The Prevalence of Arrhythmias, Including Premature Supraventricular and Ventricular Beats and Other Electrocardiographic Patterns, in 24-Hour Holter Monitoring in Patients with Overweight and Obesity
Abstract
:1. Introduction
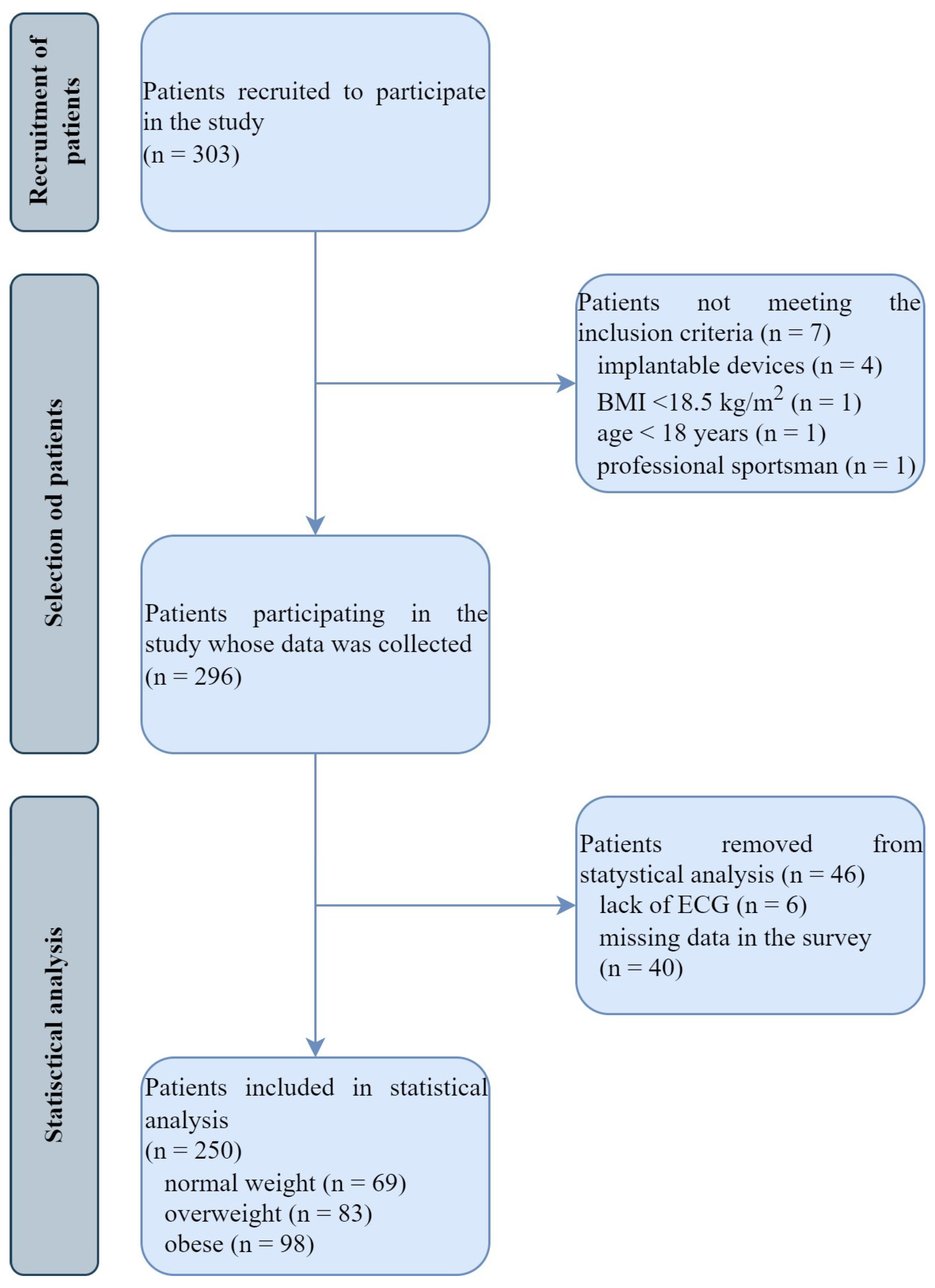
2. Materials and Methods
2.1. Study Population
2.2. Trial Design
2.3. Statistical Analysis
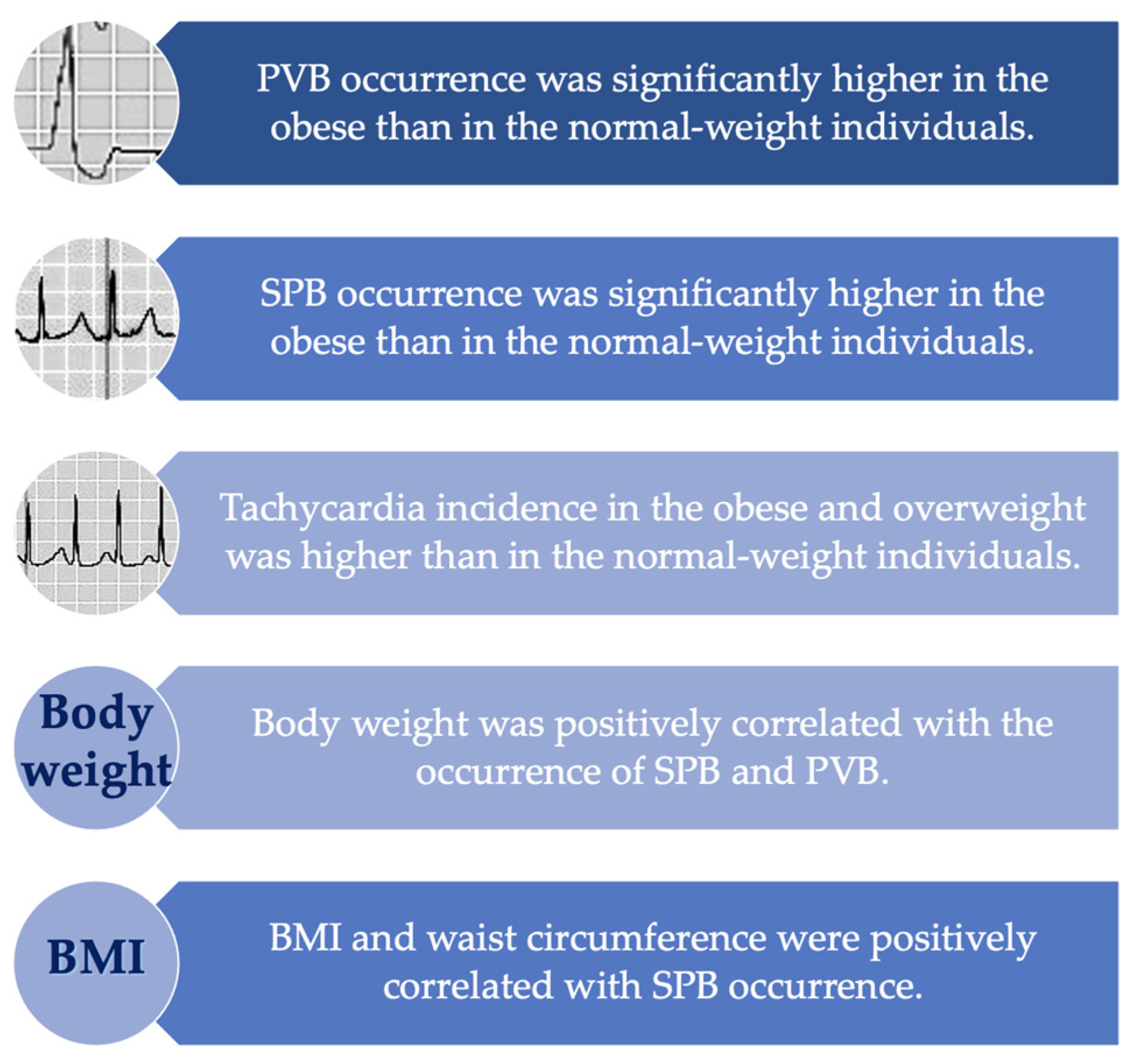
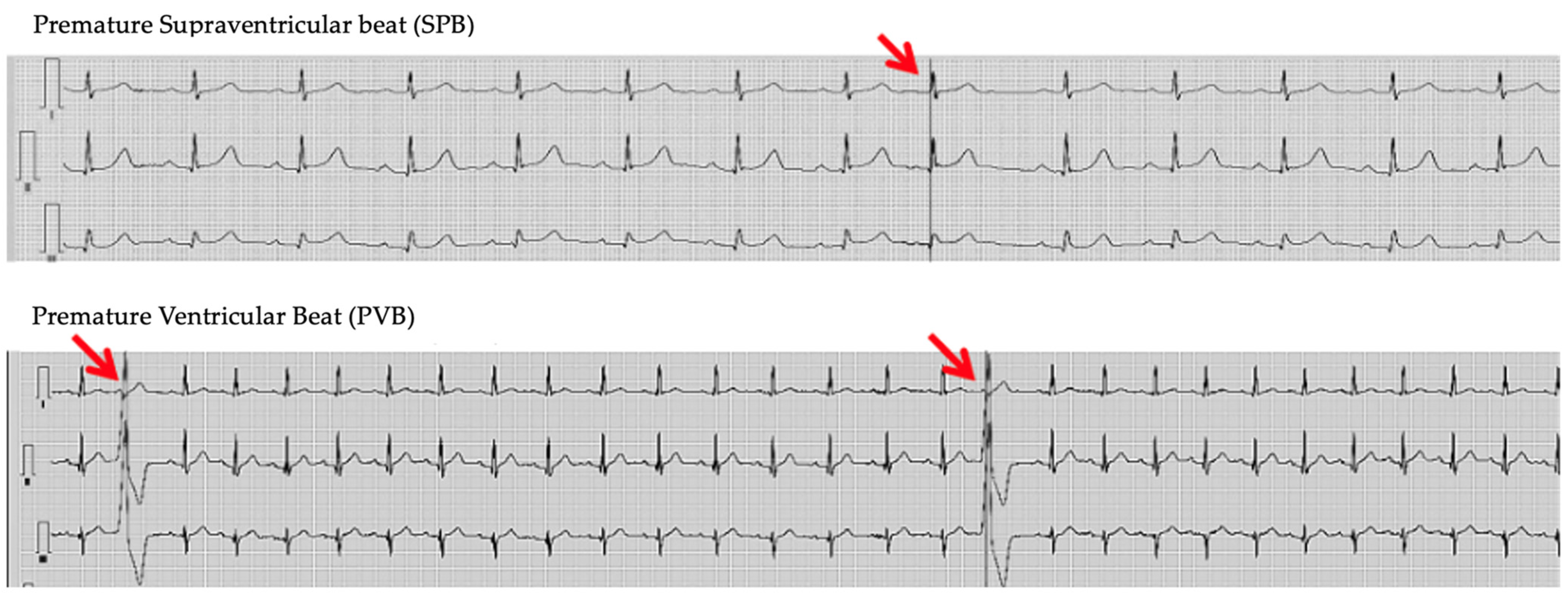
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 August 2024).
- Eurostat Report. Over Half of Adults in the EU Are Overweight. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Overweight_and_obesity_-_BMI_statistics#Obesity_by_age_group (accessed on 8 August 2024).
- Raport: Odsetek Osób w Wieku Powyżej 15 Lat Według Indeksu Masy Ciała (BMI), GUS. 2019. Available online: https://stat.gov.pl/obszary-tematyczne/zdrowie/zdrowie/odsetek-osob-w-wieku-powyzej-15-lat-wedlug-indeksu-masy-ciala-bmi,23,1.html (accessed on 8 August 2024).
- Goudis, C.A.; Korantzopoulos, P.; Ntalas, I.V.; Kallergis, E.M.; Ketikoglou, D.G. Obesity and atrial fibrillation: A comprehensive review of the pathophysiological mechanisms and links. J. Cardiol. 2015, 66, 361–369. [Google Scholar] [CrossRef]
- Lavie, C.J.; Pandey, A.; Lau, D.H.; Alpert, M.A.; Sanders, P. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis: Effects of Weight Loss and Exercise. J. Am. Coll. Cardiol. 2017, 70, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, M.R.; Ananya, F.N. Impact of Weight Loss on Atrial Fibrillation. Cureus 2023, 15, e46232. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.W.; Hylek, E.M.; Apovian, C.M. Obesity begets atrial fibrillation: A contemporary summary. Circulation 2013, 128, 401–405. [Google Scholar] [CrossRef] [PubMed]
- A Healthy Lifestyle—WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 8 August 2024).
- Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. Available online: https://www.who.int/publications/i/item/9789241501491 (accessed on 8 August 2024).
- Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [CrossRef]
- Di Fusco, S.A.; Mocini, E.; Gulizia, M.M.; Gabrielli, D.; Grimaldi, M.; Oliva, F.; Colivicchi, F. ANMCO (Italian Association of Hospital Cardiologists) scientific statement: Obesity in adults-an approach for cardiologists. Eat Weight Disord. 2024, 29, 1. [Google Scholar] [CrossRef]
- Templin, T.; Cravo Oliveira Hashiguchi, T.; Thomson, B.; Dieleman, J.; Bendavid, E. The overweight and obesity transition from the wealthy to the poor in low- and middle-income countries: A survey of household data from 103 countries. PLoS Med. 2019, 16, e1002968. [Google Scholar] [CrossRef]
- Skovgaard, D.; Haahr, P.; Lester, R.; Clark, K.; Paglialunga, S.; Finer, N.; Friedrichsen, M.H.; Hjerpsted, J.B.; Engelmann, M.D. Prevalence of Baseline Cardiac Arrhythmias in Participants with Overweight or Obesity in Phase 1 Clinical Trials: Analysis of 24-Hour Holter Electrocardiogram Recordings. J. Clin. Pharmacol. 2023, 63, 539–543. [Google Scholar] [CrossRef]
- Hingorani, P.; Karnad, D.R.; Rohekar, P.; Kerkar, V.; Lokhandwala, Y.Y.; Kothari, S. Arrhythmias Seen in Baseline 24-Hour Holter ECG Recordings in Healthy Normal Volunteers During Phase 1 Clinical Trials. J. Clin. Pharmacol. 2016, 56, 885–893. [Google Scholar] [CrossRef]
- Bienias, P.; Rymarczyk, Z.; Domienik-Karłowicz, J.; Lisik, W.; Sobieraj, P.; Pruszczyk, P.; Ciurzyński, M. Assessment of arrhythmias cardiac autonomic tone at a relatively young age patients with obesity class, I.I.I. Clin. Obes. 2021, 11, e12424. [Google Scholar] [CrossRef]
- Binu, A.J.; Srinath, S.C.; Cherian, K.E.; Jacob, J.R.; Paul, T.V.; Kapoor, N. A Pilot Study of Electrocardiographic Features in Patients with Obesity from a Tertiary Care Centre in Southern India (Electron). Med. Sci. 2022, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xue, J.; Li, B. Obesity and sudden cardiac death: Prevalence, pathogenesis, prevention and intervention. Front. Cell Dev. Biol. 2022, 10, 1044923. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, A. Cardiac Autonomic Neuropathy: Why Should Cardiologists Care about That? J. Diabetes Res. 2017, 2017, 5374176. [Google Scholar] [CrossRef] [PubMed]
- Fidan-Yaylali, G.; Yaylali, Y.T.; Erdogan, Ç.; Can, B.; Senol, H.; Gedik-Topçu, B.; Topsakal, S. The Association between Central Adiposity and Autonomic Dysfunction in Obesity. Med. Princ. Pract. 2016, 25, 442–448. [Google Scholar] [CrossRef]
- Valensi, P. Autonomic nervous system activity changes in patients with hypertension and overweight: Role and therapeutic implications. Cardiovasc. Diabetol. 2021, 20, 170. [Google Scholar] [CrossRef]
- McCully, B.H.; Hasan, W.; Streiff, C.T.; Houle, J.C.; Woodward, W.R.; Giraud, G.D.; Brooks, V.L.; Habecker, B.A. Sympathetic cardiac hyperinnervation and atrial autonomic imbalance in diet-induced obesity promote cardiac arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1530–H1537. [Google Scholar] [CrossRef]
- Yadav, R.L.; Yadav, P.K.; Yadav, L.K.; Agrawal, K.; Sah, S.K.; Islam, M.N. Association between obesity and heart rate variability indices: An intuition toward cardiac autonomic alteration—A risk of CVD. Diabetes Metab. Syndr. Obes. 2017, 10, 57–64. [Google Scholar] [CrossRef]
- Espinoza Salinas, A.; Brito, C.; Arenas Sánchez, G.; Peiret Villacura, L.; Molina Sotomayor, E.; Cigarroa Cuevas, I.; González Jurado, J. Autonomic function and its relationship with central obesity and hemodynamic variables in obese and overweight adults. Nutr. Hosp. 2022, 39, 320–328. [Google Scholar] [CrossRef]
- Yi, S.H.; Lee, K.; Shin, D.G.; Kim, J.S.; Kim, H.C. Differential association of adiposity measures with heart rate variability measures in Koreans. Yonsei Med. J. 2013, 54, 55–61. [Google Scholar] [CrossRef]
- Chang, W.P.; Wang, C.H.; Lin, Y.K. Influence of Obesity on Heart Rate Variability in Nurses with Age and Shift Type as Moderators. Biomed. Res. Int. 2021, 2021, 8119929. [Google Scholar] [CrossRef]
- Plourde, B.; Sarrazin, J.F.; Nault, I.; Poirier, P. Sudden cardiac death and obesity. Expert Rev Cardiovasc Ther. 2014, 12, 1099–1110. [Google Scholar] [CrossRef]
- Valencia-Flores, M.; Orea, A.; Castaño, V.A.; Resendiz, M.; Rosales, M.; Rebollar, V.; Santiago, V.; Gallegos, J.; Campos, R.M.; González, J.; et al. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes. Res. 2000, 8, 262–269. [Google Scholar] [CrossRef]
- Gupta, V.; Munjal, J.S.; Jhajj, P.; Jhajj, S.; Jain, R. Obesity and Atrial Fibrillation: A Narrative Review. Cureus 2022, 14, e31205. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Pierucci, N.; Trivigno, S.; Cipollone, P.; Piro, A.; Chimenti, C.; Della Rocca, D.G.; Miraldi, F.; Vizza, C.D.; Lavalle, C. Probability Score to Predict. Spontaneous Conversion to Sinus Rhythm. in Patients with Symptomatic Atrial Fibrillation When Less Could Be More? J. Clin. Med. 2024, 13, 1470. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, V.G.; Aroor, A.R.; Sowers, J.R. The pathophysiology of hypertension in patients with obesity. Nat. Rev. Endocrinol. 2014, 10, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Martelli, D.; Brooks, V.L. Leptin Increases: Physiological Roles in the Control of Sympathetic Nerve Activity, Energy Balance, and the Hypothalamic-Pituitary-Thyroid Axis. Int. J. Mol. Sci. 2023, 24, 2684. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.H.K.; Reddy, R.K.; Sau, A.; Sivanandarajah, P.; Ardissino, M.; Ng, F.S. Obesity as a risk factor for cardiac arrhythmias. BMJ Med. 2022, 1, e000308. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Parise, H.; Levy, D.; D’Agostino, R.B., Sr.; Wolf, P.A.; Vasan, R.S.; Benjamin, E.J. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004, 292, 2471–2477. [Google Scholar] [CrossRef]
- Tedrow, U.B.; Conen, D.; Ridker, P.M.; Cook, N.R.; Koplan, B.A.; Manson, J.E.; Buring, J.E.; Albert, C.M. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J. Am. Coll. Cardiol. 2010, 55, 2319–2327. [Google Scholar] [CrossRef]
- Huxley, R.R.; Lopez, F.L.; Folsom, A.R.; Agarwal, S.K.; Loehr, L.R.; Soliman, E.Z.; Maclehose, R.; Konety, S.; Alonso, A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011, 123, 1501–1508. [Google Scholar] [CrossRef]
- Ataklte, F.; Erqou, S.; Laukkanen, J.; Kaptoge, S. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am. J. Cardiol. 2013, 112, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiao, S.; Chen, J.; Guo, H.; Ren, L.; Sun, L.; Sun, Y.; Chen, Y. The relationship between frequent premature ventricular complexes and epicardial adipose tissue volume. Front. Endocrinol. 2023, 14, 1219890. [Google Scholar] [CrossRef] [PubMed]
- von Rotz, M.; Aeschbacher, S.; Bossard, M.; Schoen, T.; Blum, S.; Schneider, S.; Estis, J.; Todd, J.; Risch, M.; Risch, L.; et al. Risk factors for premature ventricular contractions in young and healthy adults. Heart 2017, 103, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Sabbag, A.; Sidi, Y.; Kivity, S.; Beinart, R.; Glikson, M.; Segev, S.; Goldenberg, I.; Maor, E. Obesity and exercise-induced ectopic ventricular arrhythmias in apparently healthy middle aged adults. Eur. J. Prev. Cardiol. 2016, 23, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Måneheim, A.; Engström, G.; Juhlin, T.; Persson, A.; Zaigham, S.; Johnson, L.S.B. Elevated premature ventricular complex counts on 24-hour electrocardiogram predict incident atrial fibrillation and heart failure-A prospective population-based cohort study. Heart Rhythm. O2 2022, 3, 344–350. [Google Scholar] [CrossRef]
- Aizawa, Y.; Watanabe, H.; Okumura, K. Electrocardiogram (ECG) for the Prediction of Incident Atrial Fibrillation: An Overview. J. Atr. Fibrillation. 2017, 10, 1724. [Google Scholar] [CrossRef]
| Parameter | Entire Study Group (n = 250) | Obesity (A, n = 98) | Overweight (B, n = 83) | Control Group (C, n = 69) | p < 0.05 |
|---|---|---|---|---|---|
| Age (years) | 59.94 ± 13.22 | 61.18 ± 11.07 | 53.40 ± 13.70 | 58.83 ± 15.33 | ns |
| Gender (%/n) | |||||
| Male | 41.6/104 | 50.0/49 | 50.6/42 | 18.8/13 | A vs. C: 0.001 |
| Female | 58.4/146 | 50.0/49 | 49.4/41 | 81.2/56 | B vs. C: 0.001 |
| Height (cm) | 167.37 ± 9.76 | 168.14 ± 9.65 | 168.64 ± 10.36 | 164.76 ± 8.78 | ns |
| Weight (kg) | 80.42 ± 17.50 | 95.09 ± 13.24 | 78.39 ± 11.10 | 62.02 ± 7.99 | A vs. B: 0.001 A vs. C: 0.001 B vs. C: 0.001 |
| BMI (kg/m2) | 28.64 ± 4.99 | 33.62 ± 3.26 | 27.56 ± 1.34 | 22.86 ± 1.71 | A vs. B: 0.001 A vs. C: 0.001 B vs. C: 0.001 |
| Waist (cm) | 95.99 ± 14.26 | 107.29 ± 10.16 | 95.29 ± 8.55 | 79.72 ± 7.55 | A vs. B: 0.001 A vs. C: 0.001 B vs. C: 0.001 |
| Hip circumference (cm) | 106.60 ± 12.16 | 115.23 ± 7.32 | 104.26 ± 5.23 | 93.83 ± 14.14 | A vs. B: 0.001 A vs. C: 0.001 B vs. C: 0.001 |
| WHR | 0.95 ± 0.74 | 0.93 ± 0.08 | 0.91 ± 0.09 | 1.05 ± 1.56 | ns |
| Hypertension (%/n) | 52.8/132 | 64.3/63 | 50.6/42 | 39.1/27 | A vs. C: 0.001 B vs. C: 0.048 |
| Myocardial infarction (%/n) | 6.4/16 | 7.1/7 | 7.2/6 | 4.3/3 | ns |
| Stroke (%/n) | 2.8/7 | 2.0/2 | 3.6/3 | 2.9/2 | ns |
| Atrial fibrillation (%/n) | 8.8/22 | 8.2/8 | 12.0/10 | 5.8/4 | ns |
| Deep vein thrombosis (%/n) | 3.6/9 | 7.1/7 | 1.2/1 | 1.4/1 | ns |
| Type 2 diabetes (%/n) | 13.2/33 | 21.4/21 | 10.8/9 | 4.3/3 | A vs. C: 0.002 |
| Thyroid disease (%/n) | 16.4/41 | 16.3/16 | 13.2/11 | 20.3/14 | ns |
| Smoking (%/n) | 13.2/33 | 9.2/9 | 15.8/13 | 15.9/11 | ns |
| Parameters | Mean ± SD |
|---|---|
| HR min (bpm) | 53.93 ± 0.50 |
| HR max (bpm) | 117.33 ± 1.19 |
| HR mean (bpm) | 72.73 ± 0.55 |
| SPB | 308.39 ± 88.75 |
| PVB | 374.10 ± 129.18 |
| Bradycardia | 27.94 ± 9.15 |
| Bradycardia (bpm) | 38.39 ± 4.37 |
| Tachycardia | 23.02 ± 12.70 |
| Tachycardia (bpm) | 149.16 ± 20.39 |
| VT | 0.11 ± 0.05 |
| SVT | 2.72 ± 1.38 |
| AF (average number of episodes) | 0.16 ± 0.12 |
| Ventricular rhythm | 0.06 ± 0.04 |
| Parameters | Mean ± SD |
|---|---|
| 24 h monitoring (6:00–6:00) | |
| mRR (ms) | 828.42 ± 103.06 |
| SDNN (ms) | 150.05 ± 49.14 |
| rMSSD (ms) | 36.11 ± 41.46 |
| SDSD (ms) | 26.65 ± 29.06 |
| pNN50 (%) | 8.88 ± 13.98 |
| Daily activity (6:00–22:00) | |
| mRR (ms) | 771.61 ± 102.59 |
| SDNN (ms) | 112.26 ± 38.55 |
| rMSSD (ms) | 31.79 ± 37.79 |
| SDSD (ms) | 23.37 ± 26.65 |
| pNN50 (%) | 7.02 ± 13.36 |
| Night rest (22:00–6:00) | |
| mRR (ms) | 960.74 ± 137.51 |
| SDNN (ms) | 107.57 ± 39.93 |
| rMSSD (ms) | 43.30 ± 52.70 |
| SDSD (ms) | 30.03 ± 34.42 |
| pNN50 (%) | 12.90 ± 17.38 |
| Parameter | Obesity (A, n = 98) | Overweight (B, n = 83) | Control Group (C, n = 69) | p < 0.05 |
|---|---|---|---|---|
| HR min (bpm) | 54.32 ± 0.65 | 53.52 ± 0.73 | 53.87 ± 1.32 | ns |
| HR max (bpm) | 113.38 ± 1.71 | 118.20 ± 2.18 | 118.89 ± 2.31 | ns |
| HR mean (bpm) | 72.47 ± 0.85 | 72.17 ± 0.86 | 73.78 ± 1.19 | ns |
| PVB | 573.52 ± 218.17 | 171.18 ± 59.07 | 96.87 ± 23.30 | A vs. C: 0.030 |
| SPB | 620.20 ± 301.46 | 359.19 ± 153.89 | 42.49 ± 6.18 | A vs. C: 0.042 |
| Bradycardia | 29.96 ± 18.61 | 33.20 ± 15.18 | 18.74 ± 8.51 | ns |
| Bradycardia (bpm) | 38.00 ± 4.79 | 38.89 ± 4.38 | 38.27 ± 3.85 | ns |
| Tachycardia | 35.32 ± 31.04 | 19.42 ± 10.90 | 9.88 ± 3.10 | ns |
| Tachycardia (bpm) | 147.42 ± 19.10 | 151.36 ± 24.00 | 148.16 ± 17.42 | ns |
| VT | 0.15 ± 0.09 | 0.13 ± 0.10 | 0.03 ± 0.02 | ns |
| SVT | 1.17 ± 0.44 | 3.70 ± 3.21 | 3.76 ± 3.17 | ns |
| AF (average number of episodes) | 0.06 ± 0.04 | 0.41 ± 0.36 | 0.00 ± 0.00 | ns |
| Ventricular rhythm | 0.10 ± 0.10 | 0.06 ± 0.05 | 0.01 ± 0.01 | ns |
| Parameters | Obesity (A, n = 98) | Overweight (B, n = 83) | Control group (C, n = 69) | p < 0.05 |
|---|---|---|---|---|
| 24 h monitoring (6:00–6:00) | ||||
| mRR (ms) | 815.11 ± 77.60 | 867.50 ± 120.08 | 785.49 ± 92.20 | A vs. C: 0.046 B vs. C: 0.014 |
| SDNN (ms) | 147.10 ± 40.67 | 147.35 ± 58.91 | 159.47 ± 46.17 | ns |
| rMSSD (ms) | 42.79 ± 63.12 | 29.64 ± 11.89 | 35.77 ± 25.09 | ns |
| SDSD (ms) | 30.72 ± 42.97 | 21.70 ± 9.22 | 28.11 ± 21.78 | ns |
| pNN50 (%) | 10.59 ± 20.16 | 7.72 ± 7.49 | 7.98 ± 9.85 | ns |
| Daily activity (6:00–22:00) | ||||
| mRR (ms) | 759.95 ± 80.95 | 810.30 ± 109.98 | 726.55 ± 105.14 | A vs. C: 0.045 B vs. C: 0.011 |
| SDNN (ms) | 113.56 ± 34.41 | 107.38 ± 41.11 | 118.22 ± 42.24 | ns |
| rMSSD (ms) | 36.86 ± 57.20 | 26.88 ± 11.02 | 31.54 ± 24.91 | ns |
| SDSD (ms) | 26.26 ± 38.80 | 19.62 ± 9.00 | 24.82 ± 22.02 | ns |
| pNN50 (%) | 8.61 ± 19.86 | 5.93 ± 6.32 | 6.20 ± 7.97 | ns |
| Night rest (22:00–6:00) | ||||
| mRR (ms) | 938.84 ± 102.87 | 1003.92 ± 168.56 | 925.27 ± 118.54 | ns |
| SDNN (ms) | 108.81 ± 44.16 | 101.56 ± 32.11 | 115.35 ± 45.24 | ns |
| rMSSD (ms) | 54.14 ± 80.10 | 34.82 ± 15.26 | 39.38 ± 30.72 | ns |
| SDSD (ms) | 36.93 ± 52.14 | 24.41 ± 10.78 | 27.88 ± 20.27 | ns |
| pNN50 (%) | 14.86 ± 22.46 | 11.83 ± 11.53 | 11.44 ± 16.67 | ns |
| Parameter | Body Weight (kg) | BMI (kg/m2) | Waist Circumference (cm) | Hip Circumference (cm) | WHR |
|---|---|---|---|---|---|
| HR min (bpm) | ns | ns | ns | 0.30 (p = 0.010) | ns |
| HR max (bpm) | ns | ns | ns | ns | ns |
| HR mean (bpm) | ns | ns | ns | ns | ns |
| PVB | 0.14 (p = 0.028) | ns | ns | ns | ns |
| SPB | 0.14 (p = 0.028) | 0.13 (p = 0.043) | 0.14 (p = 0.031) | ns | ns |
| Bradycardia | ns | ns | ns | ns | ns |
| Bradycardia (bpm) | ns | ns | ns | ns | ns |
| Tachycardia | ns | ns | ns | ns | ns |
| Tachycardia (bpm) | ns | ns | ns | ns | ns |
| VT | ns | ns | ns | ns | ns |
| SVT | ns | ns | ns | ns | ns |
| AF | ns | ns | ns | ns | ns |
| Ventricular rhythm | ns | ns | ns | ns | ns |
| Model for: SPB | |||
| BMI (kg/m2) | Type 2 Diabetes | Thyroid Disease | |
| Regression coefficient (RC) | 82.292 | 791.956 | 918.975 |
| SEM of Rc | 39.721 | 384.861 | 352.687 |
| p | 0.037 | 0.040 | <0.025 |
| p for the model | 0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dykiert, I.A.; Kraik, K.; Jurczenko, L.; Gać, P.; Poręba, R.; Poręba, M. The Prevalence of Arrhythmias, Including Premature Supraventricular and Ventricular Beats and Other Electrocardiographic Patterns, in 24-Hour Holter Monitoring in Patients with Overweight and Obesity. Life 2024, 14, 1140. https://doi.org/10.3390/life14091140
Dykiert IA, Kraik K, Jurczenko L, Gać P, Poręba R, Poręba M. The Prevalence of Arrhythmias, Including Premature Supraventricular and Ventricular Beats and Other Electrocardiographic Patterns, in 24-Hour Holter Monitoring in Patients with Overweight and Obesity. Life. 2024; 14(9):1140. https://doi.org/10.3390/life14091140
Chicago/Turabian StyleDykiert, Irena Anna, Krzysztof Kraik, Lidia Jurczenko, Paweł Gać, Rafał Poręba, and Małgorzata Poręba. 2024. "The Prevalence of Arrhythmias, Including Premature Supraventricular and Ventricular Beats and Other Electrocardiographic Patterns, in 24-Hour Holter Monitoring in Patients with Overweight and Obesity" Life 14, no. 9: 1140. https://doi.org/10.3390/life14091140









