Impact of Nebulized BromAc® on Mucus Plug Clearance in a Mechanically Ventilated Ex Vivo Ovine Lung Model of Obstructive Respiratory Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Preparation of Drugs (Bromelain, AC, and BromAc®)
2.3. Preparation of Mucus Simulant
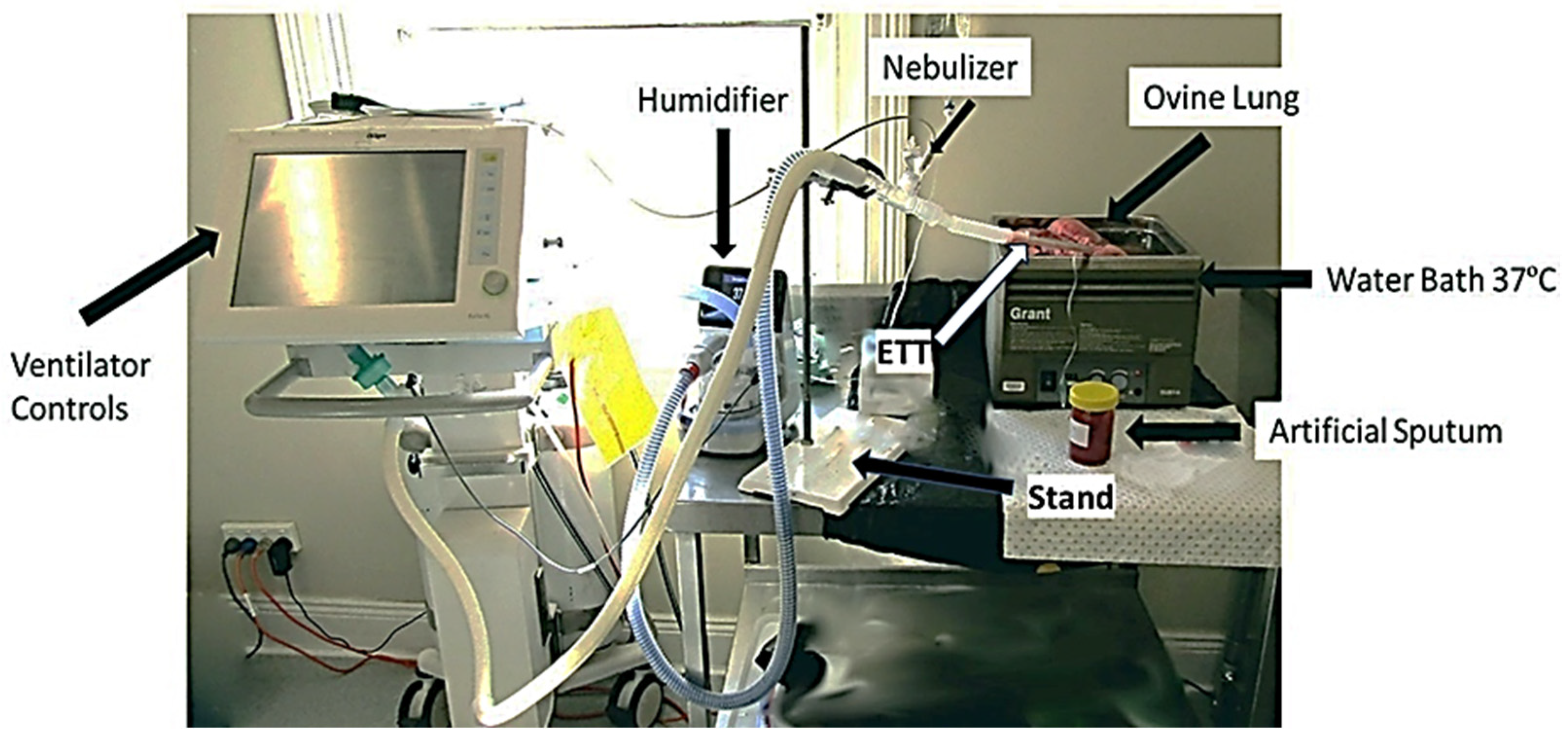
2.4. Ventilator Settings and Measurements
2.5. Mucus Simulant Administration and Nebulization of Drugs in Ovine Lung
2.6. Viscosity Measurements
2.7. Statistical Analysis
3. Results
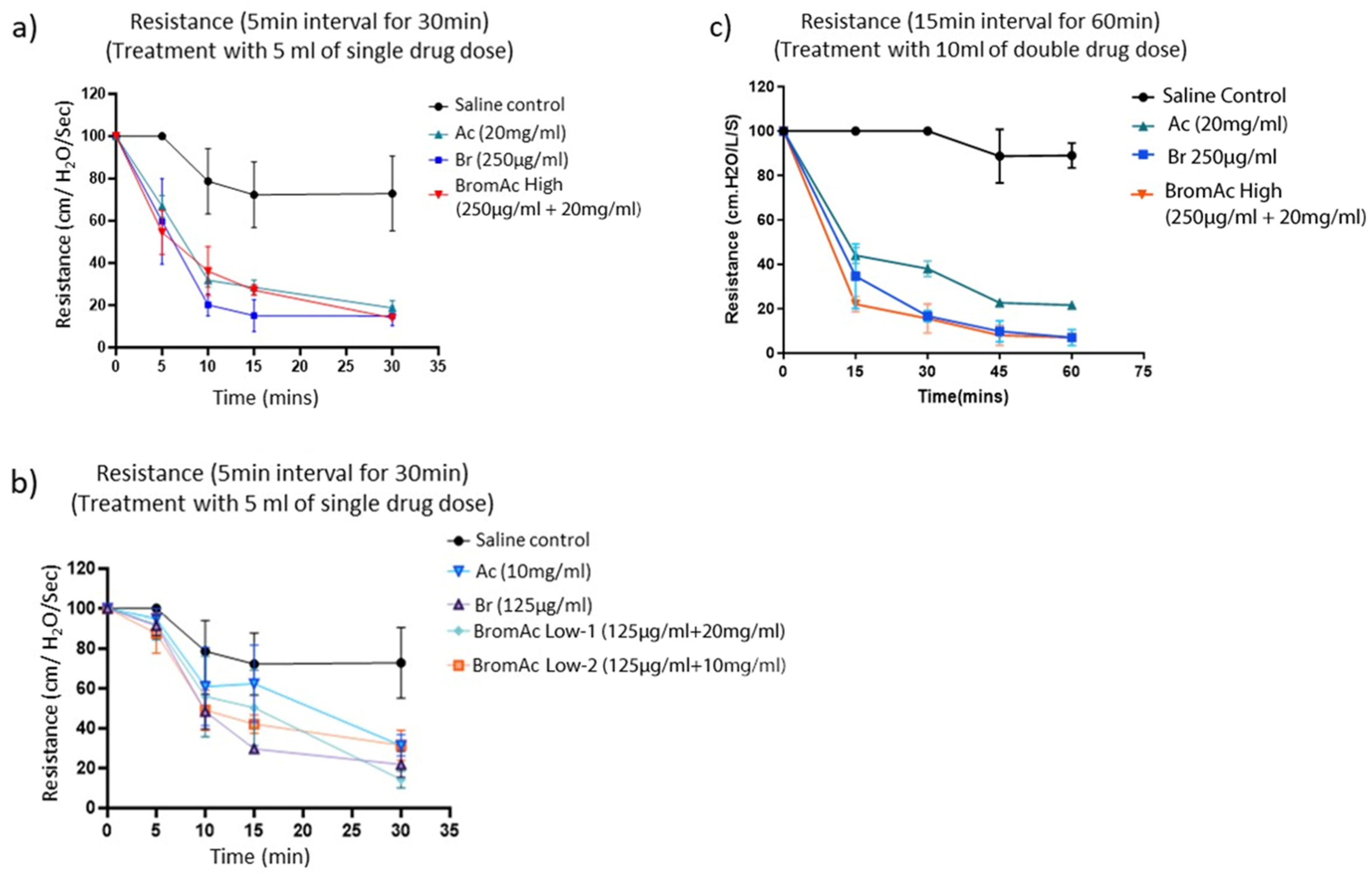
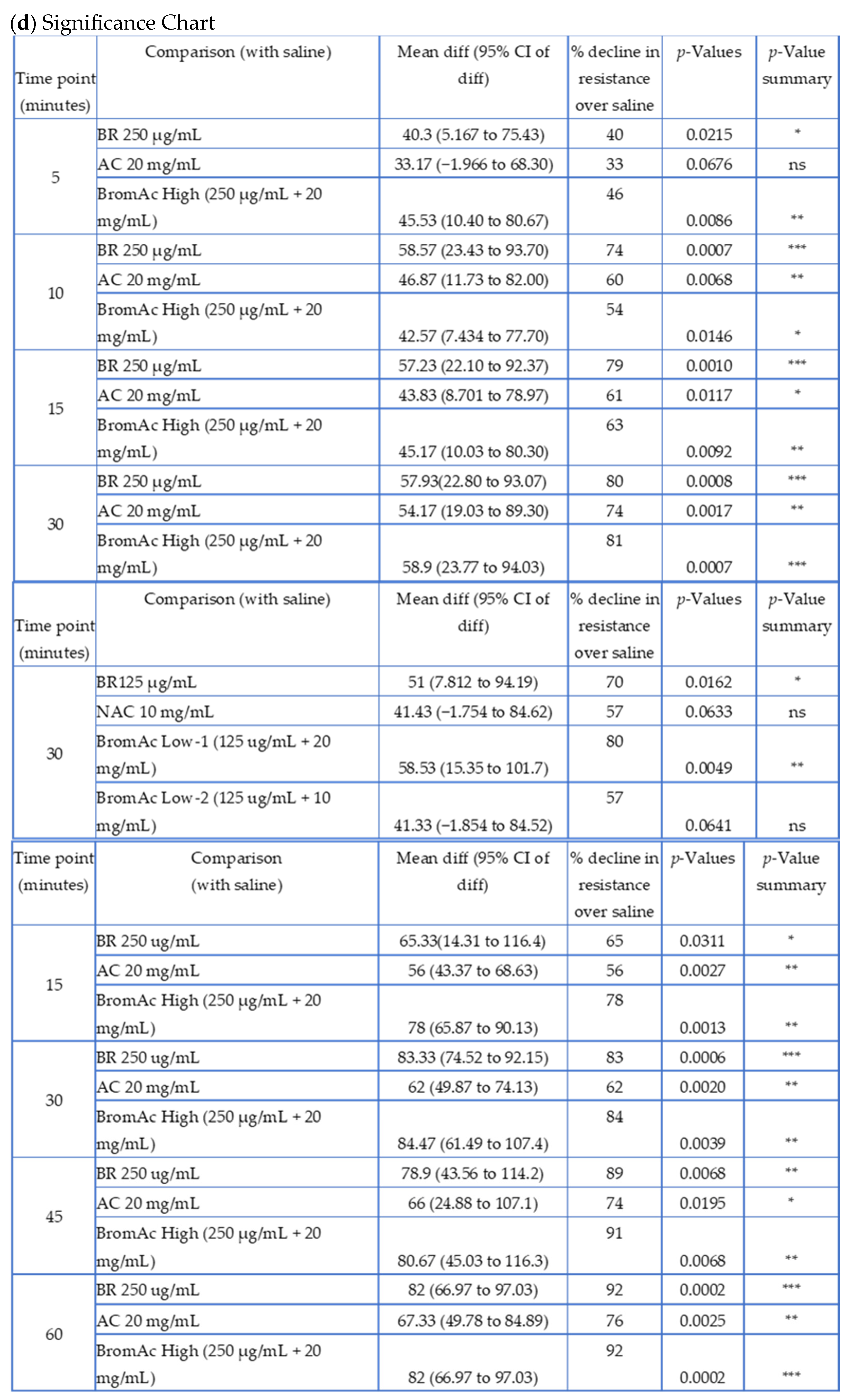
3.1. Effect of Bromelain, Ac, and BromAc on Ventilatory Resistance
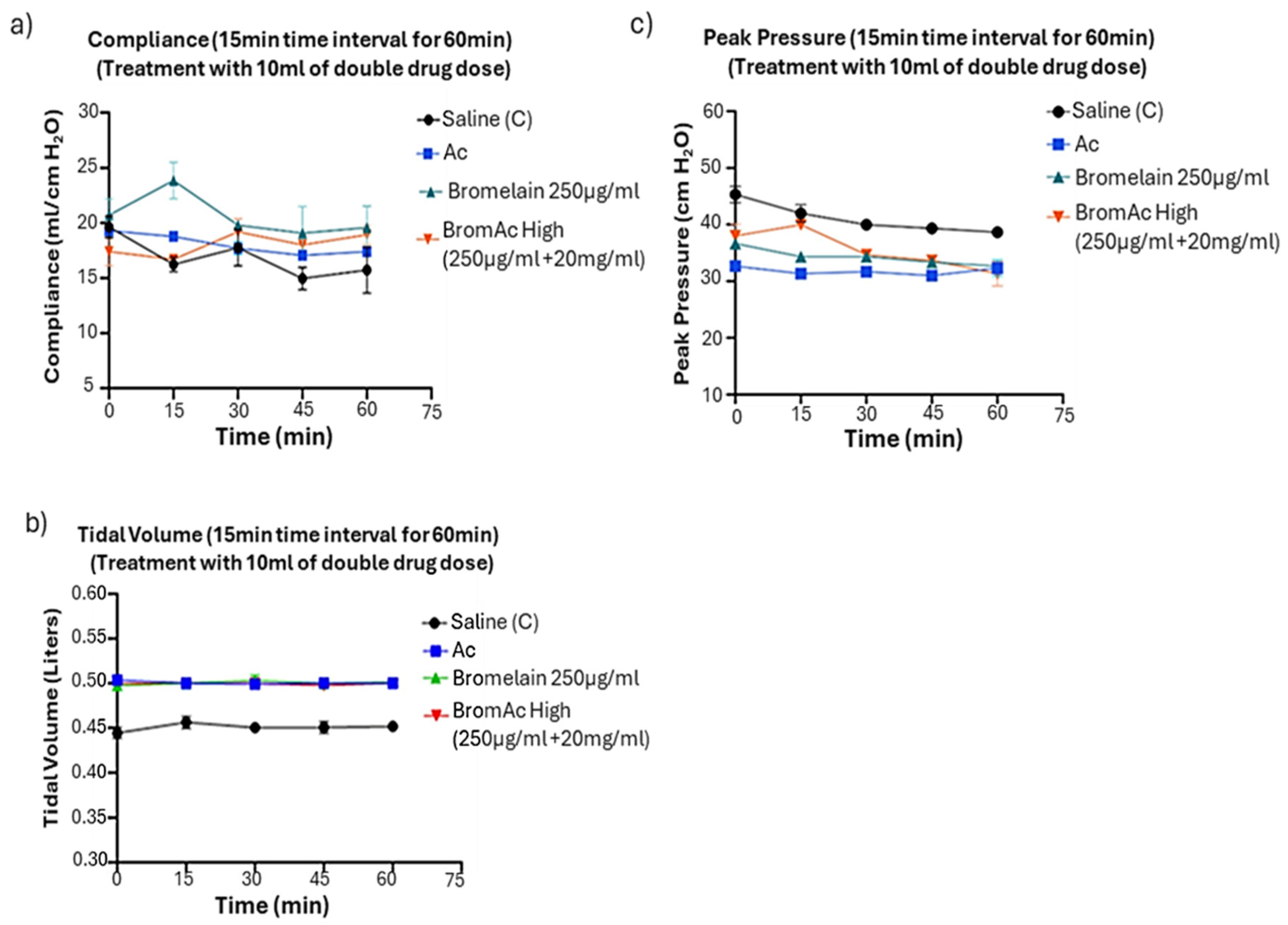
3.2. Effect of Bromelain, Ac, and BromAc on Ventilatory Compliance, Tidal Volume, and Peak Pressure
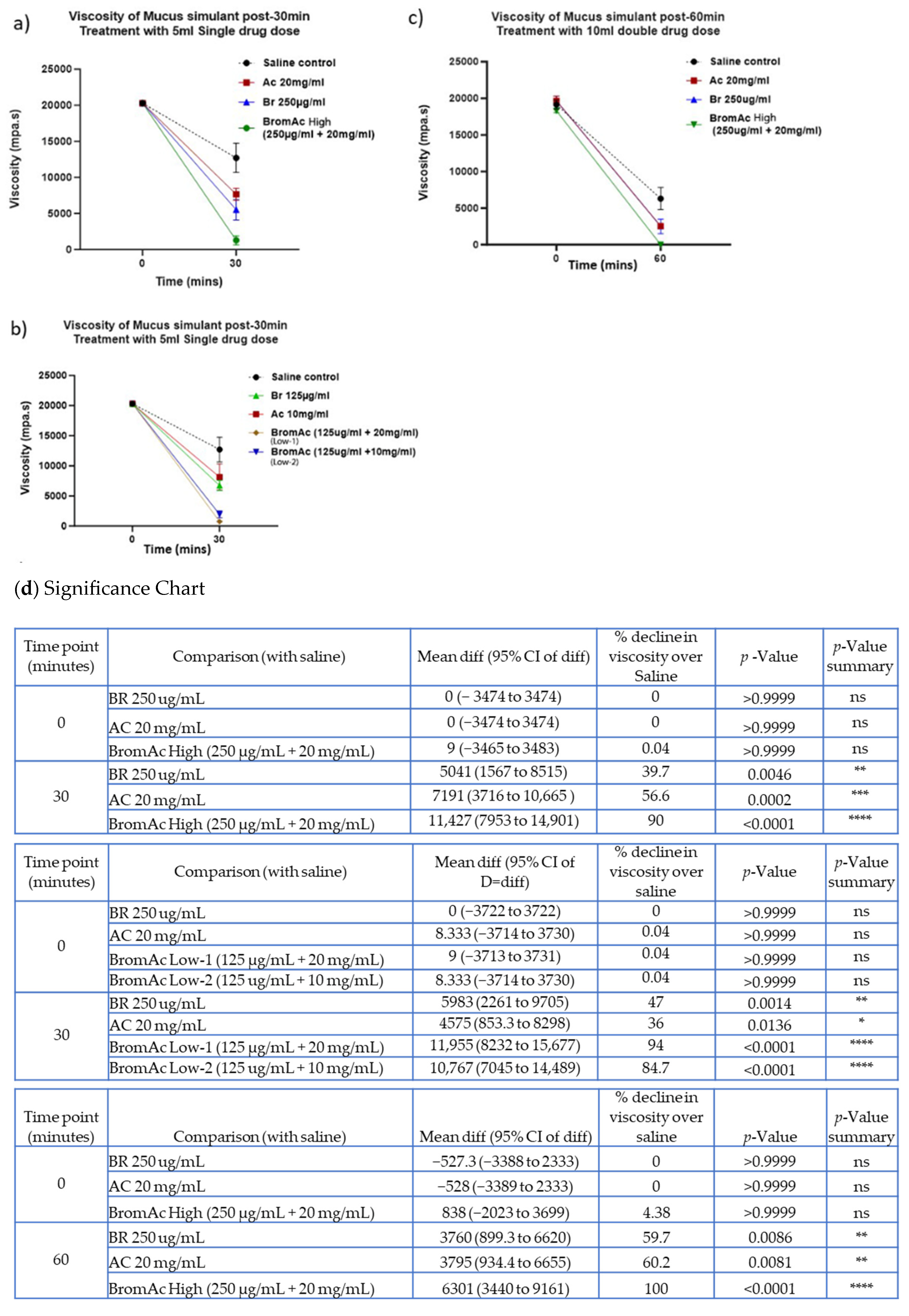
3.3. Effect of Bromelain, Acetylcysteine, and BromAc®™ on Mucus Viscosity Using Rheology
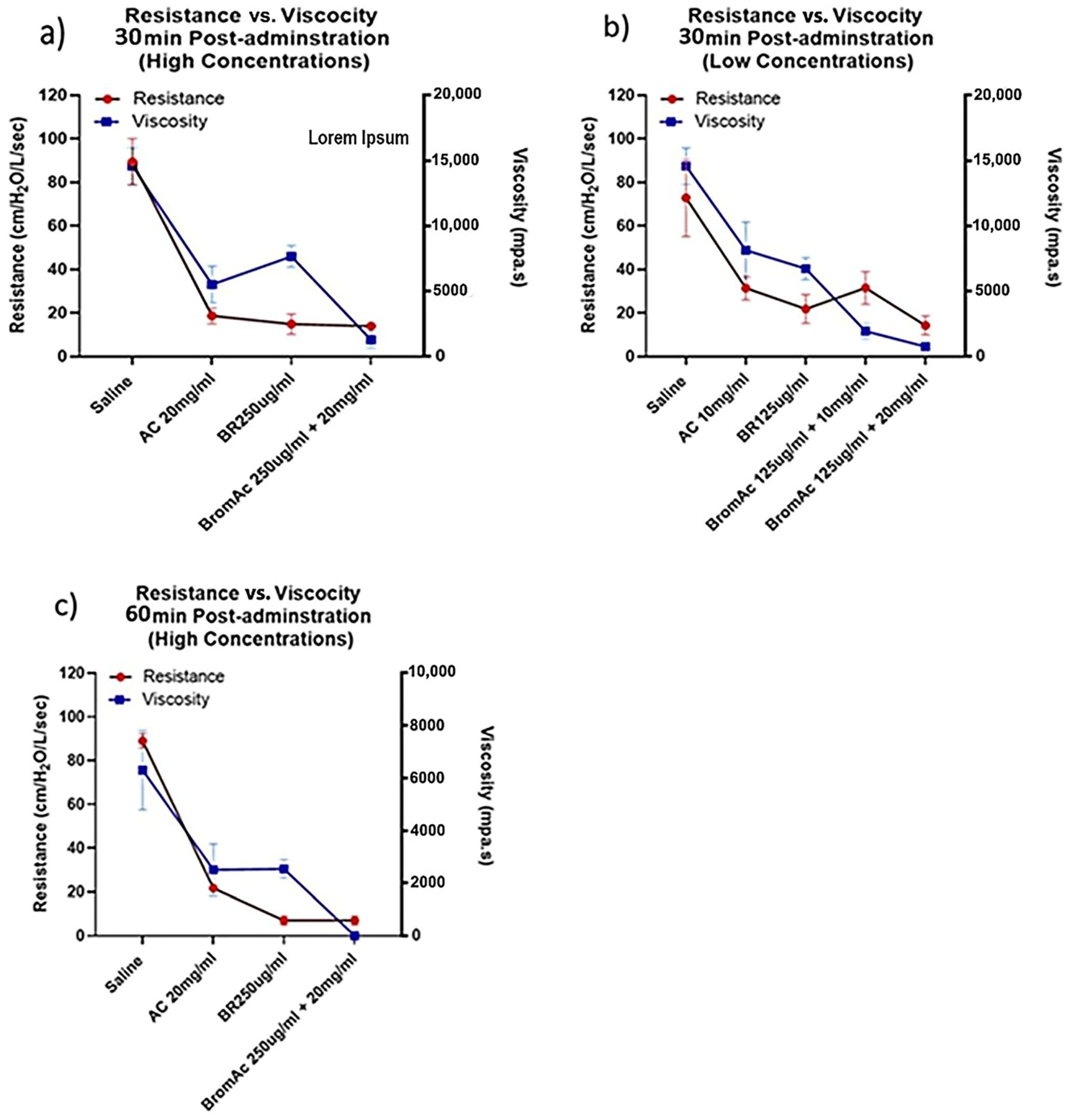
3.4. Viscosity and Ventilatory Parameters and Relationship in the Treatment Group and Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgel, P.-R.; Nadel, J.A. Plugging (publicising) to prevent mucous plugging. Eur. Respir. J. 2010, 36, 1236–1238. [Google Scholar] [CrossRef]
- Diaz, A.A.; Orejas, J.L.; Grumley, S.; Nath, H.P.; Wang, W.; Dolliver, W.R.; Yen, A.; Kligerman, S.J.; Jacobs, K.; Manapragada, P.P.; et al. Airway-Occluding Mucus Plugs and Mortality in Patients With Chronic Obstructive Pulmonary Disease. JAMA 2023, 329, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Schluger, N.W.; Koppaka, R. Lung disease in a global context. A call for public health action. Ann. Am. Thorac. Soc. 2014, 11, 407–416. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodriguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor. Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, T.; Hassani, F.; Ghaffari, N.; Ebrahimi, B.; Yarahmadi, A.; Hassanzadeh, G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J. Mol. Histol. 2020, 51, 613–628. [Google Scholar] [CrossRef]
- Kumar, S.S.; Binu, A.; Devan, A.R.; Nath, L.R. Mucus targeting as a plausible approach to improve lung function in COVID-19 patients. Med. Hypotheses 2021, 156, 110680. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.B.; Sandholdt, H.; Moller, M.E.E.; Perez-Alos, L.; Pedersen, L.; Israelsen, S.B.; Garred, P.; Benfield, T. Prediction of Respiratory Failure and Mortality in COVID-19 Patients Using Long Pentraxin PTX3. J. Innate Immun. 2022, 14, 493–501. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, Z.A.; Charles, M.; Pratap, P.; Naeem, A.; Siddiqui, Z.; Naqvi, N.; Srivastava, S. Cytokine Storm and Mucus Hypersecretion in COVID-19: Review of Mechanisms. J. Inflamm. Res. 2021, 14, 175–189. [Google Scholar] [CrossRef]
- Ottestad, W.; Sovik, S. COVID-19 patients with respiratory failure: What can we learn from aviation medicine? Brit J. Anaesth. 2020, 125, E280–E281. [Google Scholar] [CrossRef]
- Manckoundia, P.; Franon, E. Is Persistent Thick Copious Mucus a Long-Term Symptom of COVID-19? Eur. J. Case Rep. Intern. Med. 2020, 7, 002145. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Keir, H.R.; Chalmers, J.D. Pathophysiology of Bronchiectasis. Semin. Respir. Crit. Care Med. 2021, 42, 499–512. [Google Scholar] [CrossRef]
- Muñoz, G.; de Gracia, J.; Buxó, M.; Alvarez, A.; Vendrell, M. Long-term benefits of airway clearance in bronchiectasis: A randomised placebo-controlled trial. Eur. Respir. J. 2018, 51, 1701926. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, H.; Lambrecht, B.N. The Pathology of Asthma: What Is Obstructing Our View? Annu. Rev. Pathol. 2023, 18, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Trawoger, R.; Kolobow, T.; Cereda, M.; Giacomini, M.; Usuki, J.; Horiba, K.; Ferrans, V.J. Clearance of Mucus from Endotracheal Tubes during Intratracheal Pulmonary Ventilation. Anesthesiology 1997, 86, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Liu, X.; Wang, T.; Liu, F.; Zhu, A.; Lin, Y.; Luo, J.; Ye, F.; He, J.; Zhao, J.; et al. Elevated MUC1 and MUC5AC mucin protein levels in airway mucus of critical ill COVID-19 patients. J. Med. Virol. 2021, 93, 582–584. [Google Scholar] [CrossRef]
- Serra Mitjà, P.; Centeno, C.; Garcia-Olivé, I.; Antuori, A.; Casadellà, M.; Tazi, R.; Armestar, F.; Fernández, E.; Andreo, F.; Rosell, A. Bronchoscopy in Critically Ill COVID-19 Patients: Findings, Microbiological Profile, and Coinfection. J. Bronchol. Interv. Pulmonol. 2022, 29, 186–190. [Google Scholar] [CrossRef]
- Valle, S.J.; Akhter, J.; Mekkawy, A.H.; Lodh, S.; Pillai, K.; Badar, S.; Glenn, D.; Power, M.; Liauw, W.; Morris, D.L. A novel treatment of bromelain and Acetylcysteine (BromAc) in patients with peritoneal mucinous tumours: A phase I first in man study. Eur. J. Surg. Oncol. 2021, 47, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pillai, K.; Akhter, J.; Chua, T.C.; Morris, D.L. A formulation for in situ lysis of mucin secreted in pseudomyxoma peritonei. Int. J. Cancer 2014, 134, 478–486. [Google Scholar] [CrossRef]
- Coelho dos Reis, J.G.A.; Ferreira, G.M.; Lourenço, A.A.; Ribeiro, Á.L.; da Mata, C.P.d.S.M.; de Melo Oliveira, P.; Marques, D.P.d.A.; Ferreira, L.L.; Clarindo, F.A.; da Silva, M.F.; et al. Ex-vivo mucolytic and anti-inflammatory activity of BromAc in tracheal aspirates from COVID-19. Biomed. Pharmacother. 2022, 148, 112753. [Google Scholar] [CrossRef]
- Pillai, K.; Mekkawy, A.H.; Akhter, J.; Valle, S.J.; Morris, D.L. Effect of nebulised BromAc® on rheology of artificial sputum: Relevance to muco-obstructive respiratory diseases including COVID-19. bioRxiv 2021. [Google Scholar] [CrossRef]
- Serisier, D.J.; Carroll, M.P.; Shute, J.K.; Young, S.A. Macrorheology of cystic fibrosis, chronic obstructive pulmonary disease & normal sputum. Respir. Res. 2009, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, C.A.; Allen, P.G.; Wohl, M.E.; Drazen, J.M.; Janmey, P.A.; Stossel, T.P. Reduction in Viscosity of Cystic Fibrosis Sputum in Vitro by Gelsolin. Science 1994, 263, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Taussig, S.J.; Batkin, S. Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. J. Ethnopharmacol. 1988, 22, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Maurer, H.R. Bromelain: Biochemistry, pharmacology and medical use. Cell Mol. Life Sci. 2001, 58, 1234–1245. [Google Scholar] [CrossRef]
- Pillai, K.; Akhter, J.; Morris, D.L. Assessment of a novel mucolytic solution for dissolving mucus in pseudomyxoma peritonei: An ex vivo and in vitro study. Pleura Peritoneum 2017, 2, 111–117. [Google Scholar] [CrossRef]
- Akhter, J.; Pillai, K.; Chua, T.C.; Alzarin, N.; Morris, D.L. Efficacy of a novel mucolytic agent on pseudomyxoma peritonei mucin, with potential for treatment through peritoneal catheters. Am. J. Cancer Res. 2014, 4, 495–507. [Google Scholar]
- Guerini, M.; Condrò, G.; Friuli, V.; Maggi, L.; Perugini, P. N-acetylcysteine (NAC) and Its Role in Clinical Practice Management of Cystic Fibrosis (CF): A Review. Pharmaceuticals 2022, 15, 217. [Google Scholar] [CrossRef]
- Sheehan, J.K.; Richardson, P.S.; Fung, D.C.; Howard, M.; Thornton, D.J. Analysis of respiratory mucus glycoproteins in asthma: A detailed study from a patient who died in status asthmaticus. Am. J. Respir. Cell Mol. Biol. 1995, 13, 748–756. [Google Scholar] [CrossRef]
- Georas, S.N. All plugged up—Noninvasive mucus score to assess airway dysfunction in asthma. J. Clin. Investig. 2018, 128, 906–909. [Google Scholar] [CrossRef]
- Sinha, P.; Flower, O.; Soni, N. Deadspace ventilation: A waste of breath! Intensive Care Med. 2011, 37, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Carlesso, E.; Brioni, M.; Cressoni, M. Airway driving pressure and lung stress in ARDS patients. Crit. Care 2016, 20, 276. [Google Scholar] [CrossRef] [PubMed]
- Beitler, J.R.; Malhotra, A.; Thompson, B.T. Ventilator-induced Lung Injury. Clin. Chest Med. 2016, 37, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Yu, Y.; Han, T.; Zhou, J.; Bi, L. Sputum characteristics and airway clearance methods in patients with severe COVID-19. Medicine 2020, 99, e23257. [Google Scholar] [CrossRef]
- Østergaard, L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valle, N.; Eapen, M.S.; Pillai, K.; Morris, R.; Akhter, J.; Mekkawy, A.H.; Morris, D.L.; Valle, S.J. Impact of Nebulized BromAc® on Mucus Plug Clearance in a Mechanically Ventilated Ex Vivo Ovine Lung Model of Obstructive Respiratory Conditions. Life 2024, 14, 1111. https://doi.org/10.3390/life14091111
Valle N, Eapen MS, Pillai K, Morris R, Akhter J, Mekkawy AH, Morris DL, Valle SJ. Impact of Nebulized BromAc® on Mucus Plug Clearance in a Mechanically Ventilated Ex Vivo Ovine Lung Model of Obstructive Respiratory Conditions. Life. 2024; 14(9):1111. https://doi.org/10.3390/life14091111
Chicago/Turabian StyleValle, Nicole, Mathew Suji Eapen, Krishna Pillai, Richard Morris, Javed Akhter, Ahmed H. Mekkawy, David L. Morris, and Sarah J. Valle. 2024. "Impact of Nebulized BromAc® on Mucus Plug Clearance in a Mechanically Ventilated Ex Vivo Ovine Lung Model of Obstructive Respiratory Conditions" Life 14, no. 9: 1111. https://doi.org/10.3390/life14091111
APA StyleValle, N., Eapen, M. S., Pillai, K., Morris, R., Akhter, J., Mekkawy, A. H., Morris, D. L., & Valle, S. J. (2024). Impact of Nebulized BromAc® on Mucus Plug Clearance in a Mechanically Ventilated Ex Vivo Ovine Lung Model of Obstructive Respiratory Conditions. Life, 14(9), 1111. https://doi.org/10.3390/life14091111




