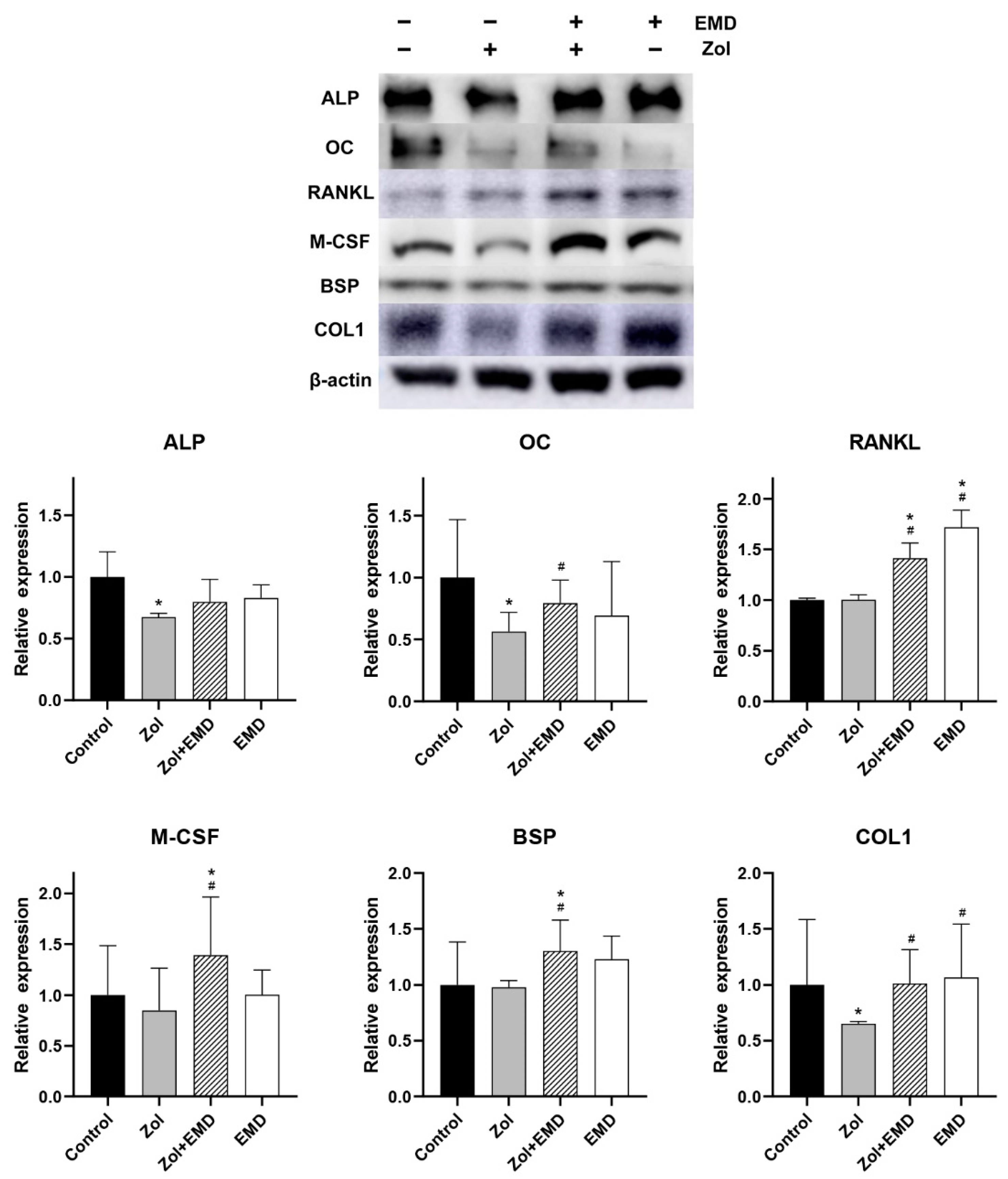
3.5. Protein Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawahara, M.; Kuroshima, S.; Sawase, T. Clinical considerations for medication-related osteonecrosis of the jaw: A comprehensive literature review. Int. J. Implant. Dent. 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.P.; Ward, L.C.; Stewart, G.O.; Will, R.K.; Criddle, R.A.; Prince, R.L.; Stuckey, B.G.; Dhaliwal, S.S.; Bhagat, C.I.; Retallack, R.W.; et al. A randomized clinical trial comparing oral alendronate and intravenous pamidronate for the treatment of Paget’s disease of bone. Bone 2004, 34, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R. Zoledronic acid to prevent skeletal complications in cancer: Corroborating the evidence. Cancer Treat. Rev. 2005, 31 (Suppl. 3), 19–25. [Google Scholar] [CrossRef]
- Coleman, R. Metastasis prevention with bone-targeted agents: A complex interaction between the microenvironment and tumour biology. J. Bone Min. Metab. 2023, 41, 290–300. [Google Scholar] [CrossRef]
- Mollica, V.; Rizzo, A.; Rosellini, M.; Marchetti, A.; Ricci, A.D.; Cimadamore, A.; Scarpelli, M.; Bonucci, C.; Andrini, E.; Errani, C.; et al. Bone Targeting Agents in Patients with Metastatic Prostate Cancer: State of the Art. Cancers 2021, 13, 546. [Google Scholar] [CrossRef]
- Fleisch, H. The role of bisphosphonates in breast cancer: Development of bisphosphonates. Breast Cancer Res. 2001, 4, 30. [Google Scholar] [CrossRef]
- Walter, C.; Klein, M.; Pabst, A.; Al-Nawas, B.; Duschner, H.; Ziebart, T. Influence of bisphosphonates on endothelial cells, fibroblasts, and osteogenic cells. Clin. Oral Investig. 2010, 14, 35–41. [Google Scholar] [CrossRef]
- Koch, F.P.; Yekta, S.S.; Merkel, C.; Ziebart, T.; Smeets, R. The impact of bisphosphonates on the osteoblast proliferation and Collagen gene expression in vitro. Head Face Med. 2010, 6, 12. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral. and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral. and Maxillofacial Surgeons’ Position. Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Pozzi, S.; Vallet, S.; Mukherjee, S.; Cirstea, D.; Vaghela, N.; Santo, L.; Rosen, E.; Ikeda, H.; Okawa, Y.; Kiziltepe, T. High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin. Cancer Res. 2009, 15, 5829–5839. [Google Scholar] [CrossRef]
- Basso, F.G.; Silveira Turrioni, A.P.; Hebling, J.; de Souza Costa, C.A. Zoledronic acid inhibits human osteoblast activities. Gerontology 2013, 59, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.J.; Niehoff, P.; Kimmig, B.; Wiltfang, J.; Açil, Y. Expression profile and synthesis of different collagen types I, II, III, and V of human gingival fibroblasts, osteoblasts, and SaOS-2 cells after bisphosphonate treatment. Clin. Oral Investig. 2010, 14, 51–58. [Google Scholar] [CrossRef]
- Patntirapong, S.; Singhatanadgit, W.; Chanruangvanit, C.; Lavanrattanakul, K.; Satravaha, Y. Zoledronic acid suppresses mineralization through direct cytotoxicity and osteoblast differentiation inhibition. J. Oral Pathol. Med. 2012, 41, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; Rojas, J.; Greig, I.R.; van’t Hof, R.J.; Ralston, S.H. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif. Tissue Int. 2008, 82, 191–201. [Google Scholar] [CrossRef]
- Giannasi, C.; Niada, S.; Farronato, D.; Lombardi, G.; Manfredi, B.; Farronato, G.; Brini, A.T. Nitrogen Containing Bisphosphonates Impair the Release of Bone Homeostasis Mediators and Matrix Production by Human Primary Pre-Osteoblasts. Int. J. Med. Sci. 2019, 16, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.B.; Herlofson, B.B.; Landin, M.A.; Reseland, J.E. Alendronate alters osteoblast activities. Acta Odontol. Scand. 2016, 74, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Komai, M.; Itoh, T.; Imano, M.; Sakamoto, K.; Shimaoka, H.; Takeda, T.; Ogawa, N.; Mashimo, K.; Fujiwara, D.; et al. Nitrogen-containing bisphosphonates inhibit RANKL- and M-CSF-induced osteoclast formation through the inhibition of ERK1/2 and Akt activation. J. Biomed. Sci. 2014, 21, 10. [Google Scholar] [CrossRef]
- Ibanez, L.; Nacher-Juan, J.; Terencio, M.C.; Ferrandiz, M.L.; Alcaraz, M.J. Osteostatin Inhibits M-CSF+RANKL-Induced Human Osteoclast Differentiation by Modulating NFATc1. Int. J. Mol. Sci. 2022, 23, 8551. [Google Scholar] [CrossRef]
- Jung, J.; Shim, G.-J.; Kim, M.; Yoon, Y.; Kim, J.-E.; Jue, S.-S.; Al-Nawas, B.; Kwon, Y.-D. Effect and timing of parathyroid hormone analog administration for preventing medication-related osteonecrosis of the jaws in a murine model. J. Cranio-Maxillofac. Surg. 2021, 49, 719–725. [Google Scholar]
- Ziebart, T.; Koch, F.; Klein, M.; Guth, J.; Adler, J.; Pabst, A.; Al-Nawas, B.; Walter, C. Geranylgeraniol–a new potential therapeutic approach to bisphosphonate associated osteonecrosis of the jaw. Oral Oncol. 2011, 47, 195–201. [Google Scholar] [CrossRef]
- Jung, J.; Park, J.S.; Chun, J.; Al-Nawas, B.; Ziebart, T.; Kwon, Y.D. Geranylgeraniol Application in Human Osteoblasts and Osteoclasts for Reversal of the Effect of Bisphosphonates. Life 2023, 13, 1353. [Google Scholar] [CrossRef]
- Stout, B.M.; Alent, B.J.; Pedalino, P.; Holbrook, R.; Gluhak-Heinrich, J.; Cui, Y.; Harris, M.A.; Gemperli, A.C.; Cochran, D.L.; Deas, D.E.; et al. Enamel matrix derivative: Protein components and osteoinductive properties. J. Periodontol. 2014, 85, e9–e17. [Google Scholar] [CrossRef]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35, 87–105. [Google Scholar] [CrossRef]
- Miron, R.J.; Chandad, F.; Buser, D.; Sculean, A.; Cochran, D.L.; Zhang, Y. Effect of Enamel Matrix Derivative Liquid on Osteoblast and Periodontal Ligament Cell Proliferation and Differentiation. J. Periodontol. 2016, 87, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, Y.; Xia, Q.; Meng, M.; Ye, Z.; Tang, Z.; Feng, H.; Chen, X.; Chen, H.; Zeng, X.; et al. Enamel matrix derivative (EMD) enhances the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs). Bioengineered 2021, 12, 7033–7045. [Google Scholar] [CrossRef]
- Song, Z.C.; Li, S.; Dong, J.C.; Sun, M.J.; Zhang, X.L.; Shu, R. Enamel matrix proteins regulate hypoxia-induced cellular biobehavior and osteogenic differentiation in human periodontal ligament cells. Biotech. Histochem. 2017, 92, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Park, J.S.; Righesso, L.; Pabst, A.M.; Al-Nawas, B.; Kwon, Y.D.; Walter, C. Effects of an oral bisphosphonate and three intravenous bisphosphonates on several cell types in vitro. Clin. Oral Investig. 2018, 22, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Misso, G.; Porru, M.; Stoppacciaro, A.; Castellano, M.; De Cicco, F.; Leonetti, C.; Santini, D.; Caraglia, M. Evaluation of the in vitro and in vivo antiangiogenic effects of denosumab and zoledronic acid. Cancer Biol. Ther. 2012, 13, 1491–1500. [Google Scholar] [CrossRef]
- Rodan, G.A.; Reszka, A.A. Bisphosphonate mechanism of action. Curr. Mol. Med. 2002, 2, 571–577. [Google Scholar] [CrossRef]
- Yang, Z. Small GTPases: Versatile signaling switches in plants. Plant Cell 2002, 14, S375–S388. [Google Scholar] [CrossRef]
- Zafar, S.; Coates, D.; Cullinan, M.; Drummond, B.; Milne, T.; Seymour, G. Effects of zoledronic acid and geranylgeraniol on the cellular behaviour and gene expression of primary human alveolar osteoblasts. Clin. Oral Investig. 2016, 20, 2023–2035. [Google Scholar] [CrossRef] [PubMed]
- Dunford, J.E.; Rogers, M.J.; Ebetino, F.H.; Phipps, R.J.; Coxon, F.P. Inhibition of protein prenylation by bisphosphonates causes sustained activation of Rac, Cdc42, and Rho GTPases. J. Bone Miner. Res. 2006, 21, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.; Xia, Z.; Barnett, B.; Triffitt, J.; Phipps, R.; Dunford, J.; Locklin, R.; Ebetino, F.; Russell, R. Differences between bisphosphonates in binding affinities for hydroxyapatite. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 149–155. [Google Scholar] [CrossRef]
- Otto, S.; Pautke, C.; Opelz, C.; Westphal, I.; Drosse, I.; Schwager, J.; Bauss, F.; Ehrenfeld, M.; Schieker, M. Osteonecrosis of the jaw: Effect of bisphosphonate type, local concentration, and acidic milieu on the pathomechanism. J. Oral Maxillofac. Surg. 2010, 68, 2837–2845. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Z.; Wang, L.; Jin, X.; Shen, Y.; Nan, C.; Liu, H. Minimally effective concentration of zoledronic acid to suppress osteoclasts in vitro. Exp. Ther. Med. 2018, 15, 5330–5336. [Google Scholar] [PubMed]
- Walter, C.; Pabst, A.; Ziebart, T.; Klein, M.; Al-Nawas, B. Bisphosphonates affect migration ability and cell viability of HUVEC, fibroblasts and osteoblasts in vitro. Oral Dis. 2011, 17, 194–199. [Google Scholar] [CrossRef]
- Sculean, A.; Windisch, P.; Keglevich, T.; Fabi, B.; Lundgren, E.; Lyngstadaas, P. Presence of an enamel matrix protein derivative on human teeth following periodontal surgery. Clin. Oral Investig. 2002, 6, 183–187. [Google Scholar] [CrossRef]
- Lyngstadaas, S.P.; Lundberg, E.; Ekdahl, H.; Andersson, C.; Gestrelius, S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J. Clin. Periodontol. 2001, 28, 181–188. [Google Scholar] [CrossRef]
- Miron, R.J.; Wei, L.; Bosshardt, D.D.; Buser, D.; Sculean, A.; Zhang, Y. Effects of enamel matrix proteins in combination with a bovine-derived natural bone mineral for the repair of bone defects. Clin. Oral Investig. 2014, 18, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Chen, C.L.; Lin, M.T. Enamel matrix derivative exhibits angiogenic effect in vitro and in a murine model. J. Clin. Periodontol. 2003, 30, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nagano, T.; Yamakoshi, Y.; Gomi, K.; Arai, T.; Fukae, M.; Katagiri, T.; Oida, S. Enamel matrix derivative gel stimulates signal transduction of BMP and TGF-β. J. Dent. Res. 2005, 84, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Kulakauskiene, R.; Aukstakalnis, R.; Sadzeviciene, R. Enamel matrix derivate induces periodontal regeneration by activating growth factors: A review. Stomatologija 2020, 22, 49–53. [Google Scholar] [PubMed]
- Wyganowska-Swiatkowska, M.; Urbaniak, P.; Nohawica, M.M.; Kotwicka, M.; Jankun, J. Enamel matrix proteins exhibit growth factor activity: A review of evidence at the cellular and molecular levels. Exp. Ther. Med. 2015, 9, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.; Mundy, G. Role of transforming growth factor-beta in bone remodeling. Clin. Orthop. Relat. Res. 1990, 250, 261–276. [Google Scholar] [CrossRef]
- Schwartz, Z.; Carnes, D.L., Jr.; Pulliam, R.; Lohmann, C.H.; Sylvia, V.L.; Liu, Y.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Porcine fetal enamel matrix derivative stimulates proliferation but not differentiation of pre-osteoblastic 2T9 cells, inhibits proliferation and stimulates differentiation of osteoblast-like MG63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J. Periodontol. 2000, 71, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Caluseru, O.M.; Guillemette, V.; Zhang, Y.; Gemperli, A.C.; Chandad, F.; Sculean, A. Influence of enamel matrix derivative on cells at different maturation stages of differentiation. PLoS ONE 2013, 8, e71008. [Google Scholar]
- Miron, R.J.; Saulacic, N.; Buser, D.; Iizuka, T.; Sculean, A. Osteoblast proliferation and differentiation on a barrier membrane in combination with BMP2 and TGFβ1. Clin. Oral Investig. 2013, 17, 981–988. [Google Scholar] [CrossRef]
- Zeldich, E.; Koren, R.; Dard, M.; Nemcovsky, C.; Weinreb, M. Enamel matrix derivative protects human gingival fibroblasts from TNF-induced apoptosis by inhibiting caspase activation. J. Cell. Physiol. 2007, 213, 750–758. [Google Scholar] [CrossRef]
- He, J.; King, Y.; Jiang, J.; Safavi, K.E.; Spångberg, L.S.; Zhu, Q. Enamel matrix derivative inhibits TNF-α–induced apoptosis in osteoblastic MC3T3-E1 cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 761–767. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Zhang, Y.; Caballé-Serrano, J.; Shirakata, Y.; Bosshardt, D.D.; Buser, D.; Sculean, A. Osteogain improves osteoblast adhesion, proliferation and differentiation on a bovine-derived natural bone mineral. Clin. Oral Implant. Res. 2017, 28, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Bertl, K.; An, N.; Bruckmann, C.; Dard, M.; Andrukhov, O.; Matejka, M.; Rausch-Fan, X. Effects of enamel matrix derivative on proliferation/viability, migration, and expression of angiogenic factor and adhesion molecules in endothelial cells in vitro. J. Periodontol. 2009, 80, 1622–1630. [Google Scholar] [CrossRef]
- Karima, M.M.; Van Dyke, T.E. Enamel matrix derivative promotes superoxide production and chemotaxis but reduces matrix metalloproteinase-8 expression by polymorphonuclear leukocytes. J. Periodontol. 2012, 83, 780–786. [Google Scholar] [CrossRef]
- Hagewald, S.; Pischon, N.; Jawor, P.; Bernimoulin, J.P.; Zimmermann, B. Effects of enamel matrix derivative on proliferation and differentiation of primary osteoblasts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 243–249. [Google Scholar] [CrossRef]
- He, J.; Jiang, J.; Safavi, K.E.; Spangberg, L.S.; Zhu, Q. Emdogain promotes osteoblast proliferation and differentiation and stimulates osteoprotegerin expression. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, P.; Bernimoulin, J.P.; Trackman, P.; Hagewald, S. Stimulation of osteoblasts with Emdogain increases the expression of specific mineralization markers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 304–308. [Google Scholar] [CrossRef]
- Yoneda, S. The effects of enamel matrix derivative (EMD) on osteoblastic cells. Kokubyo Gakkai Zasshi 2002, 69, 207–214. [Google Scholar] [CrossRef]
- Miron, R.J.; Hedbom, E.; Ruggiero, S.; Bosshardt, D.D.; Zhang, Y.; Mauth, C.; Gemperli, A.C.; Iizuka, T.; Buser, D.; Sculean, A. Premature osteoblast clustering by enamel matrix proteins induces osteoblast differentiation through up-regulation of connexin 43 and N-cadherin. PLoS ONE 2011, 6, e23375. [Google Scholar] [CrossRef]
- Lossdörfer, S.; Sun, M.; Götz, W.; Dard, M.; Jäger, A. Enamel matrix derivative promotes human periodontal ligament cell differentiation and osteoprotegerin production in vitro. J. Dent. Res. 2007, 86, 980–985. [Google Scholar] [CrossRef]
- Gillet, J.P.; Varma, S.; Gottesman, M.M. The clinical relevance of cancer cell lines. J. Natl. Cancer Inst. 2013, 105, 452–458. [Google Scholar] [CrossRef]
- Aimetti, M.; Stasikelyte, M.; Mariani, G.M.; Cricenti, L.; Baima, G.; Romano, F. The flapless approach with and without enamel matrix derivatives for the treatment of intrabony defects: A randomized controlled clinical trial. J. Clin. Periodontol. 2024, 51, 1112–1121. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Tamagnone, L.; Roffredo, A.; Costanzo, L. The Use of Enamel Matrix Derivative to Modulate Wound Healing of Peri-implant Soft Tissues. Int. J. Periodontics Restor. Dent. 2024, 44, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Koronna, I.; Schacher, B.; Dahmer, I.; Nickles, K.; Sonnenschein, S.K.; Kim, T.S.; Eickholz, P.; Petsos, H. Long-term stability of infrabony defects treated with enamel matrix derivative alone: A retrospective two-centre cohort study. J. Clin. Periodontol. 2023, 50, 996–1009. [Google Scholar] [CrossRef]
- Sim, I.W.; Borromeo, G.L.; Tsao, C.; Hardiman, R.; Hofman, M.S.; Papatziamos Hjelle, C.; Siddique, M.; Cook, G.J.R.; Seymour, J.F.; Ebeling, P.R. Teriparatide Promotes Bone Healing in Medication-Related Osteonecrosis of the Jaw: A Placebo-Controlled, Randomized Trial. J. Clin. Oncol. 2020, 38, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Colapinto, G.; Goker, F.; Nocini, R.; Albanese, M.; Nocini, P.F.; Sembronio, S.; Argenta, F.; Robiony, M.; Del Fabbro, M. Outcomes of a Pharmacological Protocol with Pentoxifylline and Tocopherol for the Management of Medication-Related Osteonecrosis of the Jaws (MRONJ): A Randomized Study on 202 Osteoporosis Patients. J. Clin. Med. 2023, 12, 4662. [Google Scholar] [CrossRef] [PubMed]


| Gene | 5′→3′ | |
|---|---|---|
| ALP | F | GACAAGAAGCCCTTCACTGC |
| R | AGACTGCGCCTGGTAGTTGT | |
| OC | F | GGCGCTACCTGTATCAATGG |
| R | TCAGCCAACTCGTCACAGTC | |
| RANKL | F | CACTATTAATGCCACCGAC |
| R | GGGTATGAGAACTTGGGATT | |
| M-CSF | F | TAGCCACATGATTGGGAGTG |
| R | TATCTCTGAAGCGCATGGTG | |
| BSP | F | GCGAAGCAGAAGTGGATGAAA |
| R | TGCCTCTGTGCTGTTGGTACTG | |
| COL1 | F | CCTGGTGCTAAAGGAGAAAGAGG |
| R | ATCACCACGACTTCCAGCAGGA | |
| GAPDH | F | AACAGCGACACCCACTCCTC |
| R | CATACCAGGAAATGAGCTTGACAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Choi, M.; Kwon, Y.-D.; Ohe, J.-Y.; Jung, J. The Potential of Enamel Matrix Derivative in Countering Bisphosphonate-Induced Effects in Osteoblasts. Life 2024, 14, 1088. https://doi.org/10.3390/life14091088
Kim M, Choi M, Kwon Y-D, Ohe J-Y, Jung J. The Potential of Enamel Matrix Derivative in Countering Bisphosphonate-Induced Effects in Osteoblasts. Life. 2024; 14(9):1088. https://doi.org/10.3390/life14091088
Chicago/Turabian StyleKim, Minah, Minji Choi, Yong-Dae Kwon, Joo-Young Ohe, and Junho Jung. 2024. "The Potential of Enamel Matrix Derivative in Countering Bisphosphonate-Induced Effects in Osteoblasts" Life 14, no. 9: 1088. https://doi.org/10.3390/life14091088






