Clinical Trial Findings and Drug Development Challenges for Curcumin in Infectious Disease Prevention and Treatment
Abstract
1. Introduction
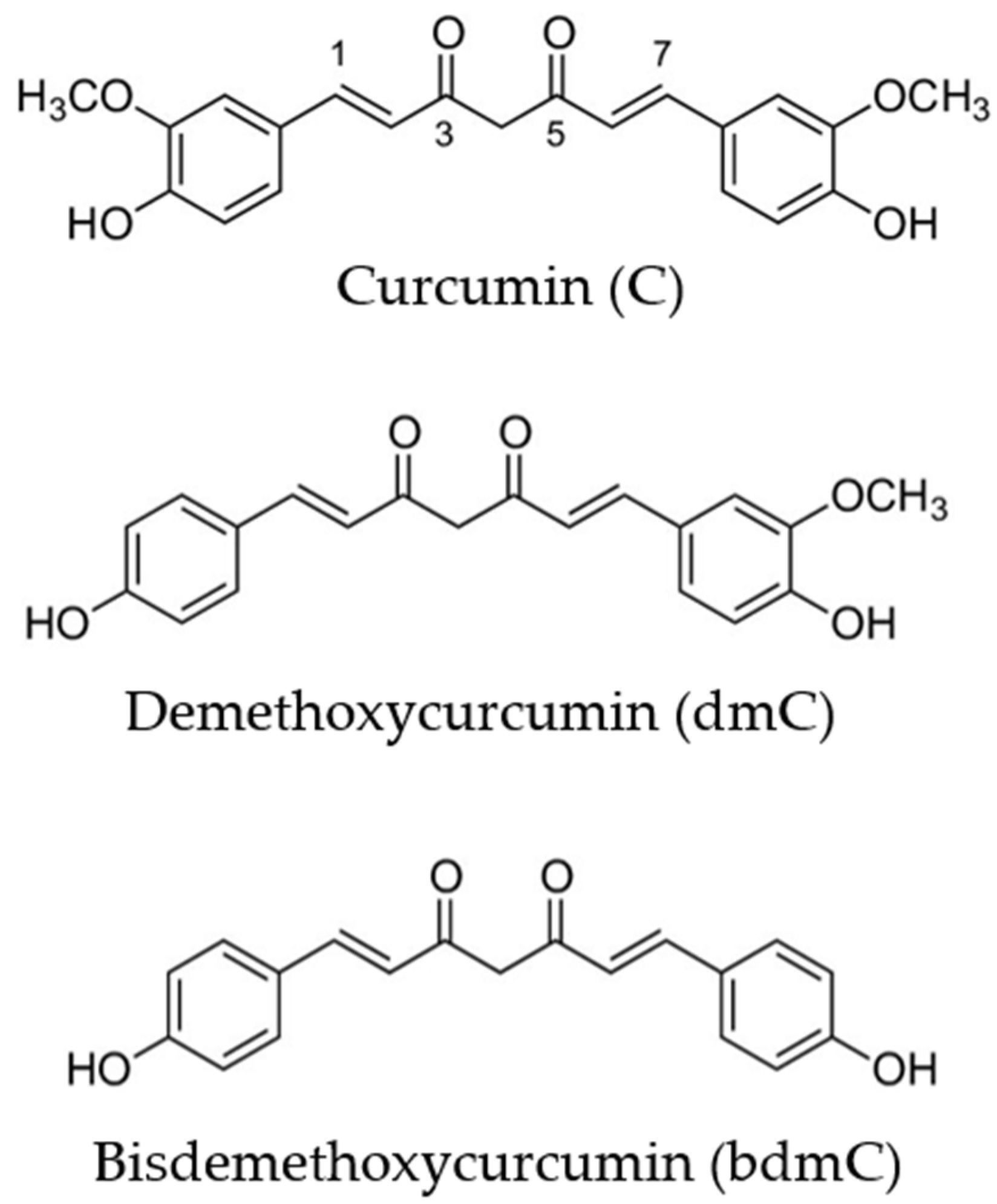
2. Bioavailability and Metabolism of Curcumin
3. Antimicrobial Properties of Curcumin
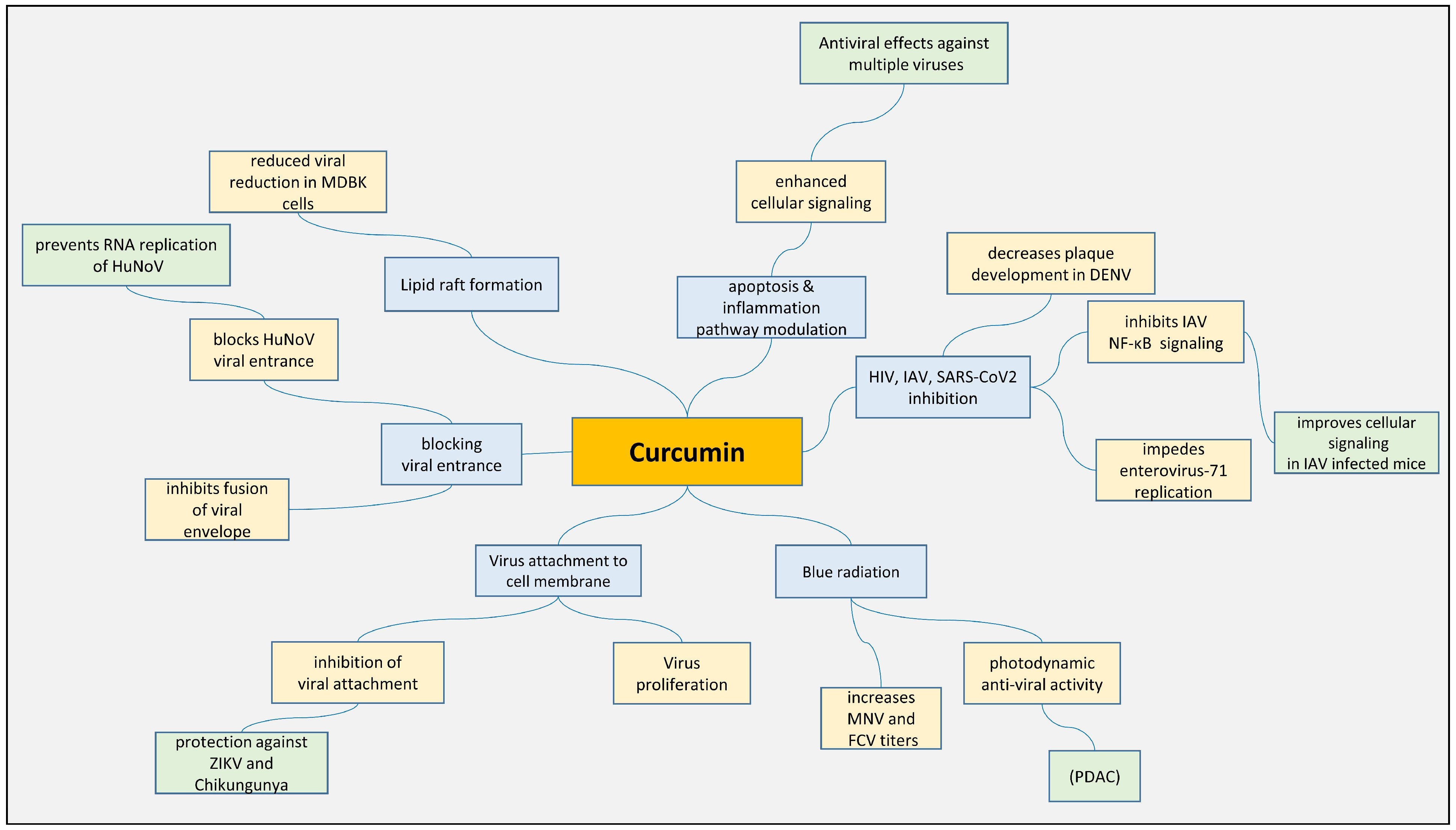
3.1. Antiviral Effect of Curcumin
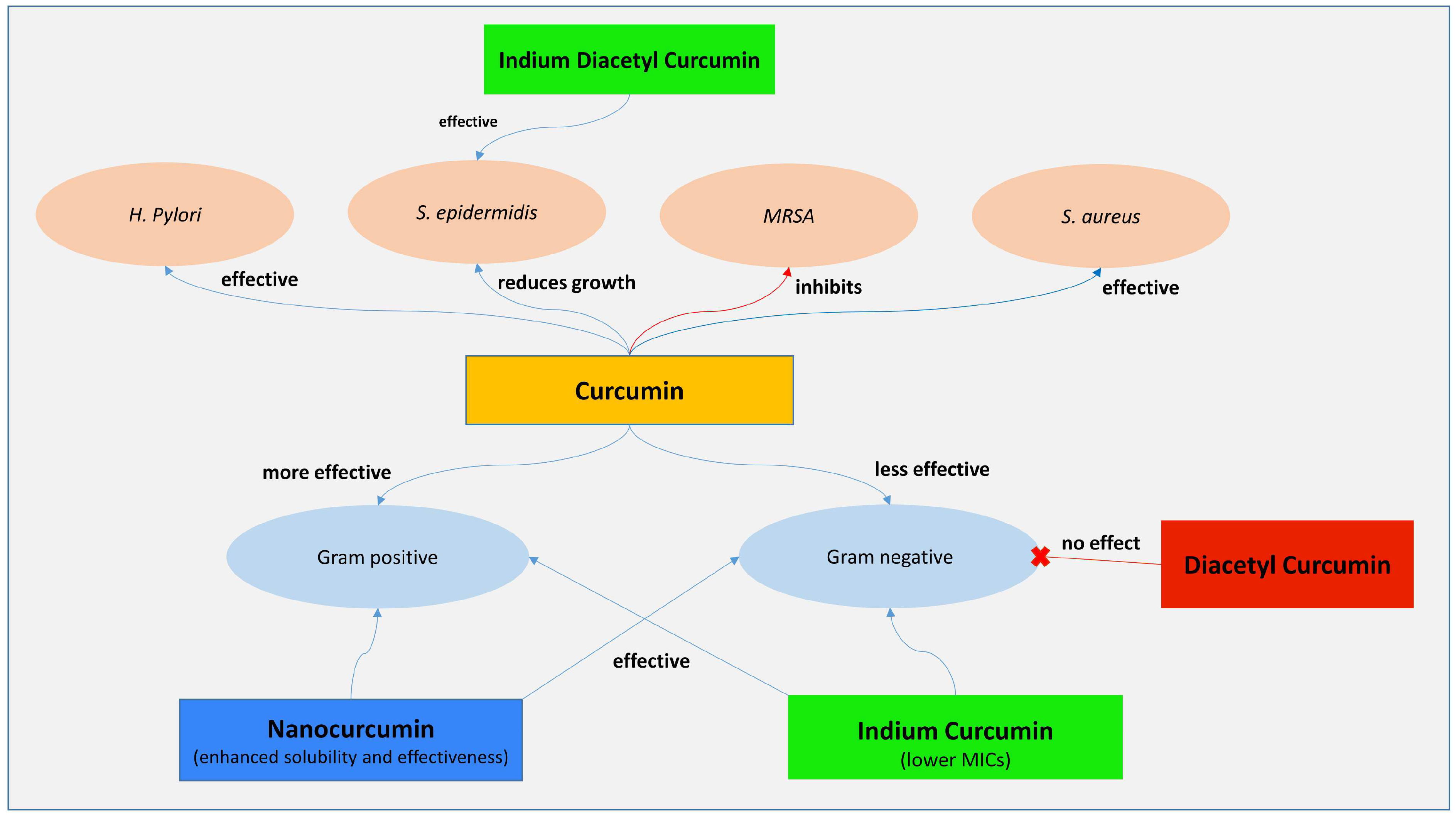
3.2. Antibacterial Effect of Curcumin
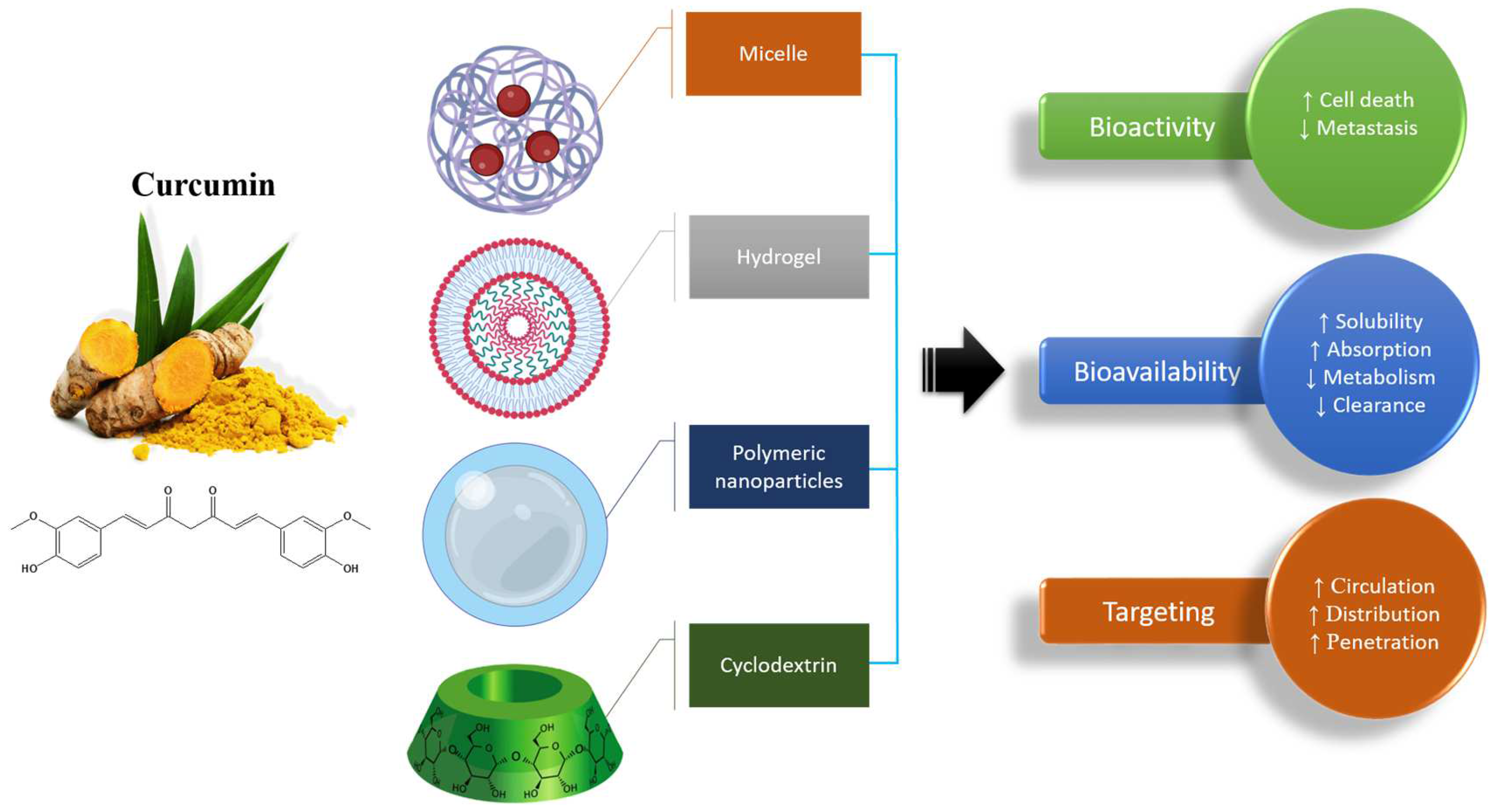
4. Nanoformulations Based on Curcumin
5. Combination Therapy Using Curcumin
6. Limited Curcumin-Based Clinical Trials
7. Side Effects Associated with Curcumin
8. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alhasawi, M.A.I.; Aatif, M.; Muteeb, G.; Alam, M.W.; El Oirdi, M.; Farhan, M. Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions. Molecules 2022, 27, 7410. [Google Scholar] [CrossRef]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Purcar, V. Curcumin: Modern Applications for a Versatile Additive. Coatings 2021, 11, 519. [Google Scholar] [CrossRef]
- Patil, S.S.; Rathod, V.K. Synergistic Effect of Ultrasound and Three Phase Partitioning for the Extraction of Curcuminoids from Curcuma longa and its Bioactivity Profile. Process. Biochem. 2020, 93, 85–93. [Google Scholar] [CrossRef]
- Patil, S.S.; Pathak, A.; Rathod, V.K. Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: A greener route for extraction of curcuminoids from Curcuma longa. Ultrason. Sonochem. 2021, 70, 105267. [Google Scholar] [CrossRef]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Degot, P.; Huber, V.; El Maangar, A.; Gramüller, J.; Rohr, L.; Touraud, D.; Zemb, T.; Gschwind, R.M.; Kunz, W. Triple role of sodium salicylate in solubilization, extraction, and stabilization of curcumin from Curcuma longa. J. Mol. Liq. 2021, 329, 115538. [Google Scholar] [CrossRef]
- Jennings, M.; Parks, R. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef]
- Hoekstra, J.C.; Leefang, P.S.H. Marketing in the era of COVID-19. Ital. J. Mark. 2020, 2020, 249–260. [Google Scholar] [CrossRef]
- Shetty, T.; Dubey, A.; Ravi, G.S.; Hebbar, S.; Shastry, C.S.; Charyulu, N. Antifungal and antioxidant therapy for the treatment of fungal infection with microemulsion gel containing curcumin and vitamin C. Asian J. Pharm. 2017, 11, 717–725. [Google Scholar]
- Sarwar, S.; Netzel, G.; Netzel, M.E.; Mereddy, R.; Phan, A.D.T.; Hong, H.T.; Cozzolino, D.; Sultanbawa, Y. Impact of Curcumin-Mediated Photosensitization on Fungal Growth, Physicochemical Properties and Nutritional Composition in Australian Grown Strawberry. Food Anal. Methods 2021, 14, 465–472. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Panichayupakaranant, P.; Lateh, L.; Yuenyongsawad, S.; Chen, H. A green method for preparation of curcuminoid-rich Curcuma longa extract and evaluation of its anticancer activity. Pharmacogn. Mag. 2019, 15, 730. [Google Scholar] [CrossRef]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Tong, T. Curcumae rhizoma: A botanical drug against infectious diseases. Front. Pharmacol. 2022, 13, 1015098. [Google Scholar] [CrossRef] [PubMed]
- Heger, M.; Van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The Molecular Basis for the Pharmacokinetics and Pharmacodynamics of Curcumin and Its Metabolites in Relation to Cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses. Molecules 2015, 20, 185–205. [Google Scholar] [CrossRef]
- Mathew, D.; Hsu, W.-L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of phytonutrient modulation of cyclooxygenase-2 (COX-2) and inflammation related to cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017, 142, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ding, X.; Zhang, D.; Ch, Y.; Wang, J.; Ndegwa, E.; Zhu, G. Curcumin inhibits bovine herpesvirus type 1 entry into MDBK cells. Acta Virol. 2015, 59, 221–227. [Google Scholar] [CrossRef]
- Colpitts, C.C.; Schang, L.M.; Rachmawati, H.; Frentzen, A.; Pfaender, S.; Behrendt, P.; Brown, R.J.; Bankwitz, D.; Steinmann, J.; Ott, M. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut 2014, 63, 1137–1149. [Google Scholar]
- Yang, M.; Lee, G.; Si, J.; Lee, S.-J.; You, H.J.; Ko, G. Curcumin shows antiviral properties against norovirus. Molecules 2016, 21, 1401. [Google Scholar] [CrossRef]
- Wu, J.; Hou, W.; Cao, B.; Zuo, T.; Xue, C.; Leung, A.W.; Xu, C.; Tang, Q.-J. Virucidal efficacy of treatment with photodynamically activated curcumin on murine norovirus bio-accumulated in oysters. Photodiagnosis Photodyn. Ther. 2015, 12, 385–392. [Google Scholar] [CrossRef]
- Randazzo, W.; Aznar, R.; Sánchez, G. Curcumin-mediated photodynamic inactivation of norovirus surrogates. Food Environ. Virol. 2016, 8, 244–250. [Google Scholar] [CrossRef]
- Gao, Y.; Tai, W.; Wang, N.; Li, X.; Jiang, S.; Debnath, A.K.; Du, L.; Chen, S. Identification of novel natural products as effective and broad-spectrum anti-zika virus inhibitors. Viruses 2019, 11, 1019. [Google Scholar] [CrossRef]
- von Rhein, C.; Weidner, T.; Henß, L.; Martin, J.; Weber, C.; Sliva, K.; Schnierle, B.S. Curcumin and Boswellia serrata gum resin extract inhibit chikungunya and vesicular stomatitis virus infections in vitro. Antivir. Res. 2016, 125, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Liu, Y.; Luo, X.; Lei, W.; Xie, L. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J. Gen. Virol. 2020, 101, 1079–1084. [Google Scholar] [CrossRef]
- Du, T.; Shi, Y.; Xiao, S.; Li, N.; Zhao, Q.; Zhang, A.; Nan, Y.; Mu, Y.; Sun, Y.; Wu, C. Curcumin is a promising inhibitor of genotype 2 porcine reproductive and respiratory syndrome virus infection. BMC Vet. Res. 2017, 13, 298. [Google Scholar] [CrossRef]
- Ranjbar, R.; Bagheri, H.; Ghasemi, F.; Guest, P.C.; Sahebkar, A. Effects of curcumin and Its analogues on infectious diseases. Adv. Exp. Med. Biol. 2021, 1291, 75–101. [Google Scholar] [PubMed]
- Mirani, A.; Kundaikar, H.; Velhal, S.; Patel, V.; Bandivdekar, A.; Degani, M.; Patravale, V. Tetrahydrocurcumin-loaded vaginal nanomicrobicide for prophylaxis of HIV/AIDS: In silico study, formulation development, and in vitro evaluation. Drug Deliv. Transl. Res. 2019, 9, 828–847. [Google Scholar] [CrossRef] [PubMed]
- Padilla-s, L.; Rodríguez, A.; Gonzales, M.M.; Gallego-g, J.C.; Castaño-o, J.C. Inhibitory effects of curcumin on dengue virus type 2-infected cells in vitro. Arch. Virol. 2014, 159, 573–579. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Pilankatta, R.; Teramoto, T.; Sajith, A.M.; Nwulia, E.; Kulkarni, A.; Padmanabhan, R. Inhibition of dengue virus by curcuminoids. Antivir. Res. 2019, 162, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Santos, C.A.D. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Expert Rev. Anti-Infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Gu, L.; Su, Y.; Wang, Q.; Zhao, Y.; Chen, X.; Deng, H.; Li, W.; Wang, G.; Li, K. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int. Immunopharmacol. 2018, 54, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Y.; Shien, J.-H.; Tiley, L.; Chiou, S.-S.; Wang, S.-Y.; Chang, T.-J.; Lee, Y.-J.; Chan, K.-W.; Hsu, W.-L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010, 119, 1346–1351. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Han, S.; Xu, J.; Guo, X.; Huang, M. Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production. Clin. Exp. Pharmacol. Physiol. 2018, 45, 84–93. [Google Scholar] [CrossRef]
- Casanova, F.; Pereira, C.F.; Ribeiro, A.B.; Castro, P.M.; Freixo, R.; Martins, E.; Tavares-Valente, D.; Fernandes, J.C.; Pintado, M.E.; Ramos, Ó.L. Biological Potential and Bioaccessibility of Encapsulated Curcumin into Cetyltrimethylammonium Bromide Modified Cellulose Nanocrystals. Pharmaceuticals 2023, 16, 1737. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Yan, Y.; Liao, S.; Li, Y.; Ye, Y.; Liu, N.; Zhao, F.; Xu, P. 3D-quantitative structure–activity relationship and antiviral effects of curcumin derivatives as potent inhibitors of influenza H1N1 neuraminidase. Arch. Pharm. Res. 2020, 43, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-I.; Chio, C.-C.; Lin, J.-Y. Inhibition of EV71 by curcumin in intestinal epithelial cells. PLoS ONE 2018, 13, e0191617. [Google Scholar] [CrossRef]
- Qin, Y.; Lin, L.; Chen, Y.; Wu, S.; Si, X.; Wu, H.; Zhai, X.; Wang, Y.; Tong, L.; Pan, B. Curcumin inhibits the replication of enterovirus 71 in vitro. Acta Pharm. Sin. B 2014, 4, 284–294. [Google Scholar] [CrossRef]
- Wen, C.-C.; Kuo, Y.-H.; Jan, J.-T.; Liang, P.-H.; Wang, S.-Y.; Liu, H.-G.; Lee, C.-K.; Chang, S.-T.; Kuo, C.-J.; Lee, S.-S. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-Rasadi, K.; Banach, M.; Sahebkar, A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020, 34, 2911–2920. [Google Scholar] [CrossRef]
- Maurya, V.K.; Kumar, S.; Prasad, A.K.; Bhatt, M.L.; Saxena, S.K. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virusdisease 2020, 31, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Angelini, P. Plant-Derived Antimicrobials and Their Crucial Role in Combating Antimicrobial Resistance. Antibiotics 2024, 13, 746. [Google Scholar] [CrossRef]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef]
- Ji, J.; Ma, Z.; Wang, Y. Advancing Gastrointestinal Health: Curcumin’s Efficacy and Nanopreparations. Molecules 2024, 29, 1659. [Google Scholar] [CrossRef]
- Barua, N.; Buragohain, A.K. Therapeutic Potential of Curcumin as an Antimycobacterial Agent. Biomolecules 2021, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, S.; Mohammadi, K.; Deilami, I.; Zandi, K.; Fouladvand, M.; Ramedani, E.; Asayesh, G. Antibacterial activity of indium curcumin and indium diacetylcurcumin. Afr. J. Biotechnol. 2008, 7, 3832–3835. [Google Scholar]
- Izui, S.; Sekine, S.; Maeda, K.; Kuboniwa, M.; Takada, A.; Amano, A.; Nagata, H. Antibacterial activity of curcumin against periodontopathic bacteria. J. Periodontol. 2016, 87, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Mandroli, P.S.; Bhat, K. An in-vitro evaluation of antibacterial activity of curcumin against common endodontic bacteria. J. Appl. Pharm. Sci. 2013, 3, 16. [Google Scholar]
- Basniwal, R.K.; Buttar, H.S.; Jain, V.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar]
- Khorsandi, K.; Hosseinzadeh, R.; Sadat Esfahani, H.; Keyvani-Ghamsari, S.; Ur Rahman, S. Nanomaterials as drug delivery systems with antibacterial properties: Current trends and future priorities. Expert Rev. Anti-Infect. Ther. 2021, 19, 1299–1323. [Google Scholar] [CrossRef]
- Varaprasad, K.; López, M.; Núñez, D.; Jayaramudu, T.; Sadiku, E.R.; Karthikeyan, C.; Oyarzúnc, P. Antibiotic copper oxide-curcumin nanomaterials for antibacterial applications. J. Mol. Liq. 2020, 300, 112353. [Google Scholar] [CrossRef]
- Mody, D.; Athamneh, A.I.; Seleem, M.N. Curcumin: A natural derivative with antibacterial activity against Clostridium difficile. J. Glob. Antimicrob. Resist. 2020, 21, 154–161. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Li, W.; Deng, X.; Deng, Y.; Niu, X. Curcumin protects mice from Staphylococcus aureus pneumonia by interfering with the self-assembly process of α-hemolysin. Sci. Rep. 2016, 6, 28254. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Pourakbari, B.; Bahador, A. Contribution of antimicrobial photo-sonodynamic therapy in wound healing: An in vivo effect of curcumin-nisin-based poly (L-lactic acid) nanoparticle on Acinetobacter baumannii biofilms. BMC Microbiol. 2022, 22, 28. [Google Scholar] [CrossRef]
- Elfaky, M.A.; Abdel-Hamid, M.I.; Khalifa, E.; Alshareef, W.A.; Mosbah, R.A.; Elazab, S.T.; Ghoneim, M.M.; Al-Sanea, M.M.; Bendary, M.M. Innovative next-generation therapies in combating multi-drug-resistant and multi-virulent Escherichia coli isolates: Insights from in vitro, in vivo, and molecular docking studies. Appl. Microbiol. Biotechnol. 2022, 106, 1691–1703. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef]
- Yun, D.G.; Lee, D.G. Antibacterial activity of curcumin via apoptosis-like response in Escherichia coli. App. Microbiol. Biotechnol. 2016, 100, 5505–5514. [Google Scholar] [CrossRef]
- Singh, R.P.; Jain, D. Evaluation of antimicrobial activity of curcuminoids isolated from turmeric. Int. J. Pharm. Life Sci. 2012, 3, 1368–1376. [Google Scholar]
- Bellio, P.; Brisdelli, F.; Perilli, M.; Sabatini, A.; Bottoni, C.; Segatore, B.; Setacci, D.; Amicosante, G.; Celenza, G. Curcumin inhibits the SOS response induced by levofloxacin in Escherichia coli. Phytomedicine 2014, 21, 430–434. [Google Scholar] [CrossRef]
- Bahari, S.; Zeighami, H.; Mirshahabi, H.; Roudashti, S.; Haghi, F. Inhibition of Pseudomonas aeruginosa quorum sensing by subinhibitory concentrations of curcumin with gentamicin and azithromycin. J. Glob. Antimicrob. Resist. 2017, 10, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Teow, S.Y.; Liew, K.; Ali, S.A.; Khoo, A.S.; Peh, S.C. Antibacterial action of curcumin against staphylococcus aureus: A brief review. J. Trop. Med. 2016, 2016, 2853045. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.A.; Kumar, R.; Ajitkumar, P.; Nagaraja, V.; Chakravortty, D. Curcumin reduces the antimicrobial activity of ciprofloxacin against Salmonella typhimurium and Salmonella typhi. J. Antimicrob. Chemother. 2013, 68, 139–152. [Google Scholar] [CrossRef]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef]
- Khanra, S.; Kumar, Y.P.; Dash, J.; Banerjee, R. In vitro screening of known drugs identified by scaffold hopping techniques shows promising leishmanicidal activity for suramin and netilmicin. BMC Res. Notes 2018, 51, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Obloza, M.; Milewska, A.; Botwina, P.; Szczepanski, A.; Medaj, A.; Bonarek, P.; Szczubialka, K.; Pyrc, K.; Nowakowska, M. Curcumin-poly(sodium 4-styrenesulfonate) conjugates as potent zika virus entry inhibitors. ACS Appl. Mater. Interfaces 2024, 16, 5426–5437. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Cwiklinski, K.; Aalinkeel, R.; Reynolds, J.L.; Sykes, D.E.; Quaye, E.; Oh, J.; Mahajan, S.D.; Schwartz, S.A. Immunomodulatory activities of curcumin-stabilized silver nanoparticles: Efficacy as an antiretroviral therapeutic. Immunol. Investig. 2017, 46, 833–846. [Google Scholar] [CrossRef]
- Sivani, B.M.; Azzeh, M.; Patnaik, R.; Pantea Stoian, A.; Rizzo, M.; Banerjee, Y. Reconnoitering the Therapeutic Role of Curcumin in Disease Prevention and Treatment: Lessons Learnt and Future Directions. Metabolites 2022, 12, 639. [Google Scholar] [CrossRef]
- Li, H.; Zhong, C.; Wang, Q.; Chen, W.; Yuan, Y. Curcumin is an APE1 redox inhibitor and exhibits an antiviral activity against KSHV replication and pathogenesis. Antivir. Res. 2019, 167, 98–103. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, Y.; Cao, X.; Liu, Y.; Ying, R.; Huang, Q.; Gao, P.; Zhang, C. Curcumin as an antiviral agent and immune-inflammatory modulator in COVID-19: A scientometric analysis. Heliyon 2023, 9, e21648. [Google Scholar] [CrossRef]
- Nabila, N.; Suada, N.K.; Denis, D.; Yohan, B.; Adi, A.C.; Veterini, A.S.; Anindya, A.L.; Sasmono, R.T.; Rachmawati, H. Antiviral Action of Curcumin Encapsulated in Nanoemulsion against Four Serotypes of Dengue Virus. Pharm. Nanotechnol. 2020, 8, 54–62. [Google Scholar] [CrossRef]
- Thongsri, P.; Pewkliang, Y.; Borwornpinyo, S.; Wongkajornsilp, A.; Hongeng, S.; Sa-Ngiamsuntorn, K. Curcumin inhibited hepatitis B viral entry through NTCP binding. Sci. Rep. 2021, 11, 19125. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Ac, K.V.; Varma, K.; Jude, S.; Amalraj, A.; Arundhathy, C.; George, R.; Sreeraj, T.; Divya, C.; Kunnumakkara, A.B.; et al. Comparative Oral Absorption of Curcumin in a Natural Turmeric Matrix with Two Other Curcumin Formulations: An Open-label Parallel-arm Study. Phytother. Res. 2017, 31, 1883–1891. [Google Scholar] [CrossRef]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef]
- Baspinar, Y.; Üstündas, M.; Bayraktar, O.; Sezgin, C. Curcumin and piperine loaded zein-chitosan nanoparticles: Development and in-vitro characterisation. Saudi Pharm. J. 2018, 26, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Kim, J.Y. Chondroprotective effect of curcumin and lecithin complex in human chondrocytes stimulated by IL-1β via an anti-inflammatory mechanism. Food Sci. Biotechnol. 2019, 28, 547–553. [Google Scholar] [CrossRef]
- Henriques, M.C.; Faustino, M.A.F.; Braga, S.S. Curcumin Innovative Delivery Forms: Paving the “Yellow Brick Road” of Antitumoral Phytotherapy. Appl. Sci. 2020, 10, 8990. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [PubMed]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Kasapoglu-Calik, M.; Ozdemir, M. Synthesis and controlled release of curcumin-β-cyclodextrin inclusion complex from nanocomposite poly(N-isopropylacrylamide/sodium alginate) hydrogels. J. Appl. Polym. Sci. 2019, 136, 47554. [Google Scholar] [CrossRef]
- Kongkaneramit, L.; Aiemsumang, P.; Kewsuwan, P. Development of curcumin liposome formulations using polyol dilution method. Songklanakarin J. Sci. Technol. 2016, 38, 605–610. [Google Scholar]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Yuan, F. Stability and release performance of curcumin-loaded liposomes with varying content of hydrogenated phospholipids. Food Chem. 2020, 326, 126973. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M.; Venditti, F.; Ceglie, A.; Miguel, M.; Lindman, B.; Lopez, F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surf. B Biointerfaces 2018, 168, 29–34. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, N.; Ye, J.; Yang, B.; Sun, Y.; Kuang, H. Curcumin’s prevention of inflammation-driven early gastric cancer and its molecular mechanism. Chin. Herb. Med. 2022, 14, 244–253. [Google Scholar] [CrossRef]
- Guerrero, S.; Inostroza-Riquelme, M.; Contreras-Orellana, P.; Diaz-Garcia, V.; Lara, P.; Vivanco-Palma, A.; Cárdenas, A.; Miranda, V.; Robert, P.; Leyton, L.; et al. Curcumin-loaded nanoemulsion: A new safe and effective formulation to prevent tumor reincidence and metastasis. Nanoscale 2018, 10, 22612–22622. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Ko, Y.-C.; Chang, Y.-F.; Huang, S.-H.; Liu, C.J.-L. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef]
- Saber-Moghaddam, N.; Salari, S.; Hejazi, S.; Amini, M.; Taherzadeh, Z.; Eslami, S.; Rezayat, S.M.; Jaafari, M.R.; Elyasi, S. Oral nano-curcumin formulation efficacy in management of mild to moderate 28 hospitalized coronavirus disease -19 patients: An open label nonrandomized clinical trial. Phytother. Res. 2021, 35, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, P.; Hou, X.; Yan, F.; Jiang, Z.; Shi, J.; Xie, X.; Shen, J.; Fan, Q.; Wang, Z.; et al. Hybrid curcumin–phospholipid complex-near-infrared dye oral drug delivery system to inhibit lung metastasis of breast cancer. Int. J. Nanomed. 2019, 14, 3311–3330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhang, L.; He, D.; Ju, J.; Li, W. Studies on the curcumin phospholipid complex solidified with Soluplus®. J. Pharm. Pharmacol. 2018, 70, 242–249. [Google Scholar] [CrossRef]
- Gupta, A.; Costa, A.P.; Xu, X.; Lee, S.-L.; Cruz, C.N.; Bao, Q.; Burgess, D.J. Formulation and characterization of curcumin loaded polymeric micelles produced via continuous processing. Int. J. Pharm. 2020, 583, 119340. [Google Scholar] [CrossRef]
- Karavasili, C.; Andreadis, D.A.; Katsamenis, O.L.; Panteris, E.; Anastasiadou, P.; Kakazanis, Z.; Zoumpourlis, V.; Markopoulou, C.K.; Koutsopoulos, S.; Vizirianakis, I.S.; et al. Synergistic Antitumor Potency of a Self-Assembling Peptide Hydrogel for the Local Co-delivery of Doxorubicin and Curcumin in the Treatment of Head and Neck Cancer. Mol. Pharm. 2019, 16, 2326–2341. [Google Scholar] [CrossRef]
- Liu, K.; Huang, R.-L.; Zha, X.-Q.; Li, Q.-M.; Pan, L.-H.; Luo, J.-P. Encapsulation and sustained release of curcumin by a composite hydrogel of lotus root amylopectin and chitosan. Carbohydr. Polym. 2020, 232, 115810. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H.; Illias, H.A.; Ching, K.Y.; Singh, R.; Nai-Shang, L. Influence of a nonionic surfactant on curcumin delivery of nanocellulose reinforced chitosan hydrogel. Int. J. Biol. Macromol. 2018, 118, 1055–1064. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cyclodextrin nanosponge based hydrogel for the transdermal co-delivery of curcumin and resveratrol: Development, optimization, in vitro and ex vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 55–64. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.-G.; Lee, B.-T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Er, B.; Durmus, A.S.; Ozercan, I.H.; Sahin, N.; Padigaru, M.; Morde, A.; Rai, D. Protective Effect of a Novel Highly Bioavailable Formulation of Curcumin in Experimentally Induced Osteoarthritis Rat Model. Curr. Dev. Nutr. 2020, 4 (Suppl. S2), 1765. [Google Scholar] [CrossRef]
- Panda, S.K.; Parachur, V.A.; Mohanty, N.; Swain, T.; Sahu, S. A Comparative Pharmacokinetic Evaluation of a Bioavailable Curcumin Formulation Curene® with Curcumin Formulation Containing Turmeric Volatile Oil and Standard Curcuminoids 95% in Healthy Human Subjects. Funct. Foods Health Dis. 2019, 9, 134–144. [Google Scholar] [CrossRef]
- Ullah, F.; Asgarov, R.; Venigalla, M.; Liang, H.; Niedermayer, G.; Münch, G.; Gyengesi, E. Effects of a solid lipid curcumin particle formulation on chronic activation of microglia and astroglia in the GFAP-IL6 mouse model. Sci. Rep. 2020, 10, 100090. [Google Scholar] [CrossRef]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Nie, W.; Chen, L.; Miao, Y.; Zhang, X.; Chen, F.; Yu, B.; Ao, R.; Yu, B.; He, C. Fabrication of curcumin-loaded mesoporous silica incorporated polyvinyl pyrrolidone nanofibers for rapid hemostasis and antibacterial treatment. RSC Adv. 2017, 7, 7973–7982. [Google Scholar] [CrossRef]
- Virk, R.S.; Rehman, M.A.U.; Munawar, M.A.; Schubert, D.W.; Goldmann, W.H.; Dusza, J.; Boccaccini, A.R. Curcumin-containing orthopedic implant coatings deposited on poly-ether-ether-ketone/bioactive glass/hexagonal boron nitride layers by electrophoretic deposition. Coatings 2019, 9, 572. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Sagitha, P.; Reshmi, C.; Sundaran, S.P.; Binoy, A.; Mishra, N.; Sujith, A. In-vitro evaluation on drug release kinetics and antibacterial activity of dextran modified polyurethane fibrous membrane. Int. J. Biol. Macromol. 2019, 126, 717–730. [Google Scholar]
- Keridou, I.; Franco, L.; Turon, P.; del Valle, L.J.; Puiggalí, J. Scaffolds with tunable properties constituted by electrospun nanofibers of polyglycolide and poly (ε-caprolactone). Macromol. Mater. Eng. 2018, 303, 1800100. [Google Scholar] [CrossRef]
- Negahdari, R.; Sharifi, S.; Ghavimi, M.A.; Memar, M.Y.; Khaneshi, B.; Maleki Dizaj, S.; Eftekhari, A.; Cucchiarini, M. Curcumin nanocrystals: Production, physicochemical assessment, and in vitro evaluation of the antimicrobial effects against bacterial loading of the implant fixture. Appl. Sci. 2020, 10, 8356. [Google Scholar] [CrossRef]
- Huang, F.; Cai, X.; Hou, X.; Zhang, Y.; Liu, J.; Yang, L.; Liu, Y.; Liu, J. A dynamic covalent polymeric antimicrobial for conquering drug-resistant bacterial infection. Exploration 2022, 2, 20210145. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Gao, Q.; Lai, S.; Liu, Y.; Zhou, S.; Yan, Y.; Zhang, J.; Wang, H.; Wang, J.; et al. Intelligent bacteria-targeting ZIF- composite for fluorescence imaging-guided photodynamic therapy of drug-resistant superbug infections and burn wound healing. In Exploration; Wiley: Hoboken, NJ, USA, 2024; p. 20230113. [Google Scholar]
- Shin, B.; Park, W. Zoonotic diseases and phytochemical medicines for microbial infections in veterinary science: Current state and future perspective. Front. Vet. Sci. 2018, 5, 166. [Google Scholar] [CrossRef]
- Güran, M.; Şanlıtürk, G.; Kerküklü, N.R.; Altundağ, E.M.; Yalçın, A.S. Combined effects of quercetin and curcumin on anti-inflammatory and antimicrobial parameters in vitro. Eur. J. Pharmacol. 2019, 859, 172486. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liao, Q.; Xie, M.; Tao, H.; Wang, H.L. Synergistic effect of hypocrellin B and curcumin on photodynamic inactivation of Staphylococcus aureus. Microb. Biotechnol. 2021, 14, 692–707. [Google Scholar] [CrossRef]
- Ratrey, P.; Dalvi, S.V.; Mishra, A. Enhancing aqueous solubility and antibacterial activity of curcumin by complexing with cell-penetrating octaarginine. ACS Omega 2020, 5, 19004–19013. [Google Scholar] [CrossRef]
- Sharma, G.; Dang, S.; Kalia, M.; Gabrani, R. Synergistic antibacterial and anti-biofilm activity of nisin like bacteriocin with curcumin and cinnamaldehyde against ESBL and MBL producing clinical strains. Biofouling 2020, 36, 710–724. [Google Scholar] [CrossRef]
- Altundağ, E.M.; Toprak, K.; Şanlıtürk, G.; Güran, M.; Özbilenler, C.; Kerküklü, N.R.; Yılmaz, A.M.; Yalçın, A.S. Synergistic combination of histone deacetylase inhibitor suberoylanilide hydroxamic acid and natural flavonoid curcumin exhibits anticancer and antibacterial activity. Anticancer Agents Med. Chem. 2020, 21, 1301–1308. [Google Scholar] [CrossRef]
- Bhatia, E.; Sharma, S.; Jadhav, K.; Banerjee, R. Combinatorial liposomes of berberine and curcumin inhibit biofilm formation and intracellular methicillin resistant Staphylococcus aureus infections and associated inflammation. J. Mater. Chem. B 2021, 9, 864–875. [Google Scholar] [CrossRef]
- Ibrahima, S.; Solimana, O.; Sultana, M. Synergistic antimicrobial effect of xylitol with curcumin: Water vapor barrier, mechanical and thermal properties of PSS/PVA packaging films. Int. J. Appl. Eng. Res. 2017, 12, 10360–10366. [Google Scholar]
- Dai, C.; Wang, Y.; Sharma, G.; Shen, J.; Velkov, T.; Xiao, X. Polymyxins–curcumin combination antimicrobial therapy: Safety implications and efficacy for infection treatment. Antioxidants 2020, 9, 506. [Google Scholar] [CrossRef]
- Gottumukkala, S.N.; Koneru, S.; Mannem, S.; Mandalapu, N. Effectiveness of sub gingival irrigation of an indigenous 1% curcumin solution on clinical and microbiological parameters in chronic periodontitis patients: A pilot randomized clinical trial. Contemp. Clin. Dent. 2013, 2, 186–191. [Google Scholar] [CrossRef]
- Leite, D.P.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of photodynamic therapy with blue light and curcumin as mouth rinse for oral disinfection: A randomized controlled trial. Photomed Laser Surg. 2014, 32, 627–632. [Google Scholar] [CrossRef]
- Bhatia, M.; Urolagin, S.S.; Pentyala, K.B.; Urolagin, S.B.; Menaka, K.B.; Bhoi, S. Novel therapeutic approach for the treatment of periodontitis by curcumin. J. Clin. Diagn. Res. 2014, 12, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Khonche, A.; Biglarian, O.; Panahi, Y.; Valizadegan, G.; Soflaei, S.S.; Ghamarchehreh, M.E.; Majeedn, M.; Sahebkar, A. Adjunctive therapy with curcumin for peptic ulcer: A randomized controlled trial. Drug. Res. 2016, 8, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.H.; Abdulridha, M.K.; Najeb, A.A. Potential benefit of curcumin adjuvant therapy to the standard Helicobacter pylori eradication therapy in patients with peptic ulcer disease. Asian J. Pharm. Clin. Res. 2017, 5, 313–317. [Google Scholar]
- Judaki, A.; Rahmani, A.; Feizi, J.; Asadollahi, K.; Hafezi Ahmadi, M.R. Curcumin in combination with triple therapy regimes ameliorates oxidative stress and histopathologic changes in chronic gastritis-associated helicobacter pylori infection. Arq. Gastroenterol. 2017, 3, 177–182. [Google Scholar] [CrossRef]
- Pawar, K.S.; Mastud, R.N.; Pawar, S.K.; Pawar, S.S.; Bhoite, R.R.; Bhoite, R.R.; Kulkarni, M.V.; Deshpande, A.R. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: A randomized clinical trial. Front. Pharmacol. 2021, 12, 669362. [Google Scholar] [CrossRef]
- Ahmadi, R.; Salari, S.; Sharifi, M.D.; Reihani, H.; Rostamiani, M.B.; Behmadi, M.; Taherzadeh, Z.; Eslami, S.; Rezayat, S.M.; Jaafari, M.R.; et al. Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial. Food Sci. Nutr. 2021, 9, 4068–4075. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, R.; Forgione, F.; Bernardi, A.; Sacchi, A.; Laneri, S.; Greco, G. Clinical studies on topical curcumin. Ski. Pharmacol. Physiol. 2023, 36, 235–248. [Google Scholar] [CrossRef]
- Deodhar, S.D.; Sethi, R.; Srimal, R.C. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian J. Med. Res. 1980, 71, 632–634. [Google Scholar] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- D’Angelo, N.A.; Noronha, M.A.; Kurnik, I.S.; Câmara, M.C.; Vieira, J.M.; Abrunhosa, L.; Martins, J.T.; Alves, T.F.; Tundisi, L.L.; Ataide, J.A.; et al. Curcumin encapsulation in nanostructures for cancer therapy: A 10-year overview. Int. J. Pharm. 2021, 604, 120534. [Google Scholar] [CrossRef]
| Type of Bacteria | Experiment | Minimum Inhibitory Concentration (µg/mL) | Outcome | Reference |
|---|---|---|---|---|
| S. aureus, | In vitro | 25 | Curcumin ruptured cell membranes and inhibited all tested microorganisms, showing significant antibacterial action | [63] |
| E. coli, | ||||
| Enterococcus faecalis, and Pseudomonas aeruginosa | ||||
| E. coli | In vitro | 12 | Curcumin strongly inhibited E. coli | [64] |
| Klebsiella pneumonia, | In vitro | 34 | When compared to demethoxycurcumin and bisdemethoxycurcumin, curcumin exhibited far stronger antibacterial action | [65] |
| Bacillus subtilis, | ||||
| Enterobacter aerogenes, | ||||
| E. coli, S. aureus, | ||||
| Proteus mirabilis, and | ||||
| P. aeruginosa | ||||
| E. coli | In vitro | 8 | Curcumin inhibited the levofloxacin-induced SOS reaction in E. coli | [66] |
| P. aeruginosa | In vitro | 8–512 | Antibacterial synergy was seen when curcumin, azithromycin, and gentamicin were combined | [67] |
| S. aureus | In vivo and in vitro | 2–16 | Curcumin healed S. aureus-infected mice | [60,68] |
| Salmonella typhimurium and | In vivo and in vitro | 0.5–2 | The mouse model exhibited the strong antibacterial action of curcumin | [69] |
| Salmonella typhi | ||||
| H. pylori | In vivo and in vitro | 5–50 | In mice, curcumin eradicated H. pylori that caused stomach damage | [70] |
| Type of Virus | Curcumin | Outcome | Reference |
|---|---|---|---|
| Hepatitis B virus | 10 μM | Changes in the effects of curcumin on hepatitis B virus entrance | [71] |
| Chikungunya virus and Zika virus | 5 μM | The antiviral effects of curcumin on the chikungunya and Zika viruses | [22,72] |
| Human immunodeficiency virus 1 (HIV-1) | 20 nM | Immunomodulatory effects of HIV-1 on silver nanoparticles stabilized with curcumin | [73] |
| Enterovirus 71 (EV71) | 40 μM | The activity of curcumin against EV71 virus | [43,74] |
| Kaposi’s sarcoma-associated herpesvirus (KSHV or HHV8) | 20 μM | The antiviral effects of curcumin on the replication and development of KSHV | [75] |
| Zika virus | 25 μM | Zika virus inhibitory effects were observed in the activity | [76] |
| Dengue virus | 40 μM | Curcumin has antiviral efficacy against several serotypes of dengue virus | [30,77] |
| Human parainfluenza virus type 3 | 10–30 μM | Inhibitory effects of curcumin on the replication of the human parainfluenza virus | [78] |
| Disease | Number of Participants | Dose | Result | Reference |
|---|---|---|---|---|
| Chronic periodontitis | 23 | 1% curcumin solution, 0.2% chlorhexidine gluconate, and saline were used as irrigants | The curcumin group reduced bleeding scores more effectively than the control group | [125] |
| Oral plaque | 27 | LED lighting generating blue light with a curcumin concentration of 30 mg/L | Curcumin possesses the capability to disintegrate mouth plaque; salivary microbes can be reduced using the blue LED device that contains curcumin | [126] |
| Periodontitis | 25 | Locally applied 1% curcumin gel to treat periodontitis | A curcumin gel application dramatically lowered the periopathogen microbiological count; when used after scaling and root planing, curcumin gel effectively inhibits the growth of oral germs | [127] |
| Peptic ulcer | 30 | Curcumin (500 mg) along with piperine (5 mg) | Dyspepsia symptoms improved | [128] |
| Peptic ulcer | 21 | Clarithromycin (500 mg) + amoxicillin (1 g) + esomeperazole (20 mg) + curcumin (500 mg) | Reduction in pro-inflammatory IL-1B level; improved healing | [129] |
| Chronic gastritis | 24 | Omeprazole (20 mg) + amoxicillin (1 g) + metronidazole (800 mg) + curcumin (700 mg) | Reduction in oxidative DNA damage; improved clinical symptoms | [130] |
| COVID-19 | 70 mild to severe COVID-19 patients | Patients took a dietary supplement containing 2.5 milligrams of bioperine and 252 milligrams of curcumin | Reduced the length of hospital stays for patients with moderate to severe symptoms, showed early symptomatic recovery | [131] |
| COVID-19 | 30 mild to moderate COVID-19 patients | Patients in the Sinacurcumin® soft gel 40 mg group took two gels with meals: breakfast and dinner | Symptoms such as chills, cough, and smell/taste disturbances cleared up significantly faster than in the control group, with the exception of sore throat | [132] |
| COVID-19 | 115 | Dietary supplement: palmitoylethanolamideDietary supplement: curcumin | Curcumin supplementation reduced the proinflammatory response in patients recently diagnosed with COVID-19 | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Oirdi, M.; Farhan, M. Clinical Trial Findings and Drug Development Challenges for Curcumin in Infectious Disease Prevention and Treatment. Life 2024, 14, 1138. https://doi.org/10.3390/life14091138
El Oirdi M, Farhan M. Clinical Trial Findings and Drug Development Challenges for Curcumin in Infectious Disease Prevention and Treatment. Life. 2024; 14(9):1138. https://doi.org/10.3390/life14091138
Chicago/Turabian StyleEl Oirdi, Mohamed, and Mohd Farhan. 2024. "Clinical Trial Findings and Drug Development Challenges for Curcumin in Infectious Disease Prevention and Treatment" Life 14, no. 9: 1138. https://doi.org/10.3390/life14091138
APA StyleEl Oirdi, M., & Farhan, M. (2024). Clinical Trial Findings and Drug Development Challenges for Curcumin in Infectious Disease Prevention and Treatment. Life, 14(9), 1138. https://doi.org/10.3390/life14091138







