MR Imaging of Hemosiderin Deposition in the Ankle Joints of Patients with Haemophilia: The Contribution of a Multi-Echo Gradient-Echo Sequence—Correlation with Osteochondral Changes and the Number and Chronicity of Joint Bleeds
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. MRI Technique
2.3. Imaging Analysis and Interpretation
2.4. Statistics
3. Results
3.1. Detection of Joint Hemosiderin Deposition with mGRE Sequence: Inter-Reader Agreement
3.2. The Association of Joint Hemosiderin Deposition with Synovial Thickening, Effusion, and Osteochondral Changes (OCC)
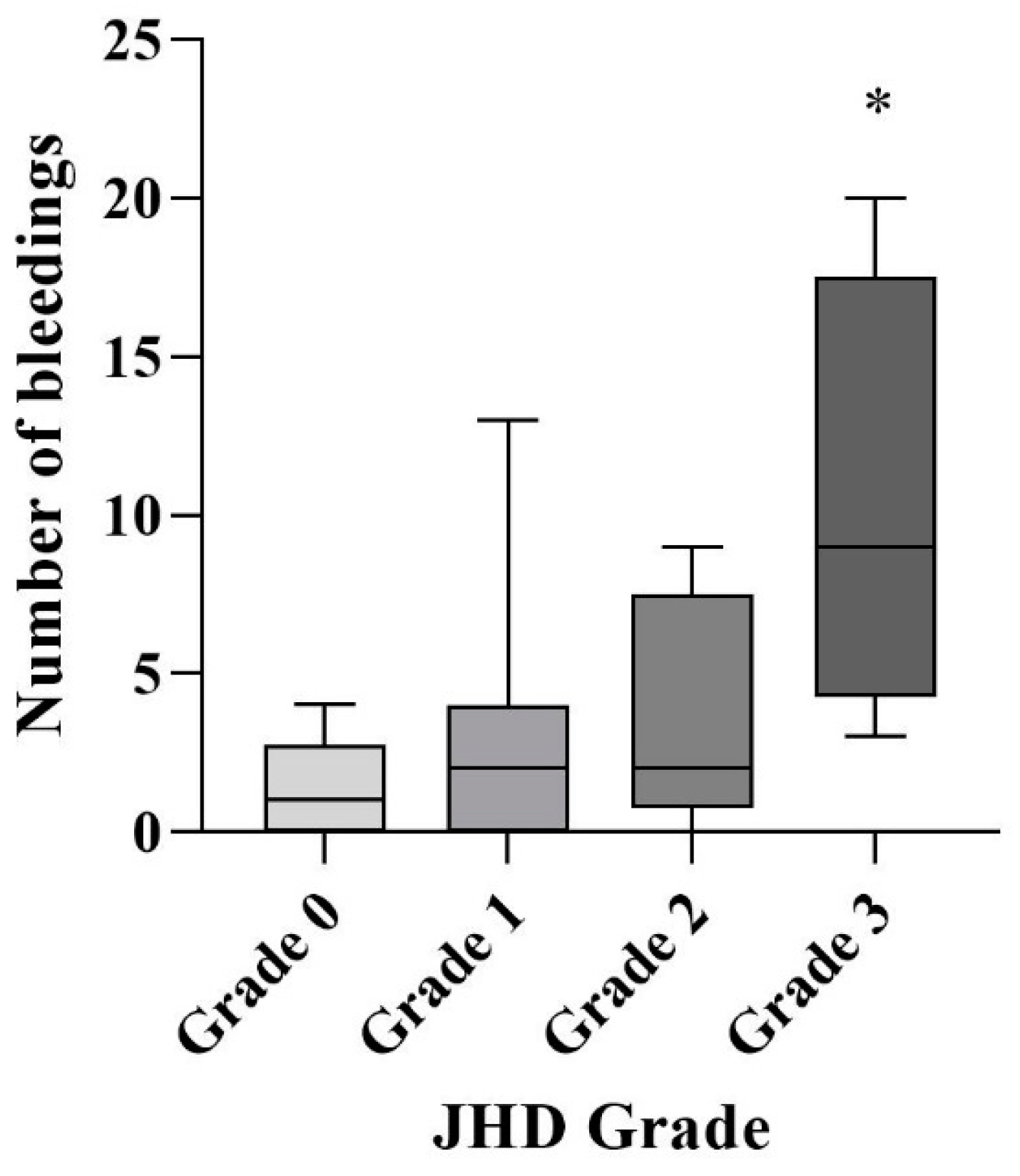
3.3. Association of Joint Hemosiderin Deposition with the Number and Chronicity of Joint Bleeds and Clinical Score
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erik, B.; Kathelijn, F.; Hart, D.P.; Mancuso, M.E.; Stephensen, D.; Shapiro, A.D.; Blanchette, V. Haemophilia. Nat. Rev. Dis. Primers 2021, 7, 45–48. [Google Scholar]
- Pulles, A.E.; Mastbergen, S.C.; Schutgens, R.E.; Lafeber, F.P.; van Vulpen, L.F. Pathophysiology of hemophilic arthropathy and potential targets for therapy. Pharmacol. Res. 2017, 115, 192–199. [Google Scholar] [CrossRef]
- van Vulpen, L.F.D.; Holstein, K.; Martinoli, C. Joint disease in haemophilia: Pathophysiology, pain and imaging. Haemophilia 2018, 24 (Suppl. S6), 44–49. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.E.; Michalopoulou, A.; Fischer, K.; Motwani, J.; Andersson, N.G.; Pergantou, H.; Ranta, S.; the PedNet Study Group. Long-term joint outcomes in adolescents with moderate or severe haemophilia A. Haemophilia 2022, 28, 1054–1061. [Google Scholar] [CrossRef]
- Dettoraki, A.; Michalopoulou, A.; Mazarakis, M.; Saslis, S.; Stamati, I.; Kapsimali, Z.; Pergantou, H. Clinical application of extended half-life factor VIII in children with severe haemophilia A. Haemophilia 2022, 28, 619–624. [Google Scholar] [CrossRef]
- Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; Valentino, L.A.; et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007, 357, 535–544. [Google Scholar]
- Seuser, A.; Khayat, C.D.; Negrier, C.; Sabbour, A.; Heijnen, L. Evaluation of early musculoskeletal disease in patients with haemophilia: Results from an expert consensus. Blood Coagul. Fibrinolysis 2018, 29, 509–520. [Google Scholar] [CrossRef]
- Pergantou, H.; Matsinos, G.; Papadopoulos, A.; Platokouki, H.; Aronis, S. Comparative study of validity of clinical, X-ray and magnetic resonance imaging scores in evaluation and management of haemophilic arthropathy in children. Haemophia 2006, 12, 241–247. [Google Scholar] [CrossRef]
- Kraft, J.; Blanchette, V.; Babyn, P.; Feldman, B.; Cloutier, S.; Israels, S.; Pai, M.; Rivard, G.; Gomer, S.; McLIMONT, M.; et al. Magnetic resonance imaging and joint outcomes in boys with severe hemophilia A treated with tailored primary prophylaxis in Canada. J. Thromb. Haemost. 2012, 10, 2494–2502. [Google Scholar] [CrossRef]
- Zwagemaker, A.F.; Kloosterman, F.R.; Hemke, R.; Gouw, S.C.; Coppens, M.; Romano, L.G.R.; Kruip, M.J.H.A.; Cnossen, M.H.; Leebeek, F.W.G.; Hutten, B.A.; et al. Joint status of patients with non-severe hemophilia A. J. Thromb. Haemost. 2022, 20, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, M.N.D.; Iervolino, S.; Soscia, E.; Tosetto, A.; Coppola, A.; Schiavulli, M.; Marrone, E.; Ruosi, C.; Salvatore, M.; Di Minno, G. Magnetic resonance imaging and ultrasound evaluation of “healthy” joints in young subjects with severe haemophilia A. Haemophilia 2013, 19, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, M.; Kurnik, K.; Pfluger, T.; Bidlingmaier, C. Identification and long-term observation of early joint damage by magnetic resonance imaging in clinically asymptomatic joints in patients with haemophilia A or B despite prophylaxis. Haemophilia 2012, 18, 369–374. [Google Scholar] [CrossRef]
- Keshava, S.N.; Gibikote, S.; Doria, A.S. Imaging evaluation of hemophilia: Musculoskeletal approach. Semin. Thromb. Hemost. 2015, 41, 880–893. [Google Scholar] [PubMed]
- Doria, A.S. State-of-the-art imaging techniques for the evaluation of haemophilic arthropathy: Present and future. Haemophilia 2010, 16 (Suppl. S5), 107–114. [Google Scholar] [CrossRef]
- Lundin, B.; Manco-Johnson, M.L.; Ignas, D.M.; Moineddin, R.; Blanchette, V.S.; Dunn, A.L.; Gibikote, S.V.; Keshava, S.N.; Ljung, R.; Manco-Johnson, M.J.; et al. An MRI scale for assessment of haemophilic arthropathy from the International Prophylaxis Study Group. Haemophilia 2012, 18, 962–970. [Google Scholar] [CrossRef]
- Doria, A.S.; Keshava, S.N.; Mohanta, A.; Jarrin, J.; Blanchette, V.; Srivastava, A.; Moineddin, R.; Kavitha, M.L.; Hilliard, P.; Poonnoose, P.; et al. Diagnostic accuracy of ultrasound for assessment of hemophilic arthropathy: MRI correlation. SAJR 2015, 204, W336–W347. [Google Scholar] [CrossRef]
- Pergantou, H.; Platokouki, H.; Matsinos, G.; Papakonstantinou, O.; Papadopoulos, A.; Xafaki, P.; Petratos, D.; Aronis, S. Assessment of the progression of haemophilic arthropathy in children. Haemophilia 2010, 16, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Stimec, J.; Dover, S.; Pullenayegum, E.; Blanchette, V.S.; Doria, A.S.; Feldman, B.M.; Carcao, M.; Rivard, G.E.; Israels, S.J.; Chan, A.K.; et al. Magnetic resonance imaging in boys with severe hemophilia A: Serial and end-of-study findings from the Canadian Hemophilia Primary Prophylaxis Study. Res. Pract. Thromb. Haemost. 2021, 5, e12565–e12578. [Google Scholar] [CrossRef]
- Foppen, W.; van der Schaaf, I.C.; Beek, F.J.A.; Mali, W.P.T.M.; Fischer, K. MRI predicts 5-year joint bleeding and development of arthropathy on radiographs in hemophilia. Blood Adv. 2020, 4, 113–121. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Haacke, E.M. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics 2009, 29, 1433–1449. [Google Scholar] [CrossRef]
- Foppen, W.; Fischer, K.; van der Schaaf, I.C. Imaging of haemophilic arthropathy: Awareness of pitfalls and need for standardization. Haemophilia 2017, 23, 645–647. [Google Scholar] [CrossRef]
- Martinoli, C.; Alberighi, O.D.C.; Di Minno, G.; Graziano, E.; Molinari, A.C.; Pasta, G.; Russo, G.; Santagostino, E.; Tagliaferri, A.; Tagliafico, A.; et al. Development and definition of a simplified scanning procedure and scoring method for haemophilia early arthropathy detection with ultrasound (HEAD-US). Thromb. Haemost. 2013, 109, 1170–1179. [Google Scholar] [CrossRef]
- Gallastegui, N.; Steiner, B.; Aguero, P.; Bailey, C.; Kruse-Jarres, R.; Quon, D.; Hanacek, C.; Volland, L.; Barnes, R.; von Drygalski, A. The role of point-of-Care Musculoskeletal Ultrasound for Routine Joint evaluation and management in the Hemophilia Clinic—A Real World Experience. BMC Musculoskelet. Disord. 2022, 23, 1111–1121. [Google Scholar] [CrossRef]
- Bakeer, N.; Dover, S.; Babyn, P.; Feldman, B.M.; von Drygalski, A.; Doria, A.S.; Ignas, D.M.; Abad, A.; Bailey, C.; Beggs, I.; et al. Musculoskeletal ultrasound in hemophilia: Results and recommendations from a global survey and consensus meeting. Res. Pract. Thromb. Haemost. 2021, 5, 12531–12541. [Google Scholar] [CrossRef]
- Martinoli, C.; Di Minno, M.N.; Pasta, G.; Tagliafico, A. Hemosiderin detection with ultrasound: Reality or myth? AJR 2016, 206, W30. [Google Scholar] [CrossRef] [PubMed]
- von Drygalski, A.; Moore, R.E.; Nguyen, S.; Barnes, R.F.W.; Volland, L.M.; Hughes, T.H.; Du, J.; Chang, E.Y. Advanced hemophilic arthropathy: Sensitivity of soft tissue discrimination with musculoskeletal ultrasound. J. Ultrasound Med. 2018, 37, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Daruge, P.; Dertkigil, S.S.J.; Fernandes, E.D.A.; Negrao, J.R.; Mitraud, S.d.A.V.; Sakuma, E.T.I.; Fernandes, A.R.C.; Zhang, N.; Huo, A.; et al. Imaging of haemophilic arthropathy in growing joints: Pitfalls in ultrasound and MRI. Haemophilia 2017, 23, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Den Uijl, I.E.M.; De Schepper, A.M.A.; Camerlinck, M.; Grobbee, D.E.; Fischer, K. Magnetic resonance imaging in teenagers and young adults with limited haemophilic arthropathy: Baseline results from a prospective study. Haemophilia 2011, 17, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, B.; Polat, A.V.; Ozturk, M.; Aslan, K.; Tomak, L.; Selcuk, M.B. Contribution of 3-T susceptibility-weighted MRI to detection of intraarticular hemosiderin accumulation in patients with hemophilia. AJR 2018, 210, 1141–1147. [Google Scholar] [CrossRef]
- Eerdekens, M.; Peerlinck, K.; Staes, F.; Pialat, J.; Hermans, C.; Lobet, S.; Deschamps, K. Clinical gait features are associated with MRI findings in patients with haemophilic ankle arthropathy. Haemophilia 2020, 26, 333–339. [Google Scholar] [CrossRef]
- Roussel, N.A.; Chantrain, V.; Foubert, A.; Lambert, C.; Hermans, C.; Meeus, M.; Guillaume, S.; Lecouvet, F.; Krüger, S.; Hilberg, T.; et al. Gaining more insight into ankle pain in haemophilia: A study exploring pain, structural and functional evaluation of the ankle joint. Haemophilia 2022, 28, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Groen, W.; van der Net, J.; Bos, K.; Abad, A.; Bergstrom, B.-M.; Blanchette, V.S.; Feldman, B.M.; Funk, S.; Helders, P.; Hilliard, P.; et al. Joint health and functional ability in children with haemophilia who receive intensive replacement therapy. Haemophilia 2011, 17, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, O.; Isaak, A.; Dalili, D.; Noebauer-Huhmann, I.M. T2-weighted hypointense tumors and tumor-like lesions. Semin. Musculoskelet. Radiol. 2019, 23, 58–75. [Google Scholar] [PubMed]
- Zhang, L.; Wei, S.; Li, J.; Wang, P.; Ge, Y. Value of 3.0T MRI T2 mapping combined with SWI for the assessment of early lesions in hemophilic arthropathy. Hematology 2022, 27, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.A.; Daghighi, M.H.; Mirza-Aghazadeh-Attari, M.; Jalili, J.; Mahmoudpour, M.; Daghighi, S. The diagnostic value of susceptibility-weighted imaging for identifying acute intraarticular hemorrhages. Skelet. Radiol. 2022, 51, 1777–1785. [Google Scholar] [CrossRef]
- Jang, H.; von Drygalski, A.; Wong, J.; Zhou, J.Y.; Aguero, P.; Lu, X.; Cheng, X.; Ball, S.T.; Ma, Y.; Chang, E.Y.; et al. Ultrashort echo time quantitative susceptibility mapping (UTE-QSM) for detection of hemosiderin deposition in hemophilic arthropathy: A feasibility study. J. Magn. Reason. Med. 2020, 84, 3246–3255. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Wong, J.H.; Berman, Z.T.; Lombardi, A.F.; Chang, E.Y.; von Drygalski, A. Bleeding with iron deposition and vascular remodelling in subchondral cysts: A newly discovered feature unique to haemophilic arthropathy. Haemophilia 2021, 27, e730–e738. [Google Scholar] [CrossRef]
- Kritsaneepaiboon, S.; Ina, N.; Chotsampancharoen, T.; Roymanee, S.; Cheewatanakornkul, S. The relationship between myocardial and hepatic T2 and T2* at 1.5T and 3T MRI in normal and iron-overloaded patients. Acta Radiol. 2018, 59, 355–362. [Google Scholar] [CrossRef]
- Plut, D.; Kotnik, B.F.; Pusnik, L.; Slak, P.; Snoj, Z.; Salapura, V. Reliability of haemophilia early arthropathy detection with ultrasound (HEAD-US) in children: A comparative manetic resonance imaging (MRI) study. Radiol. Oncol. 2022, 56, 471–478. [Google Scholar] [CrossRef]
- Prasetyo, M.; Mongan, A.E.; Chozie, N.A.; Prihartono, J.; Setiawan, S.I. Hemosiderin deposition evaluation in hemophilic ankle joints: Association between US finding and gradient-recalled echo MR imaging sequence. Insights Imaging 2021, 12, 107. [Google Scholar] [CrossRef]
- Lundin, B.; Berntorp, E.; Pettersson, H.; Wirestam, R.; Jonsson, K.; Ståhlberg, F.; Ljung, R. Gadolinium contrast agent is of limited value for magnetic resonance imaging assessment of synovial hypertrophy in hemophiliacs. Acta Radiol. 2007, 48, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Nijdam, A.; Bladen, M.; Hubert, N.; Pettersson, M.; Bartels, B.; van der Net, J.; Liesner, R.; Petrini, P.; Kurnik, K.; Fischer, K. Using routine Haemophilia Joint Health Score for international comparisons of haemophilia outcome: Standardization is needed. Haemophilia 2016, 22, 142–147. [Google Scholar] [CrossRef] [PubMed]

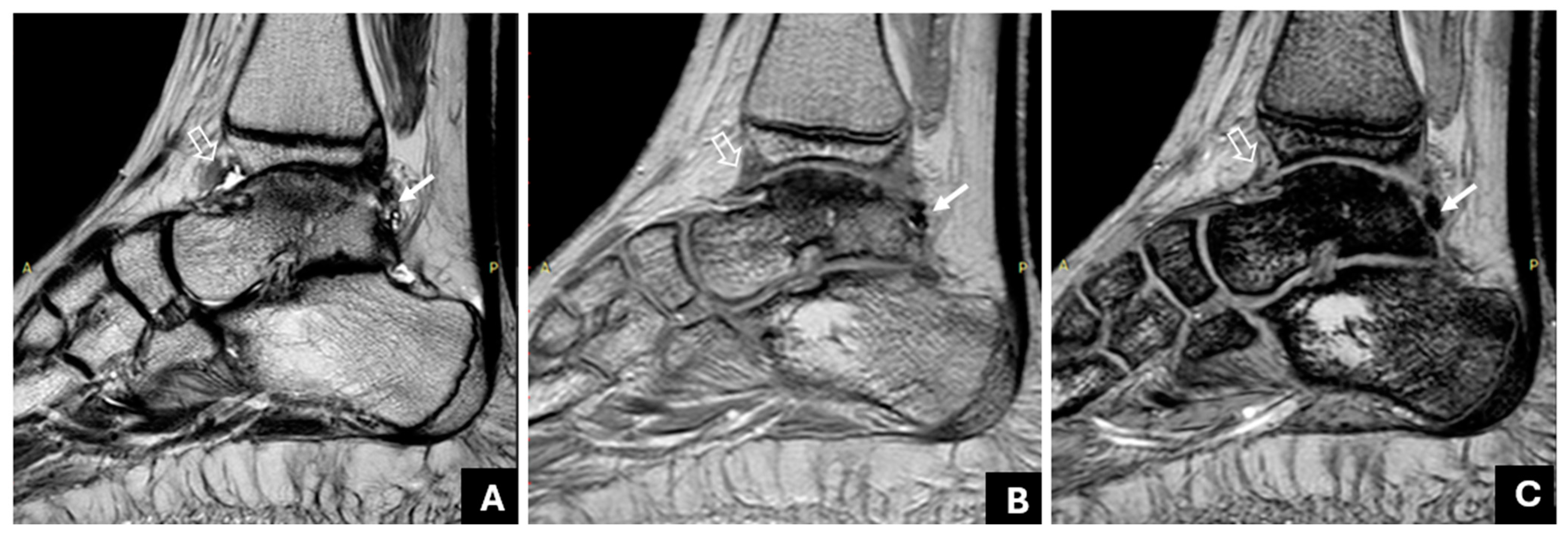

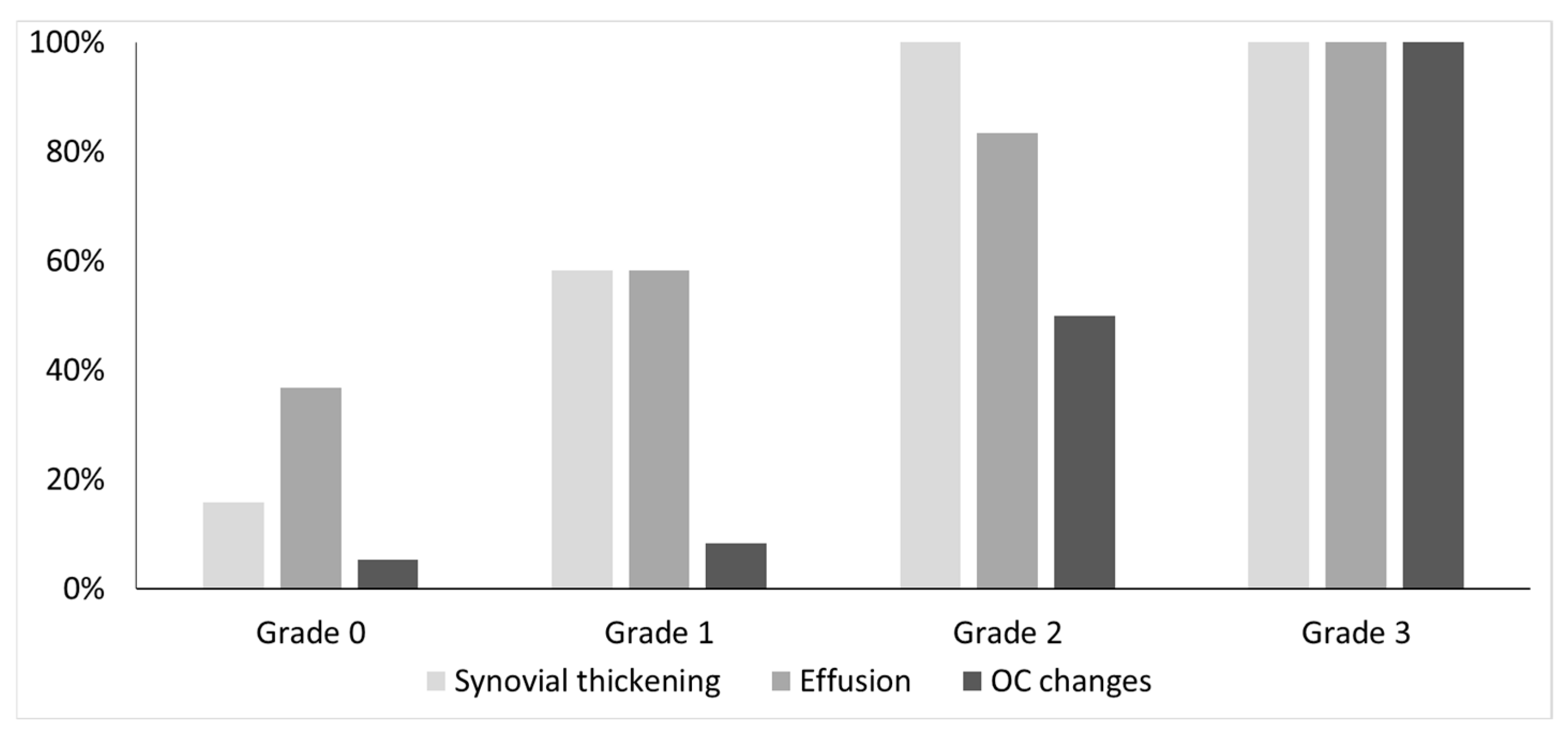
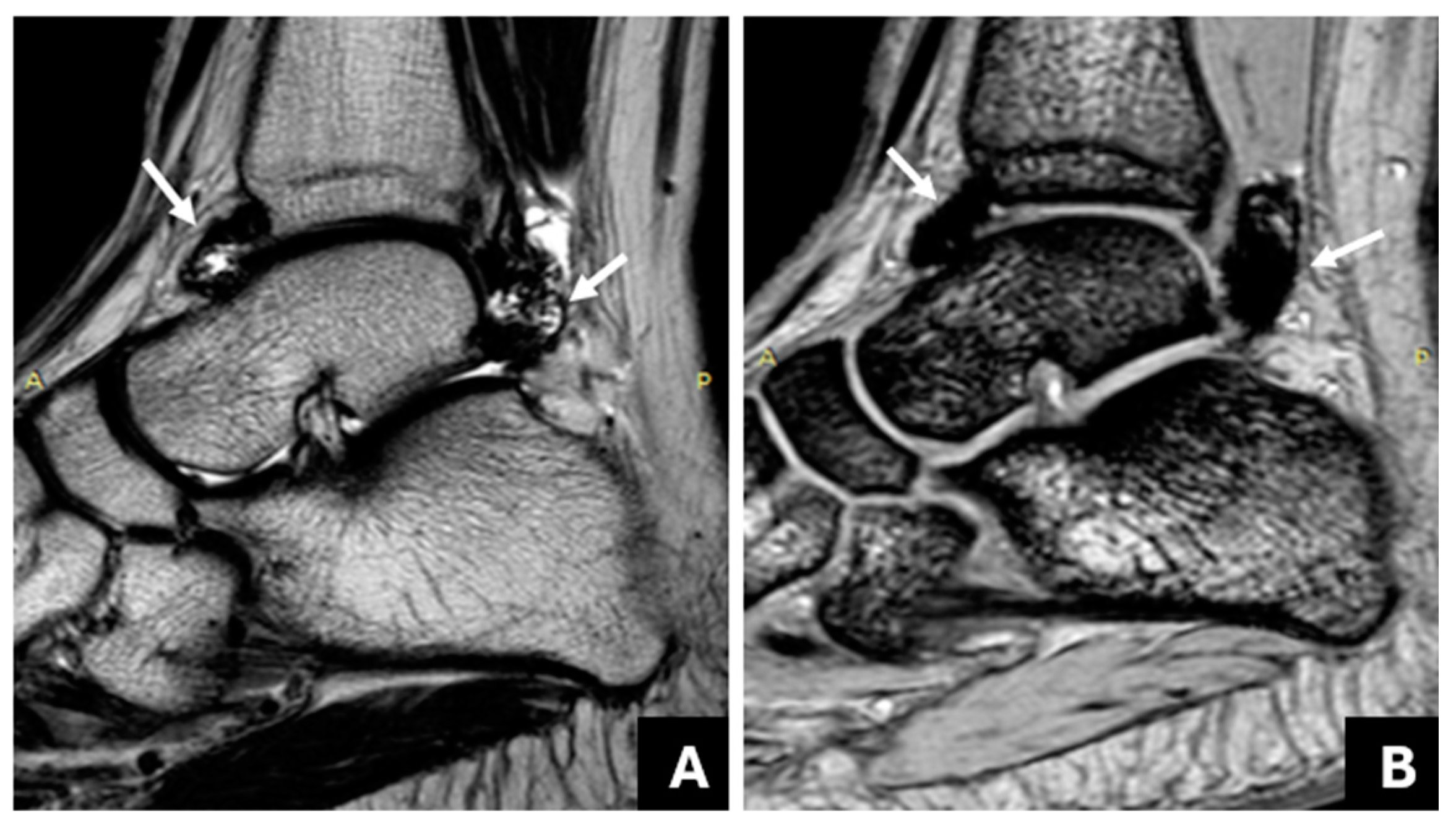
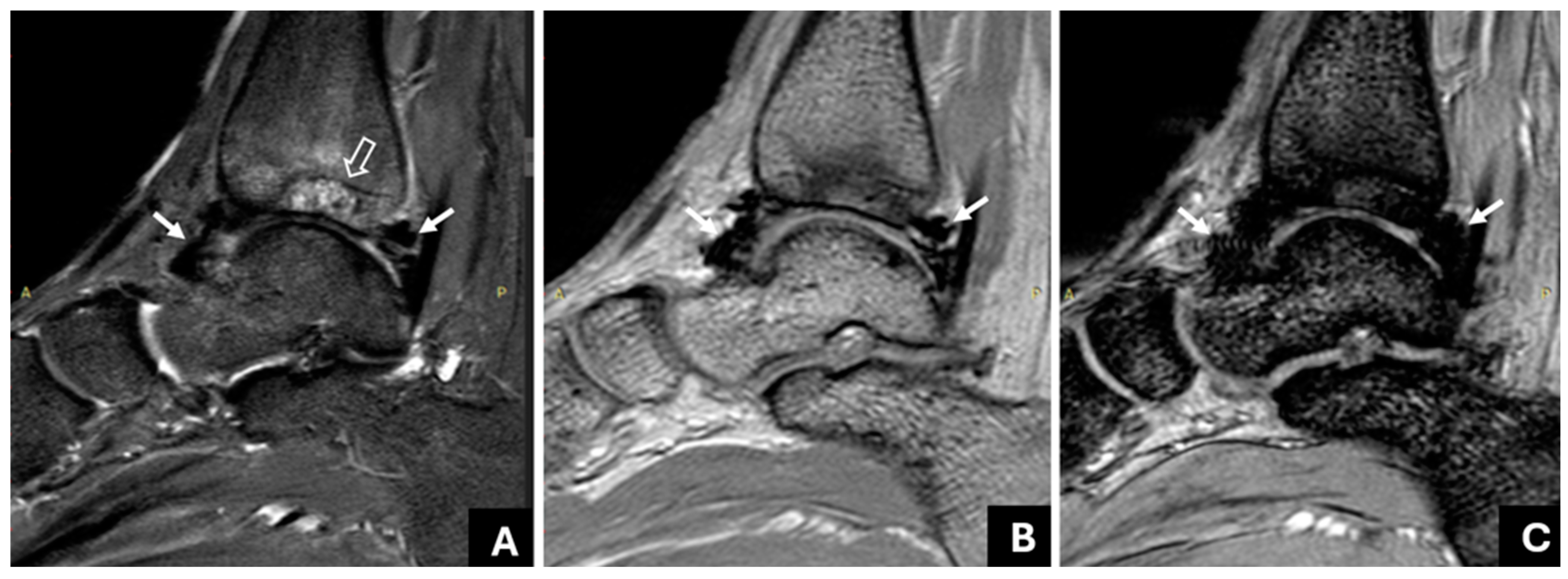
| Method | Radiologist | Sensitivity | Specificity | PPV | NPV | ICC |
|---|---|---|---|---|---|---|
| Conventional | A 1 | 85.0% (64.0–94.8%) | 95.2% (77.3–99.2%) | 94.4% (74.2–99.0%) | 87.0% (67.9–95.5%) | 0.894 (0.802–0.943) |
| B 2 | 80.0 (58.4–91.3%) | 76.2% (54.9–89.4%) | 76.2% (54.9–89.4%) | 80.0% (58.4–91.3%) | 0.724 (0.480–0.853) | |
| C 3 | 50.0% (29.9–70.1%) | 81.0% (60.0–92.3%) | 71.4% (45.4–88.3%) | 63.0% (44.2–78.5%) | 0.481 (0.049–0.720) | |
| T2* | A 1 | 90.0% (69.9–97.2%) | 100% (84.5–100%) | 100% (82.4–100%) | 91.3% (73.2–97.6%) | 0.950 (0.906–0.973) |
| B 2 | 95.0% (76.4–99.1%) | 95.2% (77.3–99.2%) | 95.% (76.4–99.1%) | 95.2% (77.3–99.2%) | 0.950 (0.906–0.973) | |
| C 3 | 95.0% (76.4–99.1%) | 100% (84.5–100%) | 100% (83.2–100%) | 95.5% (78.2–99.2%) | 0.976 (0.954–0.987) |
| Pt, No | Age | Rt/Lt Ankle | JBs, n | Years from Last JB | Clinical Score | JHD, Grades | Synovial Thickening | Effusion | OC Changes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | Rt | 13 | 1 | 1 | 1 | + | + | + |
| Lt | 1 | 7 | 2 | 0 | − | + | − | ||
| 2 | 9 | Lt | 4 | 1 | 1 | 1 | − | + | − |
| 3 | 13 | Rt | 0 | 0 | 0 | 0 | − | − | − |
| Lt | 4 | 9 | 1 | 0 | + | + | − | ||
| 4 | 12 | Rt | 0 | 0 | 0 | 1 | + | + | − |
| Lt | 1 | 9 | 0 | 0 | − | − | − | ||
| 5 | 12 | Rt | 2 | 0.5 | 0 | 0 | + | − | − |
| Lt | 4 | 1 | 0 | 0 | + | − | − | ||
| 6 | 16 | Rt | 4 | 0 | 0 | 1 | − | − | − |
| Lt | 2 | 2 | 0 | 1 | + | + | − | ||
| 7 | 16 | Rt | 0 | 0 | 0 | 0 | − | − | − |
| Lt | 1 | 5 | 0 | 1 | − | − | − | ||
| 8 | 14 | Rt | 4 | 4 | 0 | 1 | + | + | − |
| Lt | 0 | 0 | 0 | 0 | − | − | − | ||
| 9 | 16 | Rt | 2 | 7 | 1 | 1 | + | + | − |
| Lt | 3 | 2 | 1 | 3 | + | + | + | ||
| 10 | 14 | Rt | 3 | 1 | 1 | 1 | + | − | − |
| Lt | 1 | 4 | 0 | 2 | + | + | − | ||
| 11 | 15 | Rt | 7 | 6 | 3 | 2 | + | + | + |
| Lt | 9 | 3 | 0 | 2 | + | − | + | ||
| 12 | 9 | Rt | 0 | 0 | 2 | 0 | + | − | + |
| Lt | 1 | 2 | 2 | 1 | + | − | − | ||
| 13 | 16 | Rt | 2 | 8 | 0 | 2 | + | + | − |
| Lt | 20 | 1 | 1 | 3 | + | + | + | ||
| 14 | 14 | Rt | 0 | 0 | 2 | 2 | + | + | − |
| Lt | 2 | 1 | 2 | 2 | + | + | + | ||
| 15 | 17 | Rt | 0 | 0 | 0 | 0 | − | + | − |
| Lt | 4 | 12 | 0 | 0 | − | + | − | ||
| 16 | 17 | Rt | 0 | 0 | 0 | 0 | − | − | − |
| Lt | 2 | 6 | 0 | 0 | − | + | − | ||
| 17 | 17 | Rt | 8 | 0.5 | 0 | 3 | + | + | + |
| Lt | 2 | 9 | 0 | 1 | − | − | − | ||
| 18 | 17 | Rt | 1 | 5 | 2 | 0 | − | + | − |
| Lt | 2 | 1 | 2 | 1 | − | + | − | ||
| 19 | 17 | Rt | 10 | 0.5 | 1 | 3 | + | + | + |
| Lt | 0 | 0 | 0 | 0 | − | − | − | ||
| 20 | 15 | Rt | 1 | 6 | 0 | 0 | − | − | − |
| Lt | 1 | 0.5 | 0 | 0 | − | + | − | ||
| 21 | 16 | Rt | 2 | 5 | 0 | 0 | − | − | − |
| Lt | 4 | 11 | 0 | 0 | + | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papakonstantinou, O.; Karavasilis, E.; Martzoukos, E.; Velonakis, G.; Kelekis, N.; Pergantou, H. MR Imaging of Hemosiderin Deposition in the Ankle Joints of Patients with Haemophilia: The Contribution of a Multi-Echo Gradient-Echo Sequence—Correlation with Osteochondral Changes and the Number and Chronicity of Joint Bleeds. Life 2024, 14, 1112. https://doi.org/10.3390/life14091112
Papakonstantinou O, Karavasilis E, Martzoukos E, Velonakis G, Kelekis N, Pergantou H. MR Imaging of Hemosiderin Deposition in the Ankle Joints of Patients with Haemophilia: The Contribution of a Multi-Echo Gradient-Echo Sequence—Correlation with Osteochondral Changes and the Number and Chronicity of Joint Bleeds. Life. 2024; 14(9):1112. https://doi.org/10.3390/life14091112
Chicago/Turabian StylePapakonstantinou, Olympia, Efstratios Karavasilis, Epaminondas Martzoukos, Georgios Velonakis, Nikolaos Kelekis, and Helen Pergantou. 2024. "MR Imaging of Hemosiderin Deposition in the Ankle Joints of Patients with Haemophilia: The Contribution of a Multi-Echo Gradient-Echo Sequence—Correlation with Osteochondral Changes and the Number and Chronicity of Joint Bleeds" Life 14, no. 9: 1112. https://doi.org/10.3390/life14091112
APA StylePapakonstantinou, O., Karavasilis, E., Martzoukos, E., Velonakis, G., Kelekis, N., & Pergantou, H. (2024). MR Imaging of Hemosiderin Deposition in the Ankle Joints of Patients with Haemophilia: The Contribution of a Multi-Echo Gradient-Echo Sequence—Correlation with Osteochondral Changes and the Number and Chronicity of Joint Bleeds. Life, 14(9), 1112. https://doi.org/10.3390/life14091112







