Diaphragm Muscle Atrophy Contributes to Low Physical Capacity in COVID-19 Survivors
Abstract
:1. Introduction
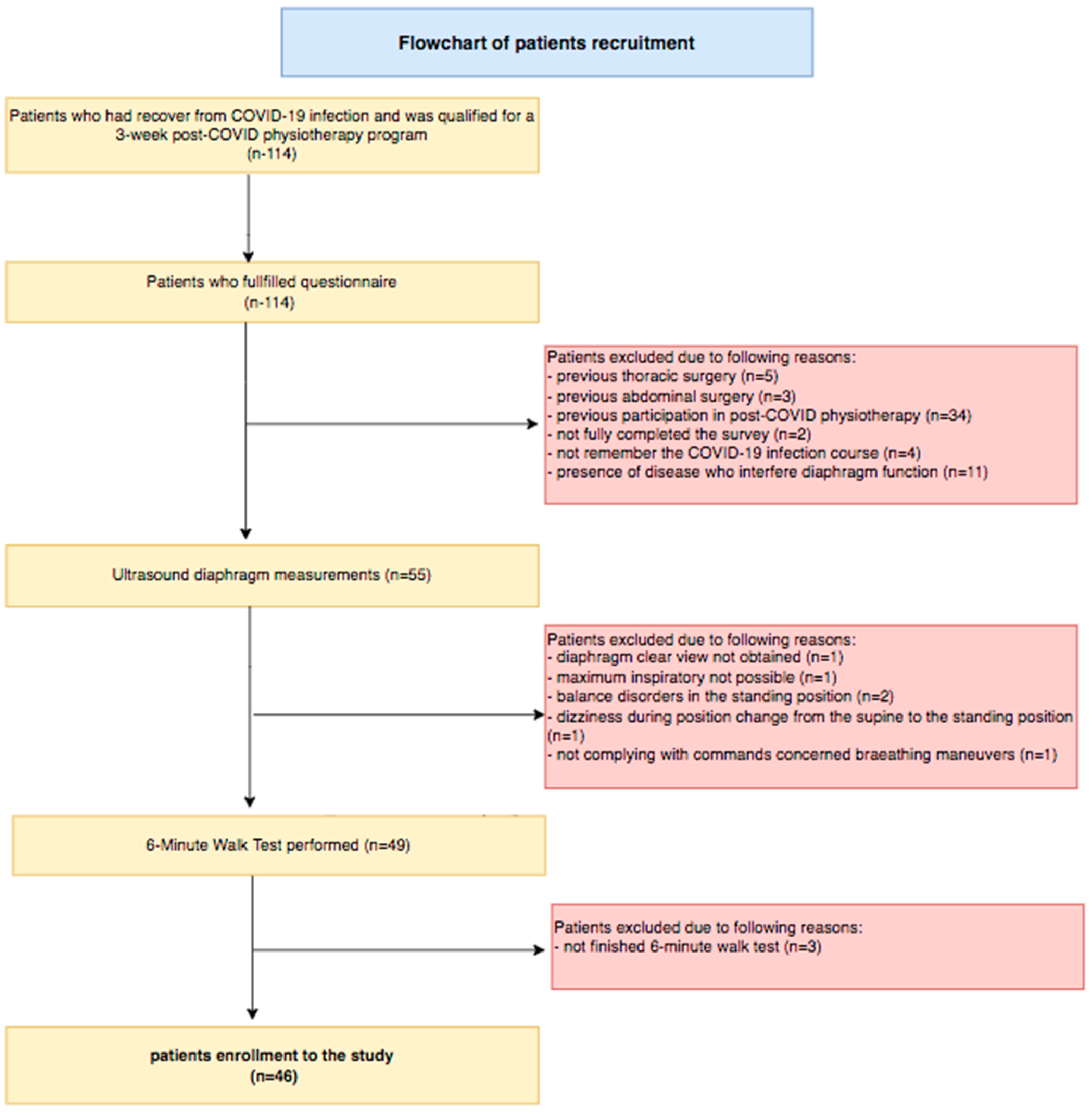
2. Materials and Methods
2.1. Study Design and Settings
2.2. Participants
2.3. Diaphragm Muscle Ultrasound
2.4. The mMRC (Modified Medical Research Council) Scale
2.5. The Six-Minute Walk Test
2.6. Rating of Perceived Exertion: The Borg Scale
2.7. MET (The Metabolic Equivalent) Score
2.8. Statistical Analysis
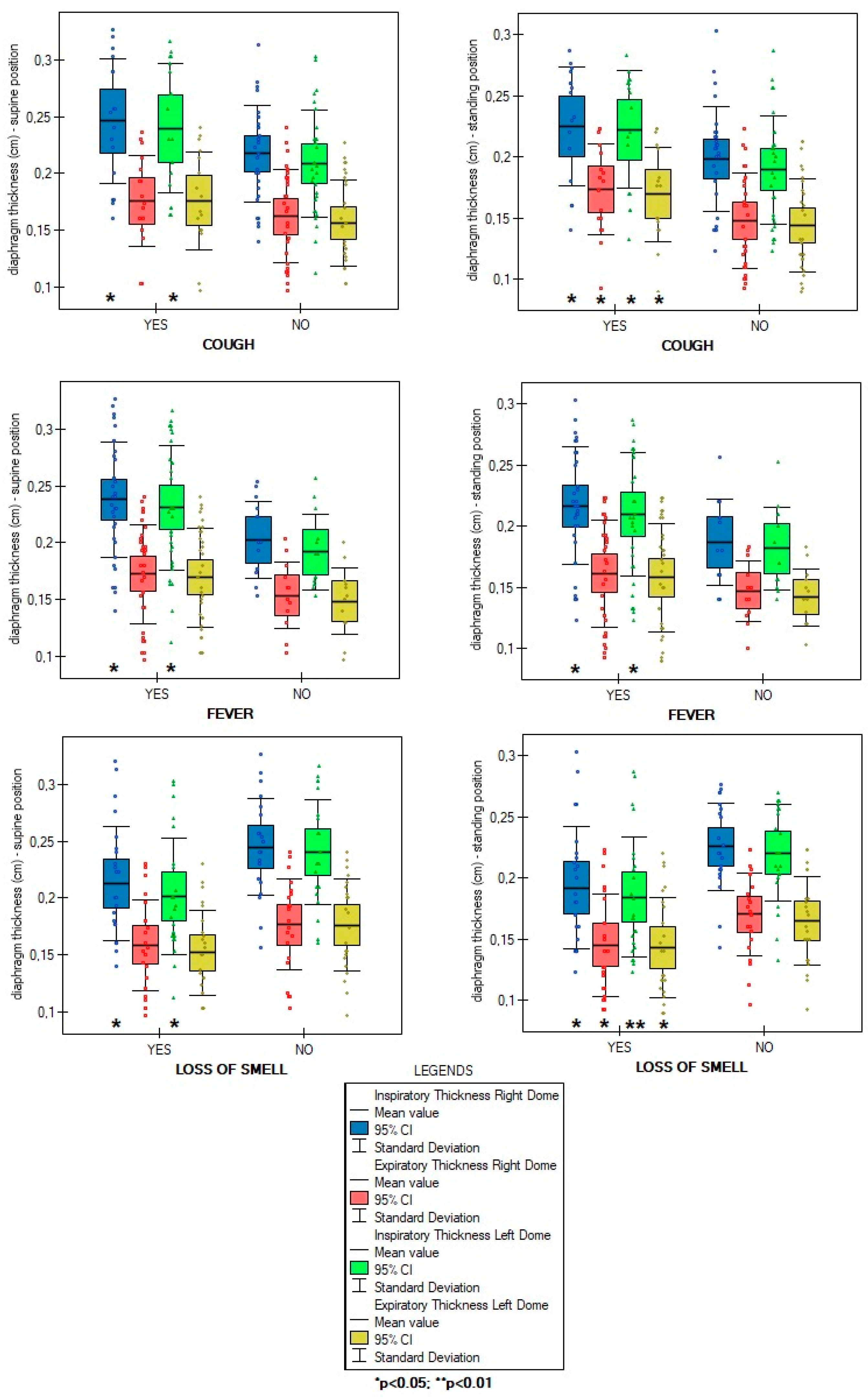
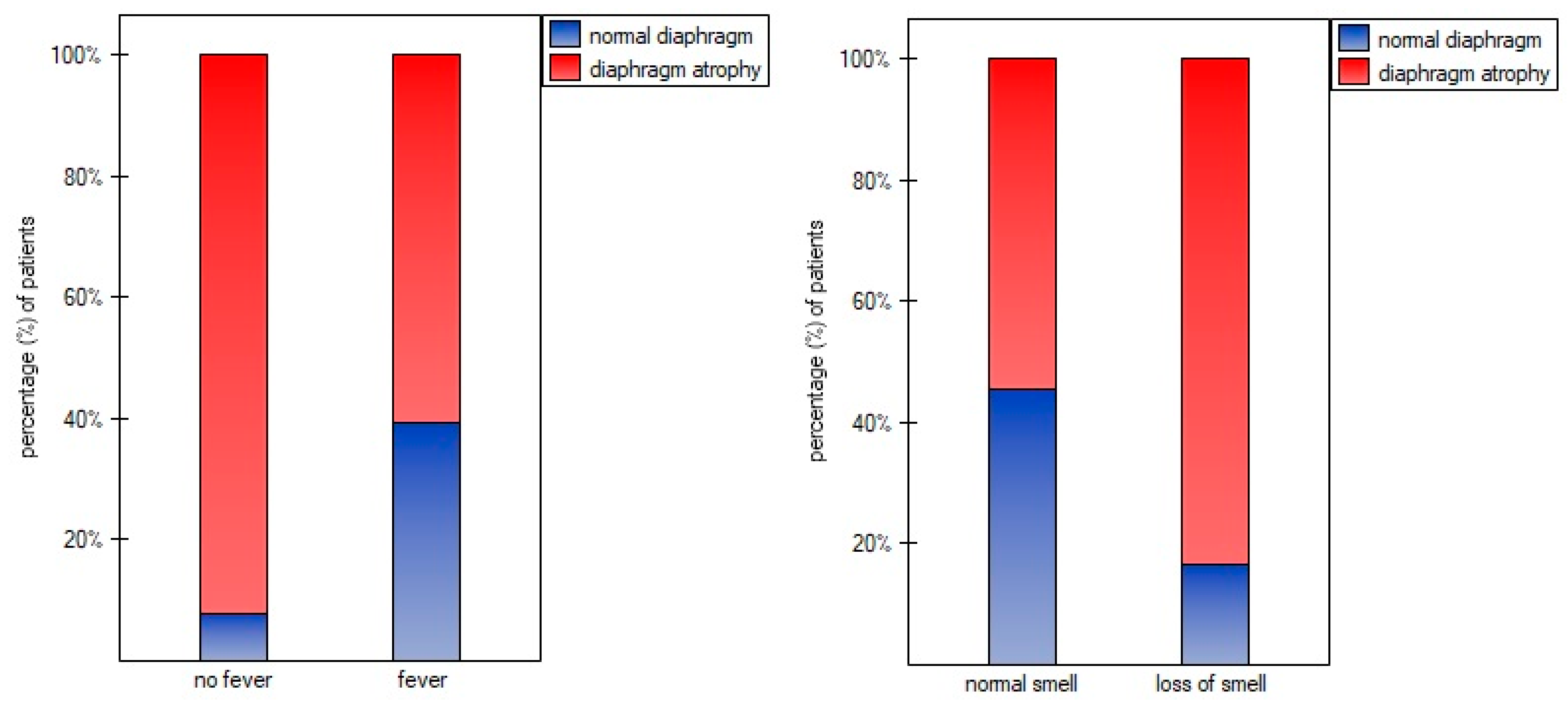
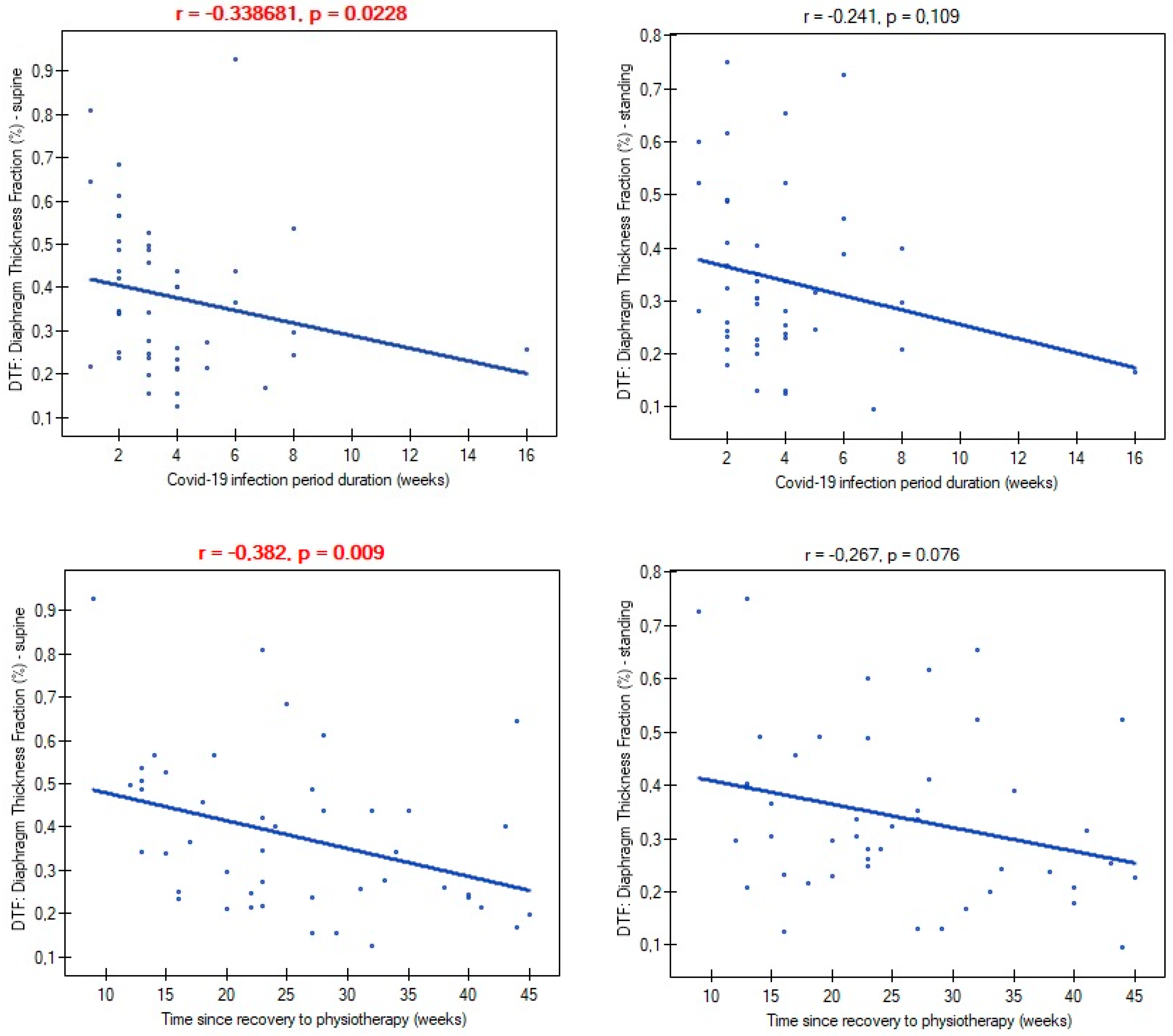
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kocjan, J.; Adamek, M.; Gzik-Zroska, B.; Czyżewski, D.; Rydel, M. Network of breathing. Multifunctional role of the diaphragm: A review. Adv. Respir. Med. 2017, 85, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Zanier, E. Anatomic connections of the diaphragm: Influence of respiration on the body system. J. Multidiscip. Healthc. 2013, 6, 281–291. [Google Scholar] [CrossRef] [PubMed]
- McCool, F.D.; Tzelepis, G.E. Dysfunction of the diaphragm. N. Engl. J. Med. 2012, 366, 932–942. [Google Scholar] [CrossRef]
- Ricoy, J.; Rodríguez-Núñez, N.; Álvarez-Dobaño, J.M.; Toubes, M.E.; Riveiro, V.; Valdés, L. Diaphragmatic dysfunction. Pulmonology 2019, 25, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Melero, M.J.; Mazzei, M.E.; Bergroth, B.; Cantardo, D.M.; Duarte, J.M.; Corti, M. Bilateral diaphragmatic paralysis in an HIV patient: Second reported case and literature review. Lung India 2014, 31, 149–151. [Google Scholar] [PubMed]
- Huh, S.; Chung, J.H.; Kwon, H.J.; Ko, H.Y. Unilateral Diaphragm Paralysis Associated with Neurosyphilis: A Case Report. Ann. Rehabil. Med. 2020, 44, 338–341. [Google Scholar] [CrossRef]
- Rudrappa, M.; Kokatnur, L.; Chernyshev, O. Neurological Respiratory Failure. Diseases 2018, 6, 7. [Google Scholar] [CrossRef]
- Betensley, A.D.; Jaffery, S.H.; Collins, H.; Sripathi, N.; Alabi, F. Bilateral diaphragmatic paralysis and related respiratory complications in a patient with West Nile virus infection. Thorax 2004, 59, 268–269. [Google Scholar] [CrossRef]
- Ratnayake, E.C.; Shivanthan, C.; Wijesiriwardena, B.C. Diaphragmatic paralysis: A rare consequence of dengue fever. BMC Infect. Dis. 2012, 12, 46. [Google Scholar] [CrossRef]
- Oike, M.; Naito, T.; Tsukada, M.; Kikuchi, Y.; Sakamoto, N.; Otsuki, Y.; Ohshima, H.; Yokokawa, H.; Isonuma, H.; Dambara, T. A case of diaphragmatic paralysis complicated by herpes-zoster virus infection. Intern. Med. 2012, 51, 1259–1263. [Google Scholar] [CrossRef]
- van der Linden, V.; Lins, O.G.; de Lima Petribu, N.C.; de Melo, A.C.M.G.; Moore, J.; Rasmussen, S.A.; Moore, C.A. Diaphragmatic paralysis: Evaluation in infants with congenital Zika syndrome. Birth Defects Res. 2019, 111, 1577–1583. [Google Scholar] [CrossRef]
- Fu, L.; Wang, B.; Yuan, T.; Chen, X.; Ao, Y.; Fitzpatrick, T.; Li, P.; Zhou, Y.; Lin, Y.-F.; Duan, Q.; et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J. Infect. 2020, 80, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.G.; Gourna Paleoudis, E.; Lesky-Di Bari, D.; Nyirenda, T.; Friedman, T.; Gupta, A.; Rasouli, L.; Zetkulic, M.; Balani, B.; Ogedegbe, C.; et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS ONE 2020, 15, e0243882. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef]
- Cesanelli, L.; Satkunskiene, D.; Bileviciute-Ljungar, I.; Kubilius, R.; Repečkaite, G.; Cesanelli, F.; Iovane, A.; Messina, G. The possible impact of COVID-19 on respiratory muscles structure and functions: A literature review. Sustainability 2022, 14, 7446. [Google Scholar] [CrossRef]
- National Health Commission of China. The Novel Coronavirus Pneumonia Diagnosis and Treatment Program, 7th version. China. 2020. Available online: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (accessed on 8 June 2024).
- GISAID. (Global Initiative on Sharing All Influenza Data). Available online: http://gisaid.com (accessed on 8 June 2024).
- Sarwal, A.; Walker, F.O.; Cartwright, M.S. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve 2013, 47, 319–329. [Google Scholar] [CrossRef]
- Boussuges, A.; Rives, S.; Finance, J.; Brégeon, F. Assessment of diaphragmatic function by ultrasonography: Current approach and perspectives. World J. Clin. Cases 2020, 8, 2408–2424. [Google Scholar] [CrossRef]
- Pinto, S.; Pinto, A.; de Carvalho, M. Phrenic nerve studies predict survival in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2012, 123, 2454–2459. [Google Scholar] [CrossRef]
- Cardenas, L.Z.; Santana, P.V.; Caruso, P.; Ribeiro de Carvalho, C.R.; Pereira de Albuquerque, A.L. Diaphragmatic Ultrasound Correlates with Inspiratory Muscle Strength and Pulmonary Function in Healthy Subjects. Ultrasound Med. Biol. 2018, 44, 786–793. [Google Scholar] [CrossRef]
- Houston, J.G.; Fleet, M.; Cowan, M.D.; McMillan, N.C. Comparison of ultrasound with fluoroscopy in the assessment of suspected hemidiaphragmatic movement abnormality. Clin. Radiol. 1995, 50, 95–98. [Google Scholar] [CrossRef]
- Mahler, D.A.; Wells, C.K. Evaluation of clinical methods for rating dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Szczegielniak, J.; Latawiec, K.J.; Łuniewski, J.; Stanisławski, R.; Bogacz, K.; Krajczy, M.; Rydel, M. A study on nonlinear estimation of submaximal effort tolerance based on the generalized MET concept and the 6MWT in pulmonary rehabilitation. PLoS ONE 2018, 13, e0191875. [Google Scholar] [CrossRef]
- Imamović, A.; Wagner, D.; Lindenmann, J.; Fink-Neuböck, N.; Sauseng, S.; Bajric, T.; Mischinger, H.J. Life threatening rupture of the diaphragm after COVID-19 pneumonia: A case report. J. Cardiothorac. Surg. 2022, 17, 145. [Google Scholar] [CrossRef]
- Shi, Z.; de Vries, H.J.; Vlaar, A.P.J.; van der Hoeven, J.; Boon, R.A.; Heunks, L.M.A.; Ottenheijm, C.A.C.; Dutch COVID-19 Diaphragm Investigators. Dutch COVID-19 Diaphragm Investigators. Diaphragm Pathology in Critically Ill Patients With COVID-19 and Postmortem Findings From 3 Medical Centers. JAMA Intern. Med. 2021, 181, 122–124. [Google Scholar] [CrossRef]
- de Boer, W.; Veldman, C.; Steenbruggen, I.; Patberg, K.W.; Van Den Berg, J. Diaphragm strength in COVID-19 patients and breathlessness. Eur. Respir. J. 2021, 58, OA4344. [Google Scholar]
- Formenti, P.; Umbrello, M.; Castagna, V.; Cenci, S.; Bichi, F.; Pozzi, T.; Bonifazi, M.; Coppola, S.; Chiumello, D. Respiratory and peripheral muscular ultrasound characteristics in ICU COVID 19 ARDS patients. J. Crit. Care 2022, 67, 14–20. [Google Scholar] [CrossRef]
- FitzMaurice, T.S.; McCann, C.; Walshaw, M.; Greenwood, J. Unilateral diaphragm paralysis with COVID-19 infection. BMJ Case Rep. 2021, 14, e243115. [Google Scholar] [CrossRef]
- Shahid, M.; Ali Nasir, S.; Shahid, O.; Nasir, S.A.; Khan, M.W. Unilateral Diaphragmatic Paralysis in a Patient with COVID-19 Pneumonia. Cureus 2021, 13, e19322. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, N.; Humphreys, C.; Gordan, P.; Okin, D. Diaphragmatic paralysis in COVID-19: A rare cause of postacute sequelae of COVID-19 dyspnoea. BMJ Case Rep. 2021, 14, e246668. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.; Garg, A.; Fakih, R.; Hamzeh, N.Y. Bilateral diaphragmatic dysfunction: A cause of persistent dyspnea in patients with post-acute sequelae of SARS-CoV-2. SAGE Open Med. Case Rep. 2022, 10, 2050313X221105990. [Google Scholar] [CrossRef]
- Borroni, B.; Gazzina, S.; Dono, F.; Mazzoleni, V.; Liberini, P.; Carrarini, C.; Russo, M.; Pontolillo, M.; Vecchiet, J.; Onofrj, M.; et al. Diaphragmatic myoclonus due to SARS-CoV-2 infection. Neurol. Sci. 2020, 41, 3471–3474. [Google Scholar] [CrossRef]
- Satici, C.; Aydin, S.; Tuna, L.; Köybasi, G.; Koşar, F. Electromyographic and Sonographic Assessment of Diaphragm Dysfunction in Patients Who Recovered from the COVID-19 Pneumonia. Tuberk. Toraks 2021, 69, 425–428. [Google Scholar] [CrossRef]
- Helmy, M.A.; Magdy Milad, L.; Osman, S.H.; Ali, M.A.; Hasanin, A. Diaphragmatic excursion: A possible key player for predicting successful weaning in patients with severe COVID-19. Anaesth. Crit. Care Pain Med. 2021, 40, 100875. [Google Scholar] [CrossRef]
- Umbrello, M.; Guglielmetti, L.; Formenti, P.; Antonucci, E.; Cereghini, S.; Filardo, C.; Montanari, G.; Muttini, S. Qualitative and quantitative muscle ultrasound changes in patients with COVID-19-related ARDS. Nutrition 2021, 91, 111449. [Google Scholar] [CrossRef]
- Corradi, F.; Vetrugno, L.; Orso, D.; Bove, T.; Schreiber, A.; Boero, E.; Santori, G.; Isirdi, A.; Barbieri, G.; Forfori, G.; et al. Diaphragmatic thickening fraction as a potential predictor of response to continuous positive airway pressure ventilation in COVID-19 pneumonia: A single-center pilot study. Respir. Physiol. Neurobiol. 2021, 284, 103585. [Google Scholar] [CrossRef]
- Corradi, F.; Isirdi, A.; Malacarne, P.; Santori, G.; Barbieri, G.; Romei, C.; Bove, T.; Vetrugno, L.; Falcone, M.; Bertini, P.; et al. Low diaphragm muscle mass predicts adverse outcome in patients hospitalized for COVID-19 pneumonia: An exploratory pilot study. Minerva Anestesiol. 2021, 87, 432–438. [Google Scholar] [CrossRef]
- Van Steveninck, A.L.; Imming, L.M. Diaphragm dysfunction prior to intubation in a patient with COVID-19 pneumonia; assessment by point of care ultrasound and potential implications for patient monitoring. Respir. Med. Case Rep. 2020, 31, 101284. [Google Scholar] [CrossRef]
- Farr, E.; Wolfe, A.R.; Deshmukh, S.; Rydberg, L.; Soriano, R.; Walter, J.M.; Franz, C.K. Diaphragm dysfunction in severe COVID-19 as determined by neuromuscular ultrasound. Ann. Clin. Transl. Neurol. 2021, 8, 1745–1749. [Google Scholar] [CrossRef]
| Variables | M ± SD [95% CI] or N (%) |
|---|---|
| Age (years): | 58.24 ± 10.70 [55.02–61.46] |
| <40 years | 2 (4.44%) |
| 41–50 years | 9 (20.0%) |
| 51–60 years | 16 (35.55) |
| 61–70 years | 13 (28.88%) |
| >70 years | 5 (11.11%) |
| Gender: | |
| male | 20 (43.47%) |
| female | 26 (56.52%) |
| Height (cm) | 1.67 ± 0.08 [1.64–1.69] |
| Weight (kg) | 79.64 ± 16.50 [74.73–84.54] |
| BMI (points): | 28.42 ± 4.83 [26.98–29.85] |
| 18.50–24.99 (normal weight) | 11 (23.91%) |
| 25.00–29.99 (overweight) | 17 (36.95%) |
| >30.00 (obesity) | 23 (50.0%) |
| COVID-19 symptoms: | |
| lack of symptoms | 0 (0.0%) |
| cough | 29 (63.04%) |
| fever | 33 (71.73%) |
| dyspnea | 33 (71.73%) |
| loss of smell | 24 (52.17%) |
| loss of taste | 23 (50.0%) |
| chest pain | 20 (43.47%) |
| neck pain | 8 (17.39%) |
| vomiting | 5 (10.87%) |
| reflux | 1 (2.17%) |
| general muscle pain | 30 (65.21%) |
| intercostal pain | 10 (21.73%) |
| mean number of symptoms per patient | 4.80 ± 1.83 [4.25–5.34] |
| COVID-19 symptom duration time (weeks) | 3.76 ± 2.59 [2.99–4.53] |
| Time since recovery to start physiotherapy (weeks) | 26.65 ± 9.98 [22.68–28.61] |
| Severity of COVID-19 disease: | |
| asymptomatic | 0 (0%) |
| mild | 12 (26.09%) |
| moderate | 31 (67.39%) |
| severe | 3 (6.52%) |
| critical | 0 (0%) |
| Oxygen therapy usage during COVID-19 course: | |
| yes | 21 (45.65%) |
| no | 25 (54.35%) |
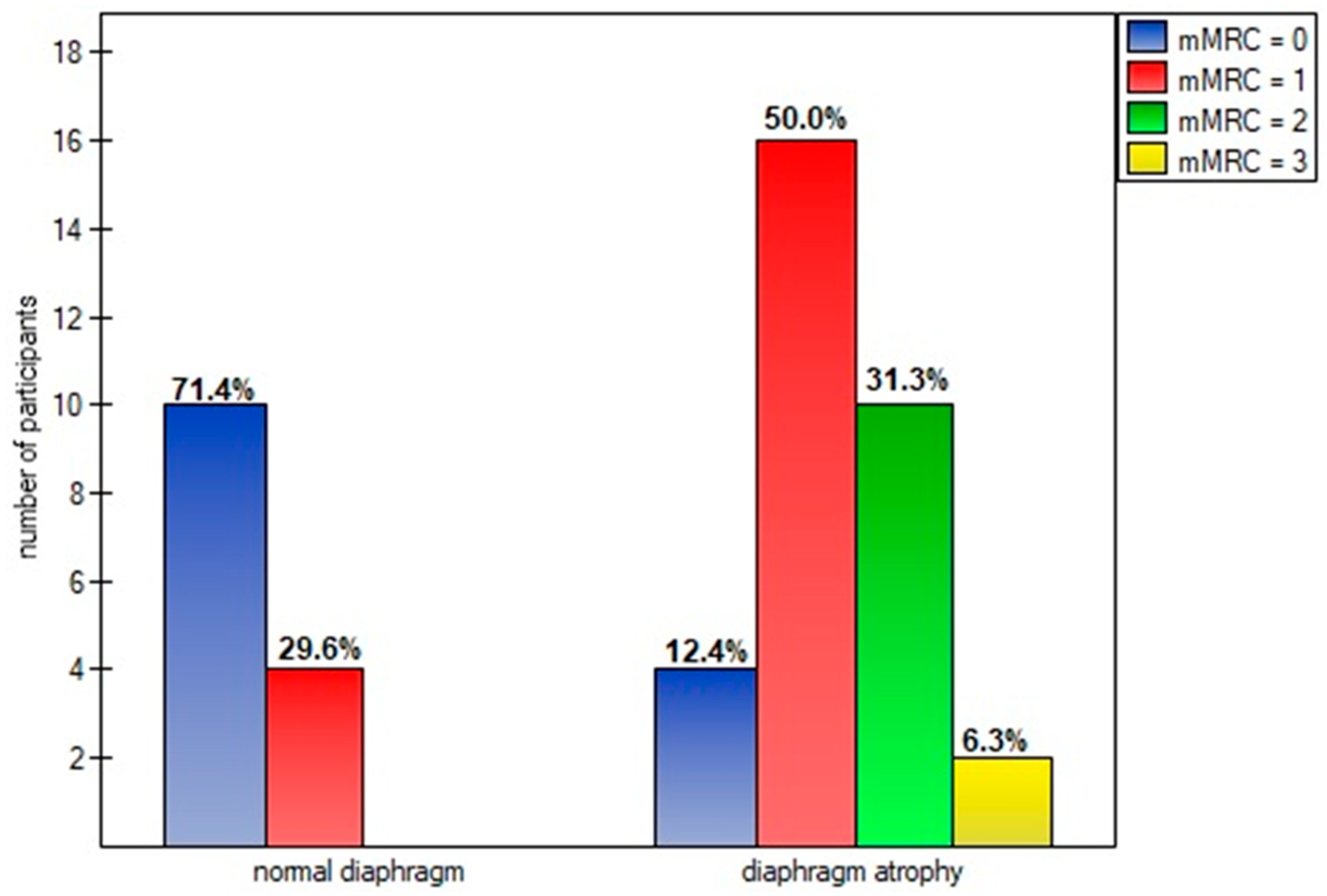
| mMRC scale (points): | 1.24 ± 0.80 [1.00–1.48] |
| grade 0 | 14 (30.43%) |
| grade 1 | 20 (43.48%) |
| grade 2 | 10 (21.74%) |
| grade 3 | 2 (4.35%) |
| 6-min walk test distance (meters) | 539.60 ± 83.29 [514.87–564.34] |
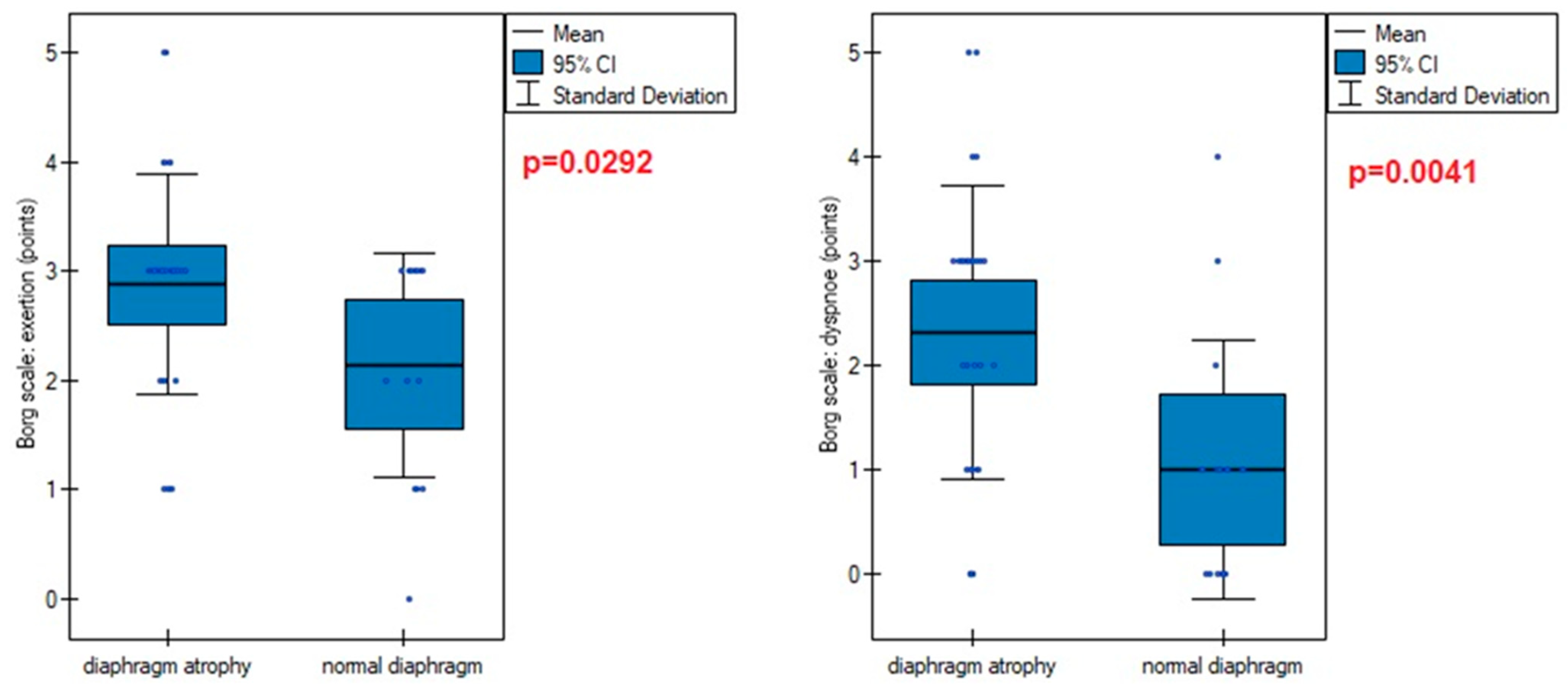
| Borg scale (points): | |
| fatigue | 2.65 ± 1.05 [2.33–2.96] |
| dyspnea | 1.91 ± 1.47 [1.47–2.35] |
| Saturation: | |
| before 6-min walk test | 95.51 + 1.78 [94.68–95.74] 95.41 + 1.90 [94.84–95.97] |
| after 6-min walk test | |
| MET (mL O2/min/kg) | 7.35 ±1.92 [6.78–7.93] |
| Variables | Supine Position | Standing Position | p-Value | |
|---|---|---|---|---|
| Diaphragm Inspiratory Thickness (cm) | left | 0.219 ± 0.052 (0.204–0.235) | 0.201 ± 0.047 (0.187–0.216) | <0.001 |
| right | 0.228 ± 0.049 (0.213–0.242) | 0.208 ± 0.046 (0.194–0.222) | <0.001 | |
| p-value | 0.085 | 0.089 | - | |
| Diaphragm Expiratory Thickness (cm) | left | 0.163 ± 0.040 (0.151–0.175) | 0.153 ± 0.039 (0.141–0.165) | 0.121 |
| right | 0.167 ± 0.040 (0.155–0.179) | 0.157 ± 0.039 (0.145–0.169) | 0.113 | |
| p-value | 0.121 | 0.093 | - | |
| Diaphragm Thickness Fraction (DTF) (%) | left | 36.26 ± 18.72 (30.70–41.82) | 33.03 ± 15.77 (28.34–37.71) | 0.118 |
| right | 38.69 ± 18.99 (33.05–44.33) | 34.42 ± 18.30 (28.98–39.85) | 0.087 | |
| p-value | 0.159 | 0.439 | - | |
| Diaphragm Thickening Ratio (DTR) | left | 1.367 ± 0.186 (1.311–1.423) | 1.332 ± 0.158 (1.284–1.380) | 0.096 |
| right | 1.390 ± 0.190 (1.332–1.447) | 1.347 ± 0.183 (1.292–1.402) | 0.093 | |
| p-value | 0.197 | 0.420 | - | |
| Variables | Saturation before 6-Min Walk Test | Saturation after 6-Min Walk Test | Borg Scale: Fatigue | Borg Scale: Dyspnea | MET | |
|---|---|---|---|---|---|---|
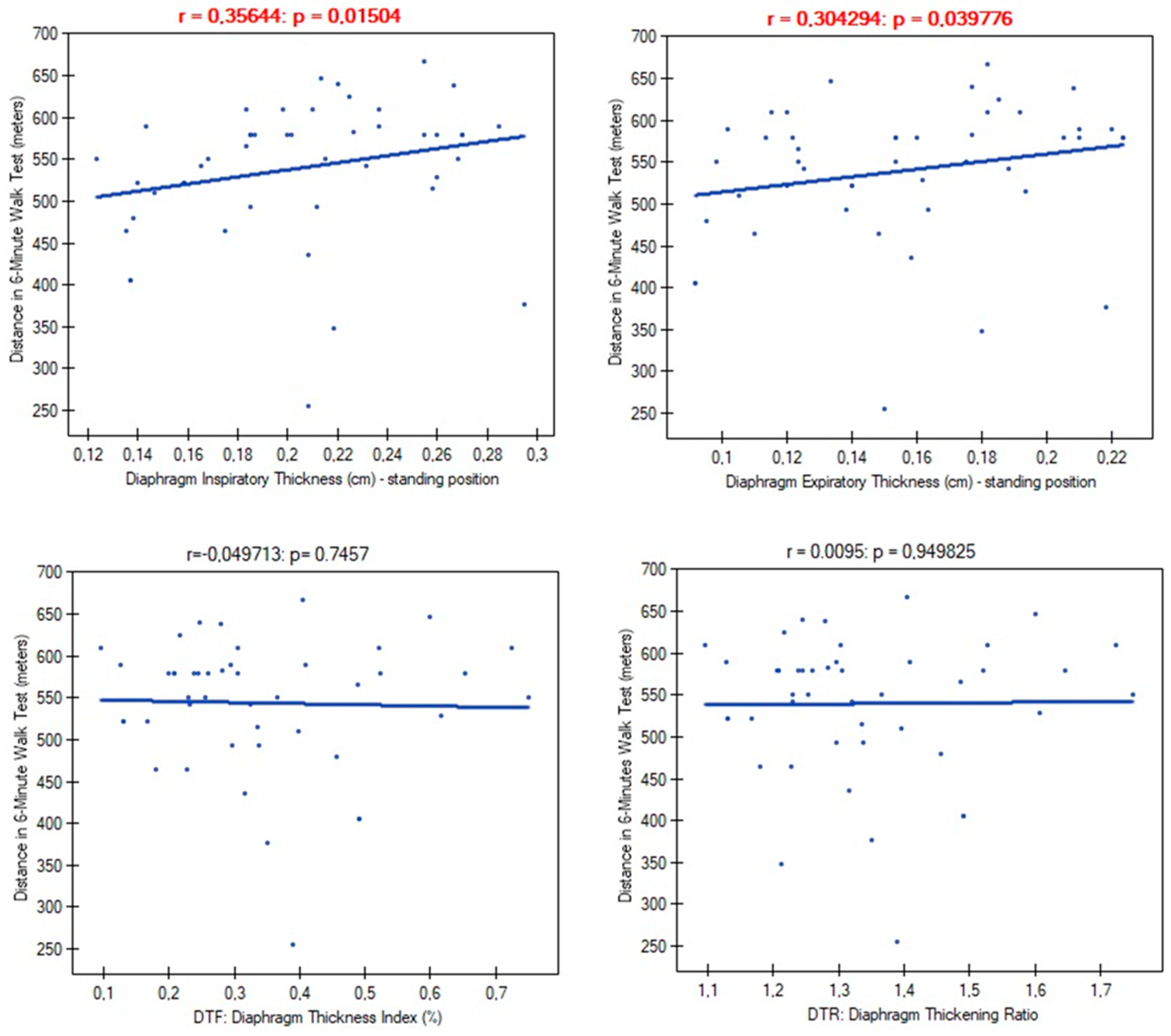
| ThIns | r | −0.021 | −0.020 | −0.534 | −0.417 | 0.323 |
| p-value | 0.8848 | 0.8914 | 0.0001 | 0.0038 | 0.0281 | |
| ThExp | r | −0.083 | −0.128 | −0.362 | −0.302 | 0.277 |
| p-value | 0.5814 | 0.3959 | 0.0134 | 0.0409 | 0.0615 | |
| DTF | r | 0.171 | 0.334 | −0.129 | −0.077 | −0.0541 |
| p-value | 0.2598 | 0.0248 | 0.3954 | 0.6117 | 0.7239 | |
| DTR | r | 0.163 | 0.324 | −0.138 | −0.098 | −0.013 |
| p-value | 0.2782 | 0.0280 | 0.3596 | 0.5129 | 0.9290 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocjan, J.; Rydel, M.; Szczegielniak, J.; Bogacz, K.; Adamek, M. Diaphragm Muscle Atrophy Contributes to Low Physical Capacity in COVID-19 Survivors. Life 2024, 14, 1117. https://doi.org/10.3390/life14091117
Kocjan J, Rydel M, Szczegielniak J, Bogacz K, Adamek M. Diaphragm Muscle Atrophy Contributes to Low Physical Capacity in COVID-19 Survivors. Life. 2024; 14(9):1117. https://doi.org/10.3390/life14091117
Chicago/Turabian StyleKocjan, Janusz, Mateusz Rydel, Jan Szczegielniak, Katarzyna Bogacz, and Mariusz Adamek. 2024. "Diaphragm Muscle Atrophy Contributes to Low Physical Capacity in COVID-19 Survivors" Life 14, no. 9: 1117. https://doi.org/10.3390/life14091117
APA StyleKocjan, J., Rydel, M., Szczegielniak, J., Bogacz, K., & Adamek, M. (2024). Diaphragm Muscle Atrophy Contributes to Low Physical Capacity in COVID-19 Survivors. Life, 14(9), 1117. https://doi.org/10.3390/life14091117






