Multiplex-PCR Detection of Clostridium tyrobutyricum, Clostridium butyricum, and Clostridium sporogenes in Raw Milk for Cheesemaking
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling
2.2. Strain Growth Condition and Spiking Tests
2.3. Enrichment of Milk Samples
2.4. DNA Extraction and Purification
2.5. Amplification
3. Results
3.1. Spiked Samples
3.2. Dairy Plant Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carminati, D.; Bonvini, B.; Francolino, S.; Ghiglietti, R.; Locci, F.; Tidona, F.; Mariut, M.; Abeni, F.; Zago, M.; Giraffa, G. Low-Level Clostridial Spores’ Milk to Limit the Onset of Late Blowing Defect in Lysozyme-Free, Grana-Type Cheese. Foods 2023, 12, 1880. [Google Scholar] [CrossRef] [PubMed]
- Chojecka, A. Susceptibility of Clostridium sporogenes Spores to Selected Reference Substances and Disinfectants. Pol. J. Microbiol. 2022, 71, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Borreani, G.; Ferrero, F.; Nucera, D.; Casale, M.; Piano, S.; Tabacco, E. Dairy Farm Management Practices and the Risk of Contamination of Tank Milk from Clostridium spp. and Paenibacillus spp. Spores in Silage, Total Mixed Ration, Dairy Cow Feces, and Raw Milk. J. Dairy Sci. 2019, 102, 8273–8289. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Torres, N.; Garde, S.; Peirotén, Á.; Ávila, M. Impact of Clostridium spp. on Cheese Characteristics: Microbiology, Color, Formation of Volatile Compounds and off-Flavors. Food Control 2015, 56, 186–194. [Google Scholar] [CrossRef]
- Brändle, J.; Domig, K.J.; Kneifel, W. Relevance and Analysis of Butyric Acid Producing Clostridia in Milk and Cheese. Food Control 2016, 67, 96–113. [Google Scholar] [CrossRef]
- Porcellato, D.; Kristiansen, H.; Finton, M.D.; Leanti La Rosa, S.; da Silva Duarte, V.; Skeie, S.B. Composition and Fate of Heat-Resistant Anaerobic Spore-Formers in the Milk Powder Production Line. Int. J. Food Microbiol. 2023, 402, 110281. [Google Scholar] [CrossRef]
- Doyle, C.J.; Gleeson, D.; Jordan, K.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. Anaerobic Sporeformers and Their Significance with Respect to Milk and Dairy Products. Int. J. Food Microbiol. 2015, 197, 77–87. [Google Scholar] [CrossRef]
- Driehuis, F.; Wilkinson, J.M.; Jiang, Y.; Ogunade, I.; Adesogan, A.T. Silage Review: Animal and Human Health Risks from Silage. J. Dairy Sci. 2018, 101, 4093–4110. [Google Scholar] [CrossRef]
- Abeni, F.; Marino, R.; Petrera, F.; Segati, G.; Galli, A.; Carminati, D. Farm Silage Facilities and Their Management for the Prevention of Anaerobic Bacteria Spore Contamination in Raw Milk. Dairy 2021, 2, 500–514. [Google Scholar] [CrossRef]
- Bermúdez, J.; González, M.J.; Olivera, J.A.; Burgueño, J.A.; Juliano, P.; Fox, E.M.; Reginensi, S.M. Seasonal Occurrence and Molecular Diversity of Clostridia Species Spores along Cheesemaking Streams of 5 Commercial Dairy Plants. J. Dairy Sci. 2016, 99, 3358–3366. [Google Scholar] [CrossRef]
- Goldsztejn, M.; Grenda, T.; Kozieł, N.; Sapała, M.; Mazur, M.; Sieradzki, Z.; Król, B.; Kwiatek, K. Potential Determinants of Clostridium spp. Occurrence in Polish Silage. J. Vet. Res. 2020, 64, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, M.; Fontana, A.; Daza, M.V.B.; Bassi, D.; Gallo, A. Clostridium tyrobutyricum Occurrence in Silages and Cattle Feed: Use of Molecular and Simulation Data to Optimize Predictive Models. Front. Microbiol. 2023, 14, 1118646. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Martin, N.H.; Wiedmann, M.; Trmčić, A. Development of a Risk Assessment Model to Predict the Occurrence of Late Blowing Defect in Gouda Cheese and Evaluate Potential Intervention Strategies. J. Dairy Sci. 2022, 105, 2880–2894. [Google Scholar] [CrossRef] [PubMed]
- Fernández García, L.; Álvarez Blanco, S.; Riera Rodríguez, F.A. Microfiltration Applied to Dairy Streams: Removal of Bacteria. J. Sci. Food Agric. 2013, 93, 187–196. [Google Scholar] [CrossRef]
- Silvetti, T.; Morandi, S.; Brasca, M. Growth Factors Affecting Gas Production and Reduction Potential of Vegetative Cell and Spore Inocula of Dairy-Related Clostridium Species. LWT 2018, 92, 32–39. [Google Scholar] [CrossRef]
- Garde, S.; Arias, R.; Gaya, P.; Nuñez, M. Occurrence of Clostridium spp. in Ovine Milk and Manchego Cheese with Late Blowing Defect: Identification and Characterization of Isolates. Int. Dairy J. 2011, 21, 272–278. [Google Scholar] [CrossRef]
- Carmen Martínez-Cuesta, M.; Bengoechea, J.; Bustos, I.; Rodríguez, B.; Requena, T.; Peláez, C. Control of Late Blowing in Cheese by Adding Lacticin 3147-Producing Lactococcus Lactis IFPL 3593 to the Starter. Int. Dairy J. 2010, 20, 18–24. [Google Scholar] [CrossRef]
- Rilla, N. Inhibition of Clostridium tyrobutyricum in Vidiago Cheese by Lactococcus lactis ssp. lactis IPLA 729, a Nisin Z Producer. Int. J. Food Microbiol. 2003, 85, 23–33. [Google Scholar] [CrossRef]
- Hassan, H.; St-Gelais, D.; Gomaa, A.; Fliss, I. Impact of Nisin and Nisin-Producing Lactococcus lactis ssp. lactis on Clostridium tyrobutyricum and Bacterial Ecosystem of Cheese Matrices. Foods 2021, 10, 898. [Google Scholar] [CrossRef]
- Anastasiou, R.; Aktypis, A.; Georgalaki, M.; Papadelli, M.; De Vuyst, L.; Tsakalidou, E. Inhibition of Clostridium tyrobutyricum by Streptococcus Macedonicus ACA-DC 198 under Conditions Mimicking Kasseri Cheese Production and Ripening. Int. Dairy J. 2009, 19, 330–335. [Google Scholar] [CrossRef]
- Ávila, M.; Calzada, J.; Muñoz-Tébar, N.; Sánchez, C.; Ortiz de Elguea-Culebras, G.; Carmona, M.; Molina, A.; Berruga, M.I.; Garde, S. Inhibitory Activity of Aromatic Plant Extracts against Dairy-Related Clostridium Species and Their Use to Prevent the Late Blowing Defect of Cheese. Food Microbiol. 2023, 110, 104185. [Google Scholar] [CrossRef] [PubMed]
- Ávila, M.; Sánchez, C.; Calzada, J.; Mayer, M.J.; Berruga, M.I.; López-Díaz, T.M.; Narbad, A.; Garde, S. Isolation and Characterization of New Bacteriophages Active against Clostridium tyrobutyricum and Their Role in Preventing the Late Blowing Defect of Cheese. Food Res. Int. 2023, 163, 112222. [Google Scholar] [CrossRef] [PubMed]
- Peruzy, M.F.; Blaiotta, G.; Aponte, M.; De Sena, M.; Murru, N. Late Blowing Defect in Grottone Cheese: Detection of Clostridia and Control Strategies. Ital. J. Food Saf. 2022, 11, 10162. [Google Scholar] [CrossRef] [PubMed]
- Brändle, J.; Fraberger, V.; Schuller, K.; Zitz, U.; Kneifel, W.; Domig, K.J. A Critical Assessment of Four Most Probable Number Procedures for Routine Enumeration of Cheese-Damaging Clostridia in Milk. Int. Dairy J. 2017, 73, 109–115. [Google Scholar] [CrossRef]
- Burtscher, J.; Rudavsky, T.; Zitz, U.; Domig, K.J. Specificity of the AMP-6000 Method for Enumerating Clostridium Endospores in Milk. Foods 2024, 13, 1192. [Google Scholar] [CrossRef] [PubMed]
- Morandi, S.; Cremonesi, P.; Silvetti, T.; Castiglioni, B.; Brasca, M. Development of a Triplex Real-Time PCR Assay for the Simultaneous Detection of Clostridium beijerinckii, Clostridium sporogenes and Clostridium tyrobutyricum in Milk. Anaerobe 2015, 34, 44–49. [Google Scholar] [CrossRef]
- Cremonesi, P.; Vanoni, L.; Silvetti, T.; Morandi, S.; Brasca, M. Identification of Clostridium beijerinckii, Cl. butyricum, Cl. sporogenes, Cl. tyrobutyricum Isolated from Silage, Raw Milk and Hard Cheese by a Multiplex PCR Assay. J. Dairy Res. 2012, 79, 318–323. [Google Scholar] [CrossRef]
- Cocolin, L.; Innocente, N.; Biasutti, M.; Comi, G. The Late Blowing in Cheese: A New Molecular Approach Based on PCR and DGGE to Study the Microbial Ecology of the Alteration Process. Int. J. Food Microbiol. 2004, 90, 83–91. [Google Scholar] [CrossRef]
- Le Bourhis, A.-G.; Doré, J.; Carlier, J.-P.; Chamba, J.-F.; Popoff, M.-R.; Tholozan, J.-L. Contribution of C. Beijerinckii and C. Sporogenes in Association with C. Tyrobutyricum to the Butyric Fermentation in Emmental Type Cheese. Int. J. Food Microbiol. 2007, 113, 154–163. [Google Scholar] [CrossRef]
- Cecere, P.; Gatto, F.; Cortimiglia, C.; Bassi, D.; Lucchini, F.; Cocconcelli, P.S.; Pompa, P.P. Colorimetric Point-of-Care Detection of Clostridium tyrobutyricum Spores in Milk Samples. Biosensors 2021, 11, 293. [Google Scholar] [CrossRef]
- Feligini, M.; Brambati, E.; Panelli, S.; Ghitti, M.; Sacchi, R.; Capelli, E.; Bonacina, C. One-Year Investigation of Clostridium spp. Occurrence in Raw Milk and Curd of Grana Padano Cheese by the Automated Ribosomal Intergenic Spacer Analysis. Food Control 2014, 42, 71–77. [Google Scholar] [CrossRef]
- Barbiero, D.; Melison, F.; Cocola, L.; Fedel, M.; Andrighetto, C.; Dea, P.D.; Poletto, L. Raman Spectroscopy Applied to Early Detection of Clostridium Infection in Milk. Appl. Spectrosc. 2024, 00037028241252693. [Google Scholar] [CrossRef]
- Cremonesi, P.; Castiglioni, B.; Malferrari, G.; Biunno, I.; Vimercati, C.; Moroni, P.; Morandi, S.; Luzzana, M. Technical Note: Improved Method for Rapid DNA Extraction of Mastitis Pathogens Directly from Milk. J. Dairy Sci. 2006, 89, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.; Marcos, P.; Horna, C.; Galan-Malo, P.; Mata, L.; Pérez, M.D.; Calvo, M.; Sánchez, L. Evaluation of Methods for DNA Extraction from Clostridium tyrobutyricum Spores and Its Detection by qPCR. J. Microbiol. Methods 2020, 169, 105818. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Küller, F.; Dreier, M.; Arias-Roth, E.; Drissner, D.; Domig, K.J. Characterization of Clostridium tyrobutyricum Strains Using Three Different Typing Techniques. Microorganisms 2020, 8, 1057. [Google Scholar] [CrossRef]
- Podrzaj, L.; Burtscher, J.; Domig, K.J. Comparative Genomics Provides Insights Into Genetic Diversity of Clostridium tyrobutyricum and Potential Implications for Late Blowing Defects in Cheese. Front. Microbiol. 2022, 13, 889551. [Google Scholar] [CrossRef]
- Bassi, D.; Fontana, C.; Zucchelli, S.; Gazzola, S.; Cocconcelli, P.S. TaqMan Real Time-Quantitative PCR Targeting the Phosphotransacetylase Gene for Clostridium tyrobutyricum Quantification in Animal Feed, Faeces, Milk and Cheese. Int. Dairy J. 2013, 33, 75–82. [Google Scholar] [CrossRef]
- Finton, M.; Skeie, S.B.; Aspholm, M.E.; Franklin-Alming, F.V.; Mekonnen, Y.B.; Kristiansen, H.; Porcellato, D. Two-Year Investigation of Spore-Formers through the Production Chain at Two Cheese Plants in Norway. Food Res. Int. 2024, 190, 114610. [Google Scholar] [CrossRef]
- Brändle, J.; Fraberger, V.; Berta, J.; Puglisi, E.; Jami, M.; Kneifel, W.; Domig, K.J. Butyric Acid Producing Clostridia in Cheese—Towards the Completion of Knowledge by Means of an Amalgamate of Methodologies. Int. Dairy J. 2018, 86, 86–95. [Google Scholar] [CrossRef]
- Reindl, A.; Dzieciol, M.; Hein, I.; Wagner, M.; Zangerl, P. Enumeration of Clostridia in Goat Milk Using an Optimized Membrane Filtration Technique. J. Dairy Sci. 2014, 97, 6036–6045. [Google Scholar] [CrossRef]
| Dairy Plant | Protocol 1 | Protocol 2 |
|---|---|---|
| A | 101 | 50 |
| B | 10 | 11 |
| C | 45 | 16 |
| D | 55 | 40 |
| total | 211 | 117 |
| Primers | Gene Target | Primer Sequence (5′-3′) | Amplification Length (bp) |
|---|---|---|---|
| Cl-SPOR-F3031 | colA | TTGGGATTTTGGGGATAACA | 549 |
| Cl-SPOR-R3579 | colA | TCCGTATCGTTGTCGTCTTG | |
| Cl-BUTY-F1329 | hydA | ATGGGTTAGGCAAGCAGAAA | 321 |
| Cl-BUTY-R1640 | hydA | GCTGGATCTGCCTTCTCATC | |
| Cl-TYRO-F1253 | enr | TGGTGTTCCACAAGAAGCTG | 210 |
| Cl-TYRO-R1462 | enr | GCAGCTGGATTTACTGCACA |
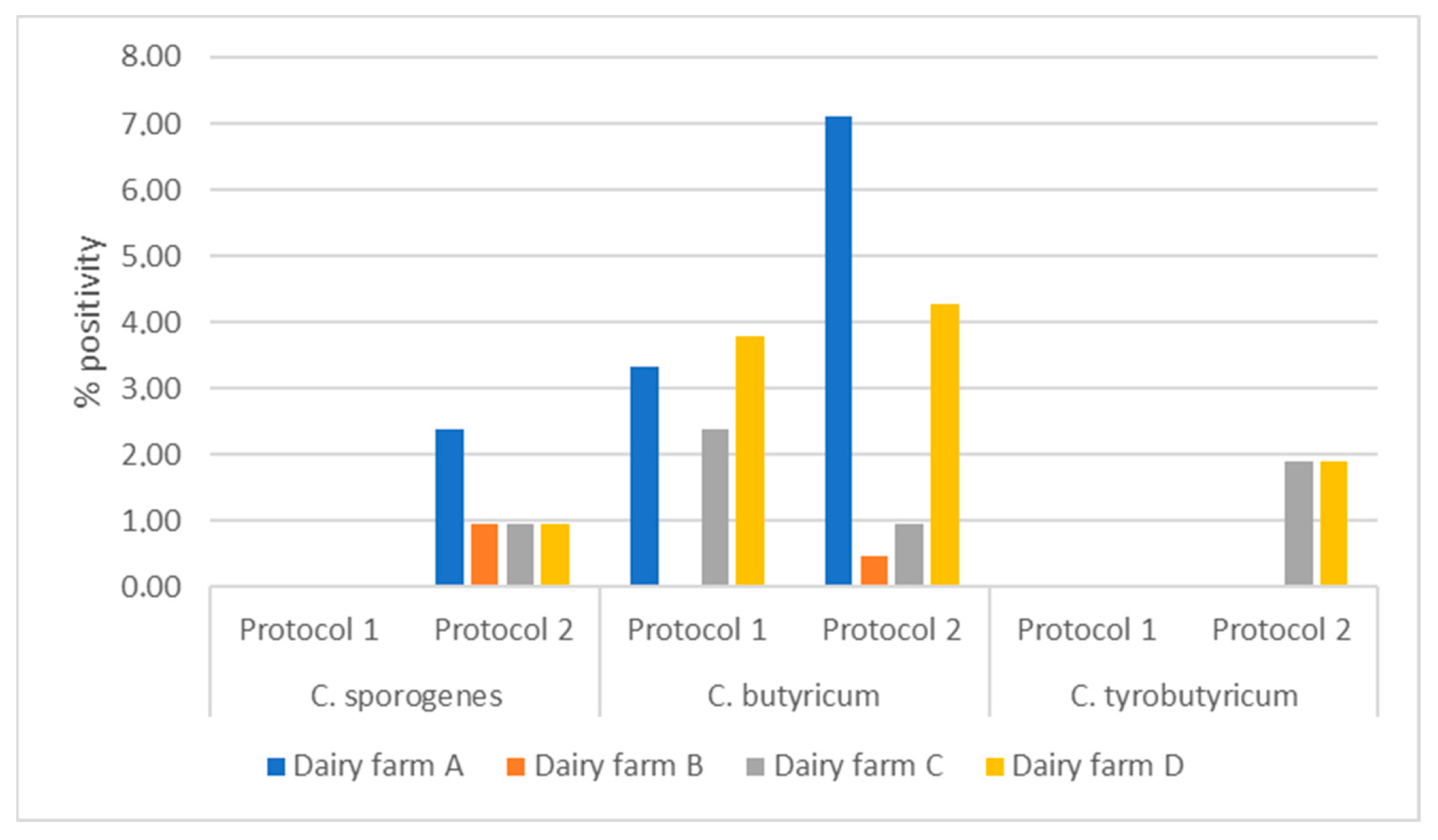
| Dairy Plant | Samples—No. | Samples Positive for C. sporogenes—No. (%) | Samples Positive for C. butyricum—No. (%) | Samples Positive for C. tyrobutyricum—No. (%) |
|---|---|---|---|---|
| Dairy plant A | 16 | 2 (12.5) | 2 (12.5) | 4 (25) |
| Dairy plant B | 11 | 2 (18.2) | 1 (9) | / |
| Dairy plant C | 50 | 5 (10) | 15 (30) | / |
| Dairy plant D | 40 | 2 (5) | 9 (22.5) | 4 (10) |
| TOTAL | 117 | 11 (9.4) | 27 (23) | 8 (6.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floris, I.; Martucci, F.; Romano, A.; Marello, G.; Ligotti, C.; Bianchi, D.M. Multiplex-PCR Detection of Clostridium tyrobutyricum, Clostridium butyricum, and Clostridium sporogenes in Raw Milk for Cheesemaking. Life 2024, 14, 1093. https://doi.org/10.3390/life14091093
Floris I, Martucci F, Romano A, Marello G, Ligotti C, Bianchi DM. Multiplex-PCR Detection of Clostridium tyrobutyricum, Clostridium butyricum, and Clostridium sporogenes in Raw Milk for Cheesemaking. Life. 2024; 14(9):1093. https://doi.org/10.3390/life14091093
Chicago/Turabian StyleFloris, Irene, Francesca Martucci, Angelo Romano, Giuseppina Marello, Carmela Ligotti, and Daniela Manila Bianchi. 2024. "Multiplex-PCR Detection of Clostridium tyrobutyricum, Clostridium butyricum, and Clostridium sporogenes in Raw Milk for Cheesemaking" Life 14, no. 9: 1093. https://doi.org/10.3390/life14091093
APA StyleFloris, I., Martucci, F., Romano, A., Marello, G., Ligotti, C., & Bianchi, D. M. (2024). Multiplex-PCR Detection of Clostridium tyrobutyricum, Clostridium butyricum, and Clostridium sporogenes in Raw Milk for Cheesemaking. Life, 14(9), 1093. https://doi.org/10.3390/life14091093







