Depositional Environment and Ecological Response of Bioconstructions: A Case Study of Southern China (Guizhou Province) in Moscovian–Gzhelian
Abstract
1. Introduction
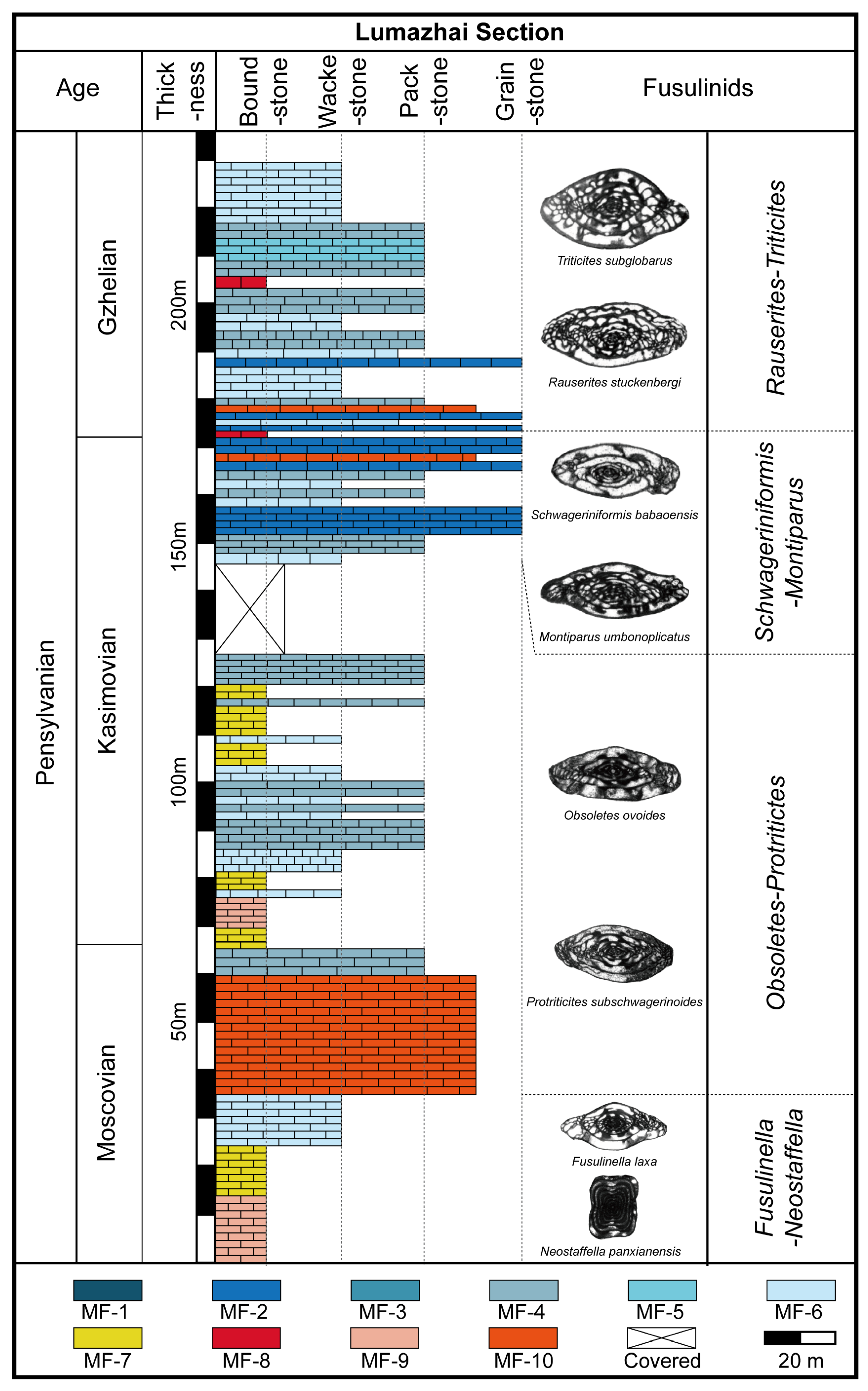
2. Geological Settings
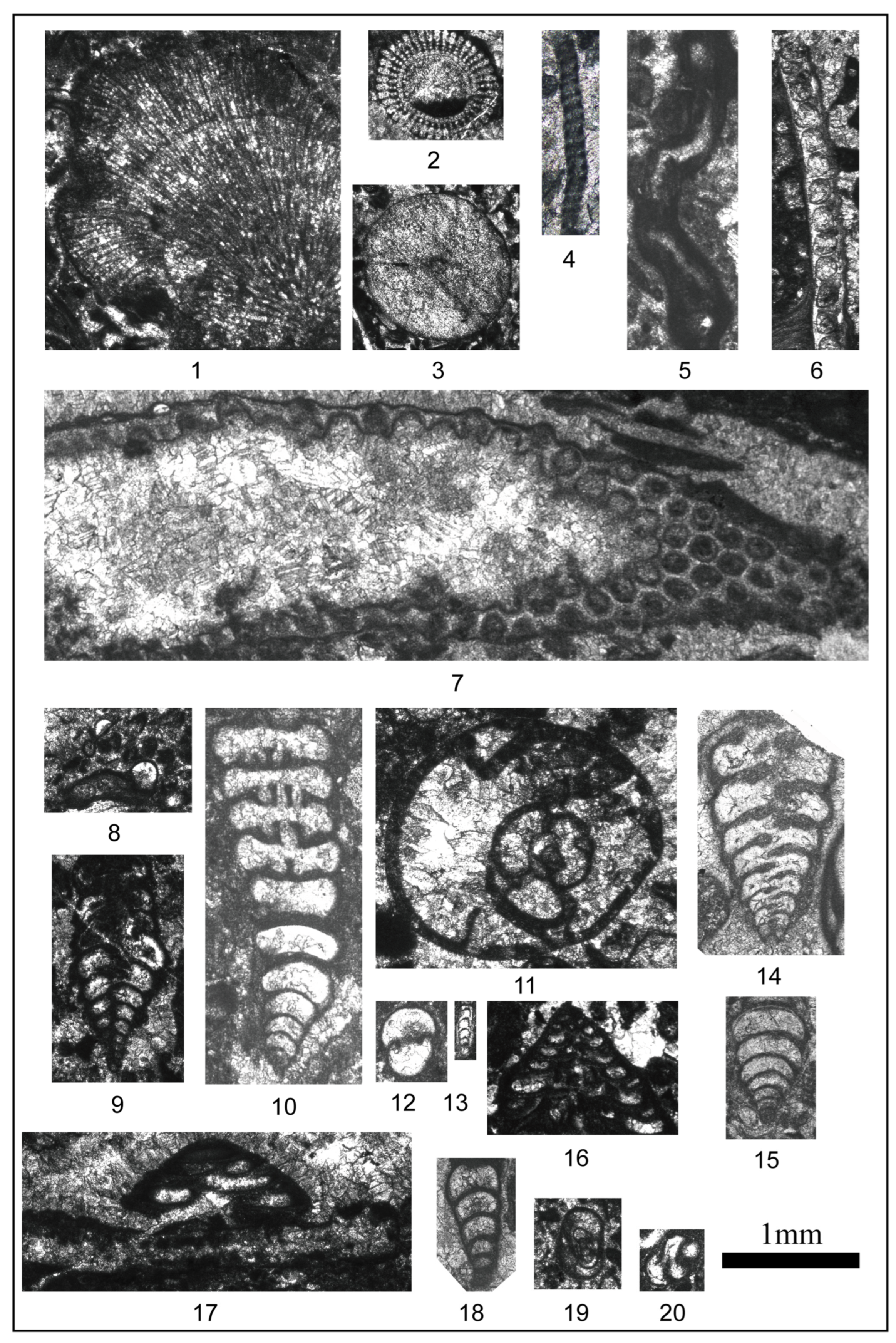
3. Methods and Database
4. Results
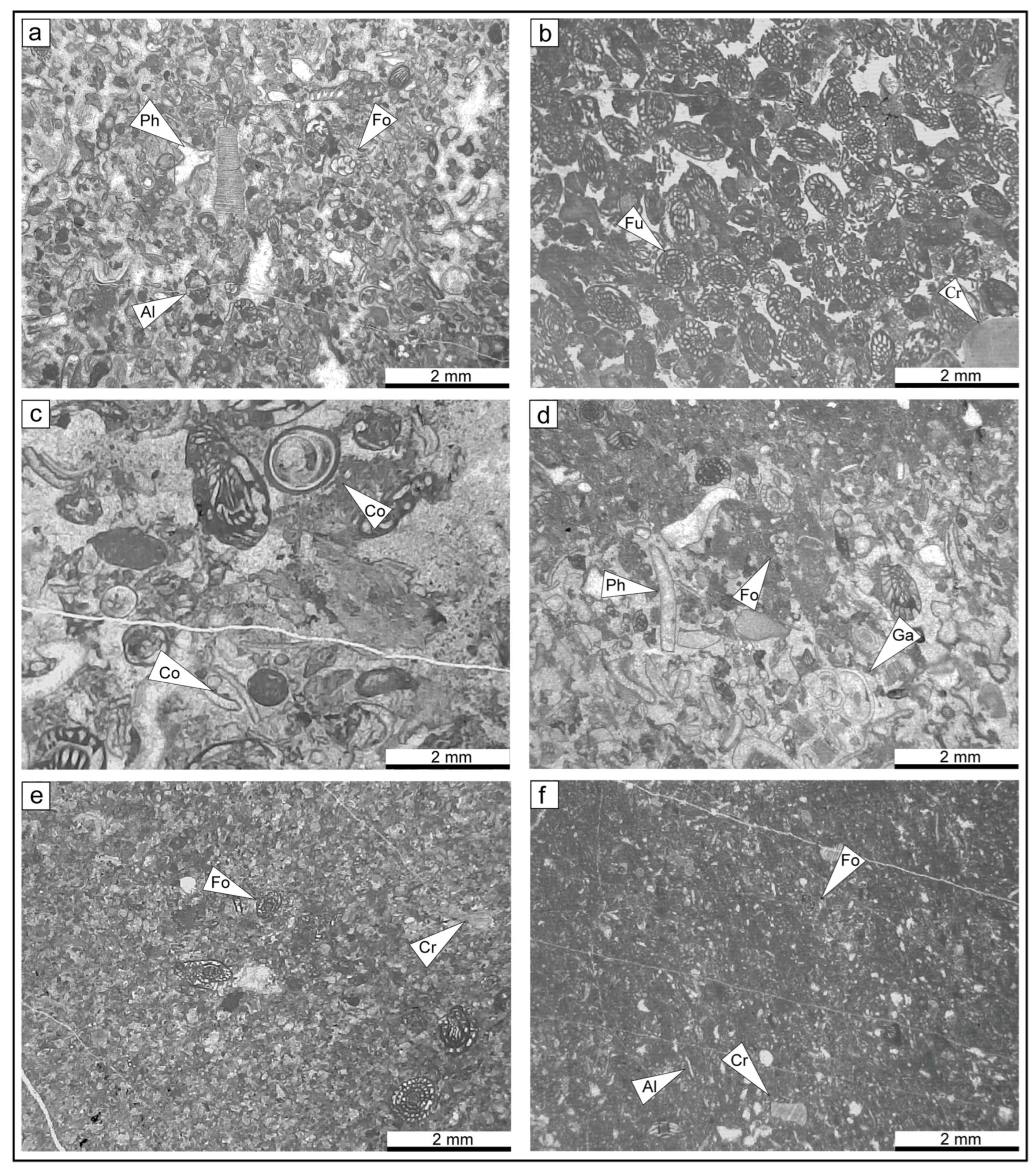
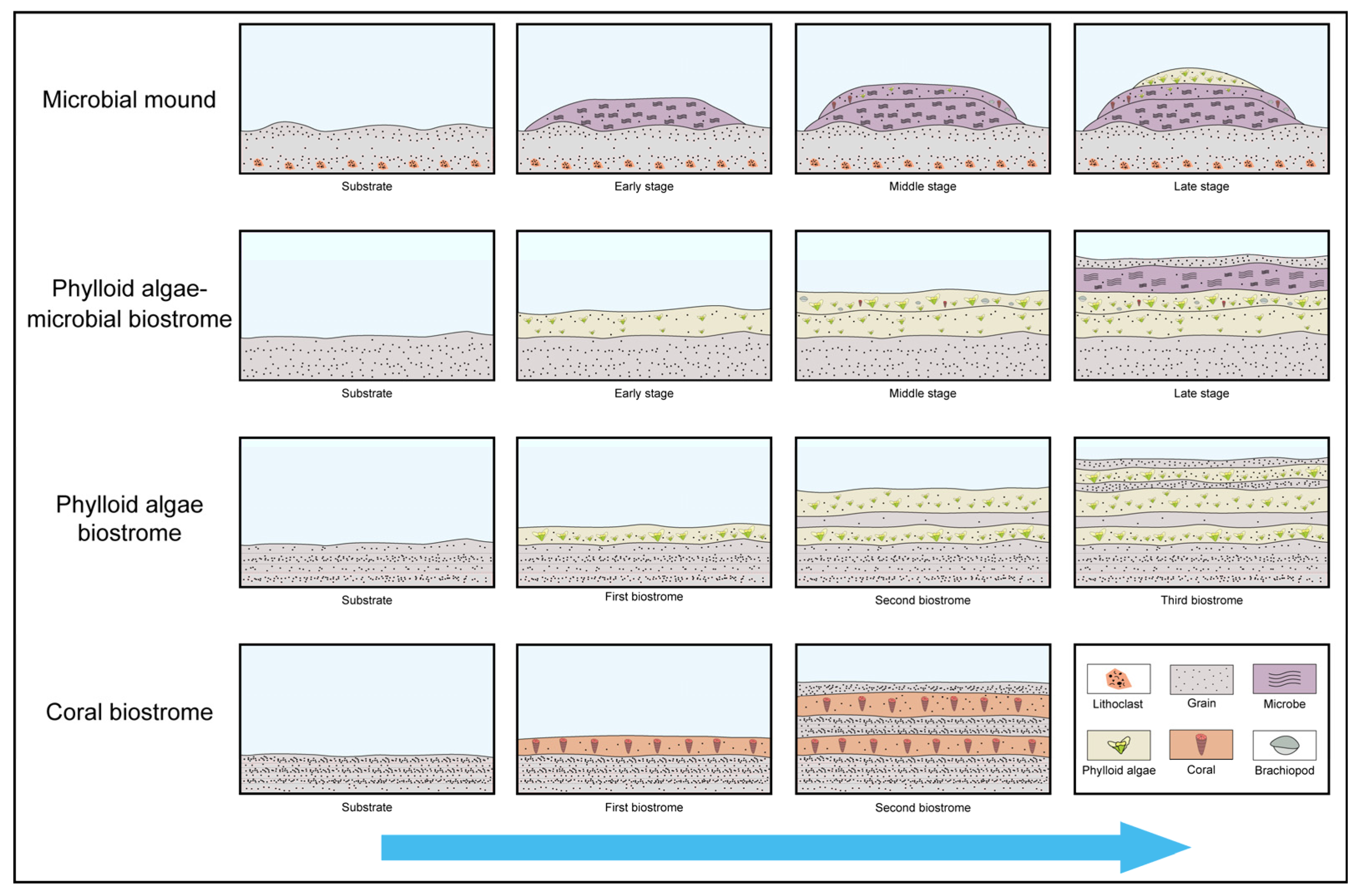
4.1. Lithofacies Types
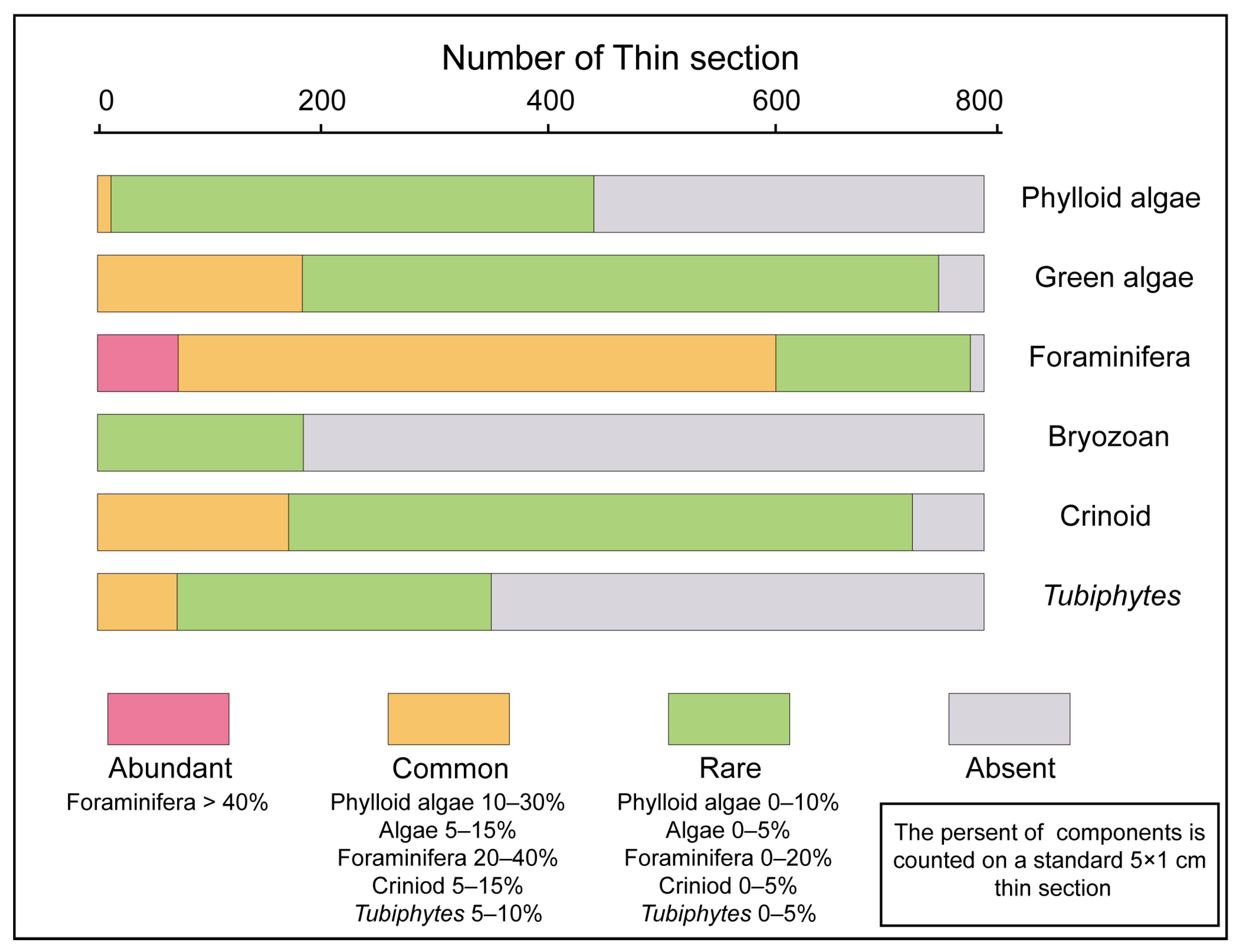
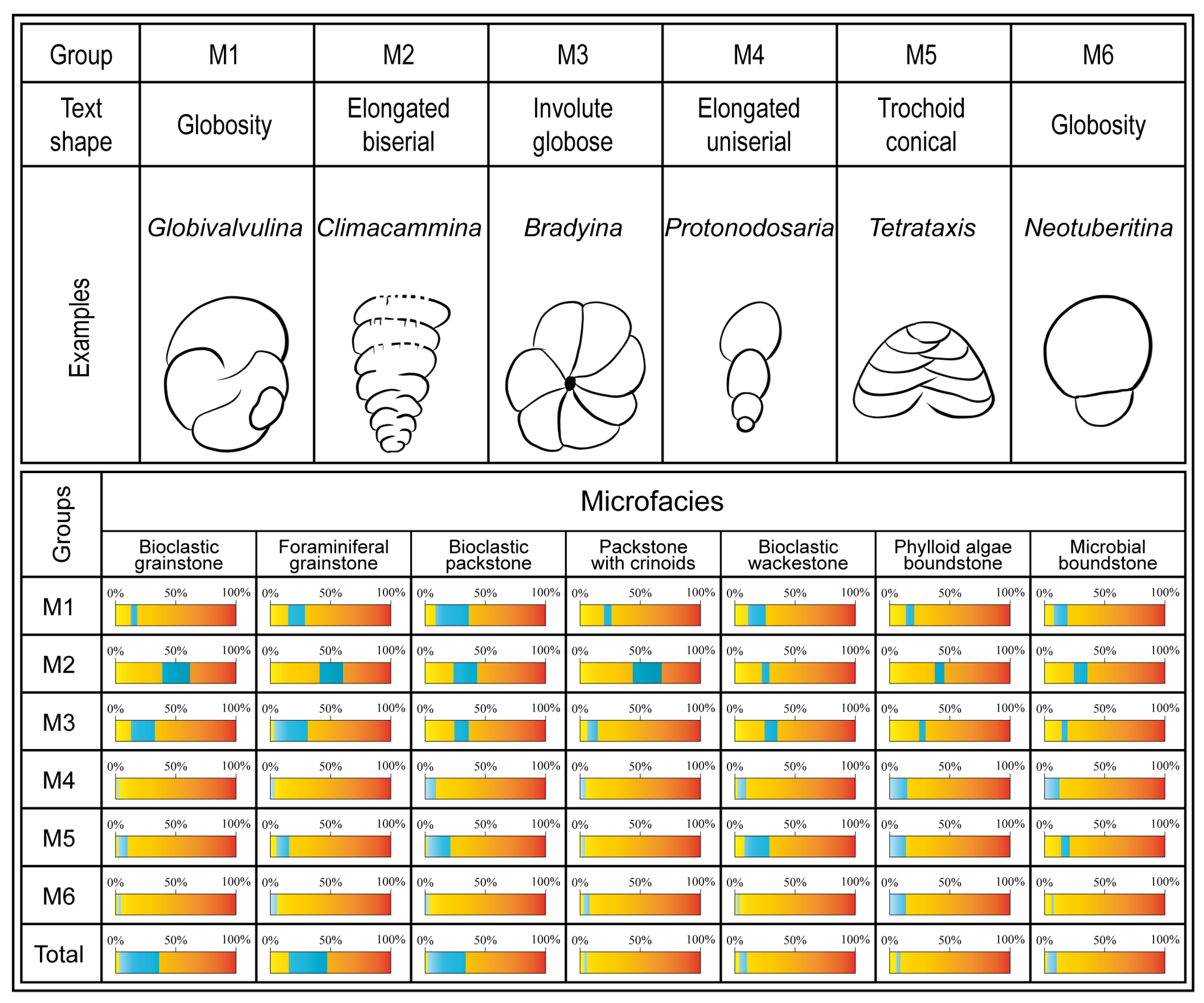
4.2. Main Skeletal Grains and Distribution in Facies
4.3. Canonical Correspondence Analysis
4.4. Theoretical Ecospace
5. Discussion
5.1. Environment Factors
5.2. Average Tiering and Average Motility
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veevers, J.J.; Powell, C.M.A. Late Paleozoic glacial episodes in Gondwanaland reflected in transgressive-regressive depositional sequences in Euramerica. GSA Bull. 1987, 98, 475–487. [Google Scholar] [CrossRef]
- Isbell, J.L.; Miller, M.F.; Wolfe, K.L.; Lenaker, P.A. Timing of late Paleozoic glaciation in Gondwana: Was glaciation responsible for the development of northern hemisphere cyclothems? In Extreme Depositional Environments: Mega End Members in Geologic Time; Chan, M.A., Archer, A.W., Eds.; Geological Society of America Special; Geological Society of America: Boulder, CO, USA, 2003; Volume 370, pp. 5–24. ISBN 9780813723709. [Google Scholar] [CrossRef]
- Isbell, J.L.; Henry, L.C.; Gulbranson, E.L.; Limarino, C.O.; Fraiser, M.L.; Koch, Z.J.; Ciccioli, P.L.; Dineen, A.A. Glacial paradoxes during the late Paleozoic ice age: Evaluating the equilibrium line altitude as a control on glaciation. Gondwana Res. 2012, 22, 1–19. [Google Scholar] [CrossRef]
- Chen, B.; Joachimski, M.M.; Wang, X.D.; Shen, S.Z.; Qi, Y.P.; Qie, W.K. Ice volume and paleoclimate history of the Late Paleozoic Ice Age from conodont apatite oxygen isotopes from Naqing (Guizhou, China). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 448, 151–161. [Google Scholar] [CrossRef]
- Huang, W.T.; Maillet, M.; Zhang, Y.L.; Guan, C.Q.; Miao, Z.W.; Samankassou, E.; Gong, E.P. The onset of the major glaciation of the LPIA: Record from South China. Int. J. Earth Sci. Geol. Rundsch. 2020, 109, 281–300. [Google Scholar] [CrossRef]
- Chappell, J. Coral morphology, diversity and reef growth. Nature 1980, 286, 249–252. [Google Scholar] [CrossRef]
- Wu, Y.S.; Fan, J.S. Quantitative evaluation of the sea-level drop at the end-Permian: Based on reefs. Acta Geol. Sin. Engl. Ed. 2003, 77, 95–102. [Google Scholar] [CrossRef]
- Hallock, P. Global change and modern coral reefs: New opportunities to understand shallow-water carbonate depositional processes. Sediment. Geol. 2005, 175, 19–33. [Google Scholar] [CrossRef]
- Gong, E.P.; Huang, W.T.; Guan, C.Q.; Zhang, Y.L.; Wang, L.F.; Yang, Z.Y.; Li, X.; Wang, J.J.; Miao, Z.W. The coupling relationship between Carboniferous reefs and the late Paleozoic ice age. Acta Geol. Sin. 2021, 95, 1671–1692, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Gong, E.P.; Guan, G.Y. Carboniferous extinction and influence on evolution of reef communities. J. Northeast. Univ. (Nat. Sci.) 1998, 19, 122–124. (In Chinese) [Google Scholar]
- Nakazawa, T. Carboniferous reef succession of the Panthalassan open-ocean setting: Example from Omi Limestone, central Japan. Facies 2001, 44, 183–210. [Google Scholar] [CrossRef]
- Bureau of Geology and Mineral Resources of Guizhou Province. Regional Geology of Guizhou Province; Geological Publishing House: Beijing, China, 1987. (In Chinese)
- Qiao, L.; Shen, S.Z. A global review of the Late Mississippian (Carboniferous) Gigantoproductus (Brachiopoda) faunas and their paleogeographical, paleoecological, and paleoclimatic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 420, 128–137. [Google Scholar] [CrossRef]
- Fang, S.X.; Hou, F.H. Bryozoan-coral patch reef of Datang age of Carboniferous period of Langping area, Tianlin County, Guangxi Province. J. Southwest. Pet. Inst. 1985, 4, 4–18, (In Chinese with English Abstract). [Google Scholar]
- Webb, G.E. Latest Devonian Early Carboniferous reefs: Depressed reef building after the Middle Paleozoic collapse. In Phanerozoic Reef Patterns; Kiessling, W., Flügel, E., Golonka, J., Eds.; SEPM Special Publication No. 72: Tulsa, OK, USA, 2002; pp. 239–269. [Google Scholar] [CrossRef]
- Gong, E.P.; Zhang, Y.L.; Guan, C.Q.; Chang, H.L. Main features of Carboniferous organic reefs in the world. J. Palaeogeogr. Chin. Ed. 2010, 12, 127–139, (In Chinese with English Abstract). [Google Scholar]
- Chen, X.H.; Gong, E.P.; Wang, T.H.; Guan, C.Q.; Zhang, Y.L.; Yang, D.Y.; Wang, H.M. The basic characteristics of Early Carboniferous coral reef at Xiadong Village in Tianlin, Guangxi, and its sedimentary environment. Acta Geol. Sin. 2013, 87, 597–608, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Yao, L.; Wang, X.D. Distribution and evolution of Carboniferous reefs in South China. Palaeoworld 2016, 25, 362–376. [Google Scholar] [CrossRef]
- Shi, Y.K.; Yang, X.N.; Liu, J.R. Early Carboniferous to Early Permian Fusulinids from Zongdi Section in Southern Guizhou; Science Press: Beijing, China, 2012; (In Chinese with English Abstract). [Google Scholar]
- Zhang, L.X.; Zhou, J.P.; Sheng, J.Z. Upper Carboniferous and Lower Permian Fusulinids from Western Guizhou; Science Press: Beijing, China, 2010; (In Chinese with English Abstract). [Google Scholar]
- Ayoub-Hannaa, W.; Huntley, J.W.; Fürsich, F.T. Significance of Detrended Correspondence Analysis (DCA) in palaeoecology and biostratigraphy: A case study from the Upper Cretaceous of Egypt. J. Afr. Earth Sci. 2013, 80, 48–59. [Google Scholar] [CrossRef]
- Hennebert, M.; Lees, A. Environmental gradients in carbonate sediments and rocks detected by correspondence analysis: Examples from the Recent of Norway and the Dinantian of southwest England. Sedimentology 1991, 38, 623–642. [Google Scholar] [CrossRef]
- Cózar, P.; Izart, A.; Somerville, I.D.; Aretz, M.; Coronado, I.; Vachard, D. Environmental controls on the development of Mississippian microbial carbonate mounds and platform limestones in southern Montagne Noire (France). Sedimentology 2019, 66, 2392–2424. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Zhang, X.; Guo, R.; Wang, W. Multivariate analyses in paleobiogeography of Devonian rugose corals for revealing the multiple accretionary processes of the southwestern Central Asian Orogenic Belt. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 615, 111455. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Verdonschot, P.F.M. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Bambach, R.K.; Bush, A.M.; Erwin, D.H. Autecology and the filling of ecospace: Key metazoan radiations. Palaeontology 2007, 50, 1–22. [Google Scholar] [CrossRef]
- Flügel, E. Microfacies of Carbonate Rocks: Analysis, Interpretation and Application; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar] [CrossRef]
- Mamet, B. Carboniferous Calcareous Algae. In Calcareous Algae; Springer: Berlin/Heidelberg, Germany, 1991; pp. 370–451. [Google Scholar]
- Sano, H.; Horibo, K.; Kumamoto, Y. Tubiphytes-archaeolithoporella-girvanella reefal facies in Permian buildup, Mino terrane, central Japan. Sediment. Geol. 1990, 68, 293–306. [Google Scholar] [CrossRef]
- Aretz, M.; Herbig, H.G. Microbial-sponge and microbial-metazoan buildups in the Late Viséan basin-fill sequence of the Jerada Massif (Carboniferous, NE Morocco). Geol. J. 2008, 43, 307–336. [Google Scholar] [CrossRef]
- Lees, A.; Miller, J. Facies variation in Waulsortian buildups, Part 2; mid-Dinantian buildups from Europe and North America. Geol. J. 1985, 20, 159–180. [Google Scholar] [CrossRef]
- Swinchatt, J.P. Algal boring: A possible depth indicator in carbonate rocks and sediments. Geol. Soc. Am. Bull. 1969, 80, 1391–1396. [Google Scholar] [CrossRef]
- Beauchamp, B.; Davies, G.R.; Nassichuk, W.W. Upper Carboniferous to Lower Permian Palaeoaplysina-phylloid algal buildups, Canadian Arctic Archipelago. In Reefs, Canada and Adjacent Areas; Geldsetzer, H.H.J., James, N.P., Tebbutt, G.E., Eds.; Canadian Society of Petroleum Geologists: Calgary, AB, Canada, 1989; Volume 13, pp. 590–599. [Google Scholar]
- Wahlman, G.P. Upper Carboniferous-Lower Permian (Bashkirian-Kungurian) mounds reefs. In Phanerozoic Reef Patterns; Kiessling, W., Flügel, E., Golonka, J., Eds.; SEPM Special Publication No. 72: Tulsa, OK, USA, 2002; pp. 271–338. [Google Scholar] [CrossRef]
- Gong, E.P.; Zhang, Y.L.; Guan, C.Q.; Samankassou, E.; Sun, B.L. Paleoecology of late Carboniferous phylloid algae in southern Guizhou, SW China. Acta Geol. Sin. Engl. Ed. 2007, 81, 566–572. [Google Scholar]
- Wilson, J.L. Carbonate Facies in Geologic History; Springer: Berlin/Heidelberg, Germany, 1975. [Google Scholar]
- Debout, L.; Denayer, J. Palaeoecology of Upper Tournaisian (Mississippian) crinoidal limestones from South Belgium. Geol. Belg. 2018, 21, 111–127. [Google Scholar] [CrossRef]
- Gong, E.P.; Samankassou, E.; Guan, C.Q.; Zhang, Y.L.; Sun, B.L. Paleoecology of Pennsylvanian phylloid algal buildups in South Guizhou, China. Facies 2007, 53, 615–623. [Google Scholar]
- Scrutton, C.T. Paleozoic corals: Their evolution and palaeoecology. Geol. Today 1999, 15, 184–193. [Google Scholar] [CrossRef]
- Egenhoff, S.O.; Peterhänsel, A.; Bechstädt, T.; Zühlke, R.; Grötsch, J. Facies architecture of an isolated carbonate platform: Tracing the cycles of the Latemàr (middle Triassic, northern Italy). Sedimentology 1999, 46, 893–912. [Google Scholar] [CrossRef]
- Bahamonde, J.R.; Kenter, J.; Porta, G.D.; Keim, L.; Immenhauser, A.; Reijmer, J. Lithofacies and depositional processes on a high, steep-margined Carboniferous (Bashkirian-Moscovian) carbonate platform slope, Sierra del Cuera, NW Spain. Sediment. Geol. 2004, 166, 145–156. [Google Scholar] [CrossRef]
- Bahamonde, J.R.; Merino-Tome, O.A.; Heredia, N. A Pennsylvanian microbial bound-stone-dominated carbonate shelf in a distal foreland margin (Picos de Europa Province, NW Spain). Sediment. Geol. 2007, 198, 167–193. [Google Scholar] [CrossRef]
- Porta, G.D.; Kenter JA, M.; Bahamonde, J.R.; Immenhauser, A.; Villa, E. Microbial boundstone dominated carbonate slope (Upper Carboniferous, N Spain): Microfacies, lithofacies distribution and stratal geometry. Facies 2003, 49, 175–207. [Google Scholar] [CrossRef]
- Veevers, J.J. Pangea: Geochronological correlation of successive environmental and stratitectonic phases in Europe and Australia. Earth-Sci. Rev. 2013, 127, 48–95. [Google Scholar] [CrossRef]
- Van der Kooij, B.; Immenhauser, A.; Steuber, T.; Bahamonde Rionda, J.R.; Meríno-Tomé, O. Controlling factors of volumetrically important marine carbonate cementation in deep slope settings. Sedimentology 2010, 57, 1491–1525. [Google Scholar] [CrossRef]
- West, R.R. Temporal changes in Carboniferous reef mound communities. Palaios 1988, 3, 152–169. [Google Scholar] [CrossRef]
- Samankassou, E.; West, R.R. Construction versus accumulation in phylloid algal mounds: An example of a small constructed mound in the Pennsylvanian of Kansas, USA. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002, 185, 379–389. [Google Scholar] [CrossRef]
- Vachard, D.; Pille, L.; Gaillot, J. Palaeozoic Foraminifera: Systematics, palaeoecology and responses to global changes. Rev. Micropaléontol. 2010, 53, 209–254. [Google Scholar] [CrossRef]
- Haynes, J.R. Foraminifera; Palgrave Macmillan: London, UK, 1981. [Google Scholar]
- Gallagher, S.J. Controls on the distribution of calcareous Foraminifera in the Lower Carboniferous of Ireland. Mar. Micropaleontol. 1998, 34, 187–211. [Google Scholar] [CrossRef]
- Holcová, K.; Slavík, L. The morphogroups of small agglutinated foraminifera from the Devonian carbonate complex of the Prague Synform, (Barrandian area, Czech Republic). Palaeogeography, Palaeoclimatol. Palaeoecol. 2013, 386, 210–224. [Google Scholar] [CrossRef]
- Della Porta, G. Facies distribution of fusulinida in a Bashkirian-Moscovian (Pennsylvanian) carbonate platform top (Cantabrian Mountains, NW Spain). J. Foraminifer. Res. 2005, 35, 344–367. [Google Scholar] [CrossRef]
- Nagy, J.; Hess, S.; Alve, E. Environmental significance of foraminiferal assemblages dominated by small-sized Ammodiscus and Trochammina in Triassic and Jurassic delta-influenced deposits. Earth-Sci. Rev. 2010, 99, 31–49. [Google Scholar] [CrossRef]
- Murray, J.W.; Alve, E.; Jones, B.W. A new look at modern agglutinated benthic foraminiferal morphogroups: Their value in palaeoecological interpretation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 309, 229–241. [Google Scholar] [CrossRef]
- Reolid, M.; Rodríguez-Tovar, F.J.; Nagy, J.; Olóriz, F. Benthic foraminiferal morphogroups of mid to outer shelf environments of the Late Jurassic (Prebetic Zone, southern Spain): Characterization of biofacies and environmental significance. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 261, 280–299. [Google Scholar] [CrossRef]
- Jones, R.W.; Charnock, M.A. “Morphogroups” of agglutinated foraminifera: Their life positions and feeding habits and potential applicability in (paleo)ecological studies. Rev. Paleobiol. 1985, 4, 311–320. [Google Scholar]
- Nagy, J. Environmental significance of foraminiferal morphogroups in Jurassic North Sea deltas. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1992, 95, 111–134. [Google Scholar]
- Nagy, J.; Gradstein, F.M.; Kaminski, M.A.; Holbourn, A.E. Foraminiferal morphogroups, paleoenvironments and new taxa from Jurassic to Cretaceous strata of Thakkhola, Nepal. In Proceedings of the Fourth International Workshop on Agglutinated Foraminifera, Krakow, Poland, 12–19 September 1993; Kaminski, M.A., Geroch, S., Gasinsk, M.A., Eds.; Grzybowski Foundation Special Publication No. 3: Kraków, Poland, 1995; pp. 191–209. [Google Scholar]
- Nagy, J.; Reolid, M.; Rodríguez-Tovar, F.J. Foraminiferal morphogroups in dysoxic shelf deposits from the Jurassic of Spitsbergen. Polar Res. 2009, 28, 214–221. [Google Scholar]
- Corliss, B.H. Morphology and microhabitat preferences of benthic foraminifera from the northwest Atlantic Ocean. Mar. Micropaleontol. 1991, 17, 195–236. [Google Scholar] [CrossRef]
- Tyszka, J. Response of middle Jurassic benthic foraminiferal morphogroups to dysoxic/anoxic conditions in the Pieniny Klippen Basin, Polish Carpathians. Palaeogeography, Palaeoclimatol. Palaeoecol. 1994, 110, 55–81. [Google Scholar] [CrossRef]
- Reolid, M.; Nagy, J.; Rodríguez-Tovar, F.J. Ecostratigraphic trends of Jurassic agglutinated foraminiferal assemblages as a response to sea-level changes in shelf deposits of Svalbard (Norway). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 293, 184–196. [Google Scholar] [CrossRef]
- Reolid, M.; Rodríguez-Tovar, F.J.; Nagy, J. Ecological replacement of Valanginian agglutinated foraminifera during a maximum flooding event in the Boreal realm (Spitsbergen). Cretac. Res. 2012, 33, 196–204. [Google Scholar] [CrossRef]
- Szydlo, A. The distribution of agglutinated Foraminifera in the Cieszyn Basin, Polish outer Carpathians. In Proceedings of the Sixth International Workshop on Agglutinated Foraminifera, Prague, Czech Republic, 1–7 September 2001; pp. 461–470. [Google Scholar]
- Murray, J.W. Ecology and Palaeoecology of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006; pp. 10–62. [Google Scholar] [CrossRef]
- Rita, P.; Reolid, M.; Duarte, L.V. Benthic foraminiferal assemblages record major environmental perturbations during the Late Pliensbachian-Early Toarcian interval in the Peniche GSSP, Portugal. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 454, 267–281. [Google Scholar] [CrossRef]
- Cossey, P.J.; Mundy DJ, C. Tetrataxis: A loosely attached limpet-like foraminifer from the Upper Palaeozoic. Lethaia 1990, 23, 311–322. [Google Scholar] [CrossRef]
- Clausen, S.; Álvaro, J.J. Encrusting strategies in a Cambrian nonreefal epibenthic community. Bull. Soc. Géologique Fr. 2002, 173, 553–559. [Google Scholar] [CrossRef]
- Wang, S.H.; Fan, J.S.; Rigby, J.K. Archaeolithoporella and Tubiphytes: Affinities and palaeoecology in Permian reefs of South China. Sci. China Ser. B Chem. Life Sci. Earth Sci. 1994, 37, 723–743, (In Chinese with English Abstract). [Google Scholar]
- Riding, R.; Li, G. Affinity of Tubiphytes. Palaeontology 1992, 35, 131–142. [Google Scholar]
- Senowbari-Daryan, B.; Flügel, E. Tubiphytes Maslov, an enigmatic fossil: Classification, fossil record and significance through time. In Studies on Fossil Benthic Algae; Senowbari-Daryan, B., Flügel, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 353–382. [Google Scholar]
- Whalen, M.T.; Day, J.; Eberli, G.P.; Homewood, P.W. Microbial carbonates as indicators of environmental change and biotic crises in carbonate systems: Examples from the late Devonian, Alberta basin, Canada. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002, 181, 127–151. [Google Scholar] [CrossRef]
- Riding, R.; Liang, L.Y. Geobiology of microbial carbonates: Metazoan and seawater saturation state influences on secular trends during the Phanerozoic. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 101–115. [Google Scholar] [CrossRef]
- Liu, J.B.; Ezaki, Y.; Yang, S.R.; Wang, H.F.; Adachi, N. Age and sedimentology of microbialites after the end-Permian mass extinction in Luodian, Guizhou Province. J. Palaeogeogr. 2007, 9, 473–486. [Google Scholar]
- Wu, Y.S.; Jiang, H.; Yang, W.; Fan, J. Microbialite of anoxic condition from Permian-Triassic transition in Guizhou, China. Sci. China Ser. D Earth Sci. 2007, 50, 1040–1051. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Gong, E.; Zhang, Y.; Guan, C.; Huang, W. Depositional Environment and Ecological Response of Bioconstructions: A Case Study of Southern China (Guizhou Province) in Moscovian–Gzhelian. Life 2024, 14, 1150. https://doi.org/10.3390/life14091150
Li X, Gong E, Zhang Y, Guan C, Huang W. Depositional Environment and Ecological Response of Bioconstructions: A Case Study of Southern China (Guizhou Province) in Moscovian–Gzhelian. Life. 2024; 14(9):1150. https://doi.org/10.3390/life14091150
Chicago/Turabian StyleLi, Xiao, Enpu Gong, Yongli Zhang, Changqing Guan, and Wentao Huang. 2024. "Depositional Environment and Ecological Response of Bioconstructions: A Case Study of Southern China (Guizhou Province) in Moscovian–Gzhelian" Life 14, no. 9: 1150. https://doi.org/10.3390/life14091150
APA StyleLi, X., Gong, E., Zhang, Y., Guan, C., & Huang, W. (2024). Depositional Environment and Ecological Response of Bioconstructions: A Case Study of Southern China (Guizhou Province) in Moscovian–Gzhelian. Life, 14(9), 1150. https://doi.org/10.3390/life14091150




