Potential Protective Effect of Hesperidin (Vitamin P) against Glyphosate-Induced Spermatogenesis Damage in Male Rats: Biochemical and Histopathological Findings on Reproductive Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Animals and Procedure
2.3. Assessments of Epididymal Spermatozoon Motility
2.4. COMET Assay
2.5. Assessments of Oxidant/Antioxidant Status
2.6. Estimation of Blood Testosterone
2.7. Examination of Histological Parameters
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonine, B.; Guillaume, M.; Philippe, D.; Marie-Hélène, P. Low concentrations of glyphosate alone affect the pubertal male rat meiotic step: An in vitro study. In Vitro Toxicol. 2022, 79, 105291. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Akiri, O.F.; Durojaiye, M.A.; Adenike, A. Combined effects of repeated administration of Bretmont Wipeout (glyphosate) and Ultrazin (atrazine) on testosterone, oxidative stress and sperm quality of Wistar rats. Toxicol. Mech. Methods 2015, 25, 70–80. [Google Scholar] [CrossRef]
- Cai, W.; Ji, Y.; Song, X.; Guo, H.; Han, L.; Zhang, F.; Liu, X.; Zhang, H.; Zhu, B.; Xu, M. Effects of glyphosate exposure on sperm concentration in rodents: A systematic review and meta-analysis. Environ. Toxicol. Pharmacol. 2017, 55, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Katsanaki, K.; Lagodonti, G.; Messini, C.; Simopoulou, M.; Dafopoulos, K.; Daponte, A. The effect of glyphosate on human sperm motility and sperm DNA fragmentation. Int. J. Environ. Res. Public. Health 2018, 15, 1117. [Google Scholar] [CrossRef] [PubMed]
- Semis, H.S.; Kandemir, F.M.; Caglayan, C.; Kaynar, O.; Genc, A.; Arıkan, S.M. Protective effect of naringin against oxaliplatin-induced peripheral neuropathy in rats: A behavioral and molecular study. J. Biochem. Mol. Toxicol. 2022, 36, e23121. [Google Scholar] [CrossRef] [PubMed]
- Shabani, M.; Jamali, Z.; Bayrami, D.; Salimi, A. Hesperidin via maintenance of mitochondrial function and antioxidant activity protects lithium toxicity in rat heart isolated mitochondria. Drug Chem. Toxicol. 2023, 27, 1–9. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Tripathi, D.N.; Jena, G.B. Hesperidin protects testicular toxicity of doxorubicin in rat: Role of NF-κB, p38 and caspase-3. Food Chem. Toxicol. 2011, 49, 838–847. [Google Scholar] [CrossRef]
- Kaya, K.; Çiftçi, O.; Çetin, A.; Doğan, H.; Başak, N. Hesperidin protects testicular and spermatological damages induced by cisplatin in rats. Andrologia 2015, 47, 793–800. [Google Scholar] [CrossRef]
- Kilic, K.; Sakat, M.S.; Yildirim, S.; Kandemir, M.F.; Gozeler, M.S.; Dortbudak, M.B.; Kucukler, S. The amendatory effect of hesperidin and thymol in allergic rhinitis: An ovalbumin-induced rat model. Eur. Arch. Oto-Rhino-L 2019, 276, 407–415. [Google Scholar] [CrossRef]
- Aksu, E.H.; Kandemir, F.M.; Kucukler, S. The effects of hesperidin on colistin-induced reproductive damage, autophagy, and apoptosis by reducing oxidative stress. Andrologia 2021, 53, e13900. [Google Scholar] [CrossRef]
- Shokoohi, M.; Khaki, A.; Shoorei, H.; Khaki, A.A.; Moghimianand, M.; Abtahi-Eivary, S.H. Hesperidin attenuated apoptotic-related genes in testicle of a male rat model of varicocoele. Andrology 2020, 8, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Che, S.; Chen, S.; Ruan, H.; Zhang, L. Hesperidin partly ameliorates the decabromodiphenyl ether-induced reproductive toxicity in pubertal mice. Environ. Sci. Pollut. Res. 2022, 29, 90391–90403. [Google Scholar] [CrossRef] [PubMed]
- Rezaeyan, A.; Fardid, R.; Haddadi, G.H.; Takhshid, M.A.; Hosseinzadeh, M.; Najafi, M.; Salajegheh, A. Evaluating radioprotective effect of hesperidin on acute radiation damage in the lung tissue of rats. J. Biomed. Phys. Eng. 2016, 6, 165. [Google Scholar]
- Turkmen, R.; Dogan, I. Determination of acute oral toxicity of glyphosate isopropylamine salt in rats. Environ. Sci. Pollut. Res. 2020, 27, 19298–19303. [Google Scholar] [CrossRef]
- Avdatek, F.; Birdane, Y.O.; Türkmen, R.; Demirel, H.H. Ameliorative effect of resveratrol on testicular oxidative stress, spermatological parameters and DNA damage in glyphosate-based herbicide-exposed rats. Andrologia 2018, 50, e13036. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Hissin, P.J.; Hilf, R.A. fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method fortotal antioxidant capacity using a new generation, morestable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Ahbab, M.A.; Barlas, N.; Karabulut, G. The toxicological effects of bisphenol A and octylphenol on the reproductive system of prepubertal male rats. Toxicol. Ind. Health 2017, 33, 133–146. [Google Scholar] [CrossRef]
- Güleş, Ö.; Doğan, G.; Ercins, U.H.; Eren, Ü. Effects of quercetin against doxorubicin-induced testicular toxicity in male rats. Biol. Bull. 2022, 49, 203–213. [Google Scholar] [CrossRef]
- Vijaya Bharathi, B.; Jaya Prakash, G.; Krishna, K.M.; Ravi Krishna, C.H.; Sivanarayana, T.; Madan, K.; Rama Raju, G.A.; Annapurna, A. Protective effect of alpha glucosyl hesperidin (G-Hesperidin) on chronic vanadium induced testicular toxicity and sperm nuclear DNA damage in male Sprague Dawley rats. Andrologia 2015, 47, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi, A.; Lombardo, F.P.; Gandini, L. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Hu, P.; Tang, J.; Li, Y.; Li, C. Effect of glyphosate on reproductive organs in male rat. Acta Histochem 2016, 118, 519–526. [Google Scholar] [CrossRef]
- Victor-Costa, A.B.; Bandeira, S.M.C.; Oliveira, A.G.; Mahecha, G.A.B.; Oliveira, C.A. Changes in testicular morphology and steroidogenesis in adult rats exposed to Atrazine. Reprod. Toxicol. 2010, 29, 323–331. [Google Scholar] [CrossRef]
- Romano, R.M.; Romano, M.A.; Bernardi, M.M.; Furtado, P.V.; Oliveira, C.A. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Arch. Toxicol. 2010, 84, 309–317. [Google Scholar] [CrossRef]
- Benachour, N.; Sipahutar, H.; Moslemi, S.; Gasnier, C.; Travert, C.; Séralini, G.E. Time-and dose-dependent effects of roundup on human embryonic and placental cells. Arch. Environ. Contam. Toxicol. 2007, 53, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Yeni, D.; İnanç, M.; Avdatek, F.; Tuncer, P.B.; Çil, B.; Türkmen, R.; Taşdemir, U. Supplementation of rosmarinic acid has reduced oxidative stress on bull spermatozoa following the freeze thawing process. Cryo Lett. 2018, 39, 156–165. [Google Scholar]
- El-Shenawy, N.S. Oxidative stress responses of rats exposed to Roundup and its active ingredient glyphosate. Environ. Toxicol. Pharmacol. 2009, 28, 379–385. [Google Scholar] [CrossRef]
- Doreswamy, K. Genotoxic consequences associated with oxidative damage in testis of mice subjected to iron intoxication. Toxicology 2005, 206, 169–178. [Google Scholar] [CrossRef]
- Turner, T.T.; Lysiak, J.J. Oxidative stress: A common factor in testicular dysfunction. J. Androl. 2008, 29, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Mancardi, D.; Rastaldo, R.; Pagliaro, P. Cardioprotection: A radical view: Free radicals in pre and postconditioning. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 781–793. [Google Scholar] [CrossRef]
- Yeni, D.; Güngör, S.; Avdatek, F.; Gülhan, M.F.; Olgaç, K.T.; Inanç, M.E.; Denk, B.; Tasdemir, U. Investigation of changes in spermatozoon characteristics, chromatin structure, and antioxidant/oxidant parameters after freeze-thawing of hesperidin (Vitamin P) doses added to ram semen. Life 2022, 12, 1780. [Google Scholar] [CrossRef]
- Inanc, M.E.; Güngör, Ş.; Avdatek, F.; Yeni, D.; Gülhan, M.F.; Olğaç, K.T.; Denk, B.; Taşdemir, U. Thymoquinone improves motility, plasma membrane integrity and DNA integrity of frozen–thawed ram semen. Andrologia 2022, 54, e14547. [Google Scholar] [CrossRef]
- Stumpp, T.; Sasso-Cerri, E.; Freymüller, E.; Miraglia, S.M. Apoptosis and testicular alterations in albino rats treated with etoposide during the prepubertal phase. Anat. Rec. Discov. Mol. Cell Evol. Biol. 2004, 279, 611–622. [Google Scholar] [CrossRef]
- Jamil, S.; Lam, I.; Majd, M.; Tsai, S.H.; Duronio, V. Etoposide induces cell death via mitochondrial-dependent actions of p53. Cancer Cell Int. 2015, 15, 79. [Google Scholar] [CrossRef]
- Alanbaki, A.A.; Mayali, H.M.; Mayali, H.K. The protective effect of quercetin and hesperidin on etoposide induced toxicity in male rats testicular. J. Pharm. Sci. Res. 2017, 9, 1394–1405. [Google Scholar]
- Belhan, S.; Özkaraca, M.; Kandemir, F.M.; Gülyüz, F.; Yıldırım, S.; Ömür, A.D.; Yener, Z. Effectiveness of hesperidin on methotrexate-induced testicular toxicity in rats. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 789–796. [Google Scholar]
- Arafa, H.M.M.; Aly, H.A.A.; Abd-Ellah, M.F.; El-Refaey, H.M. Hesperidin attenuates benzo [α] pyrene-induced testicular toxicity in rats via regulation of oxidant/antioxidant balance. Toxicol. Ind. Health 2009, 25, 417–427. [Google Scholar] [CrossRef]
- Ikpeme, E.V.; Udensi, O.; Ekaluo, U.B.; Solomon, T.O. Efficacy of ascorbic acid in reducing glyphosate-induced toxicity in rats. Biotechnol. J. Int. 2012, 2, 157–168. [Google Scholar] [CrossRef]
- Zahra, Z.; Khan, M.R.; Majid, M.; Maryam, S.; Sajid, M. Gonadoprotective ability of Vincetoxicumarnottianum extract against Bisphenol A-induced testicular toxicity and hormonal imbalance in male Sprague Dawley rats. Andrologia 2020, 52, e13590. [Google Scholar] [CrossRef] [PubMed]
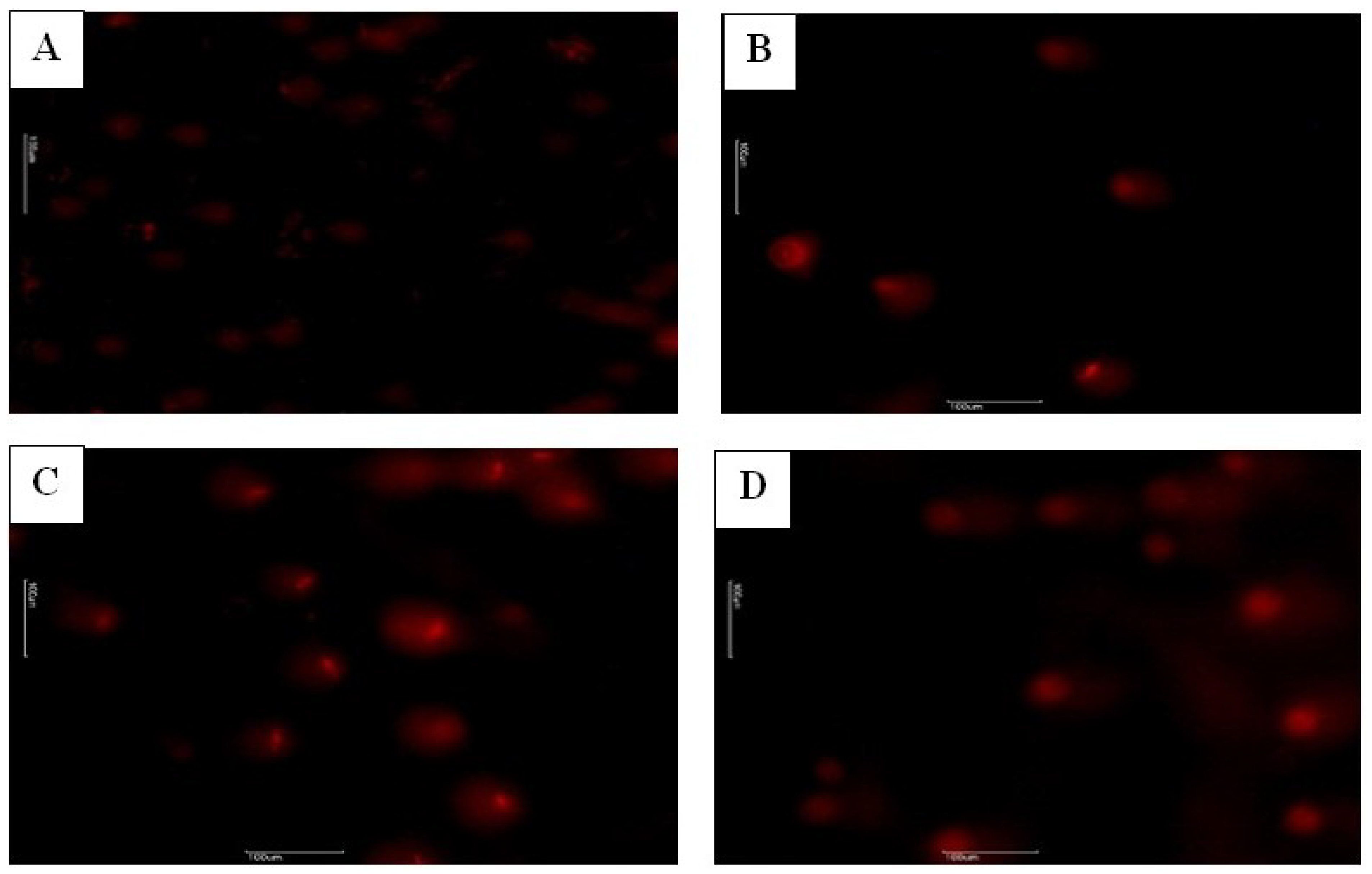
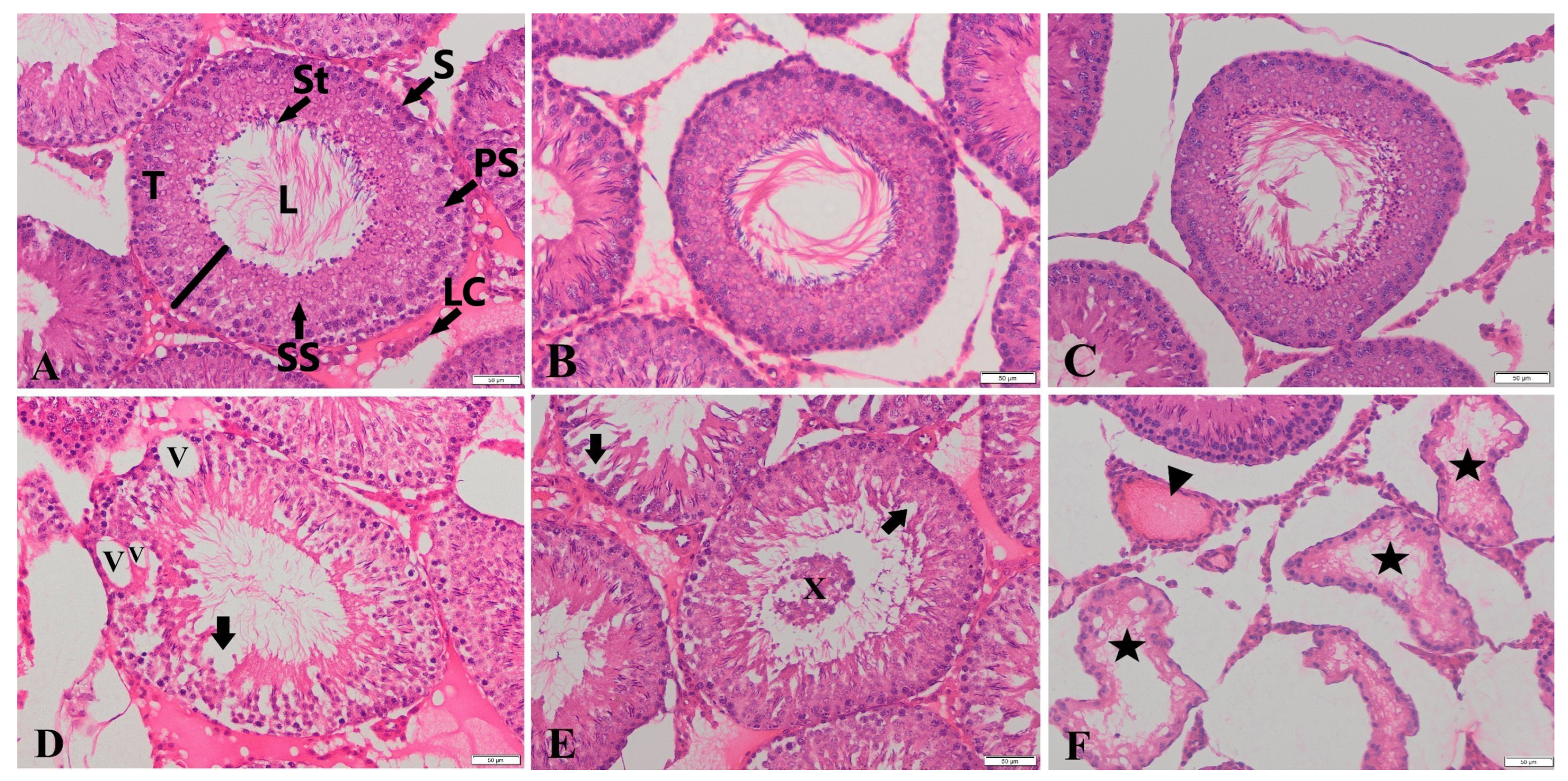
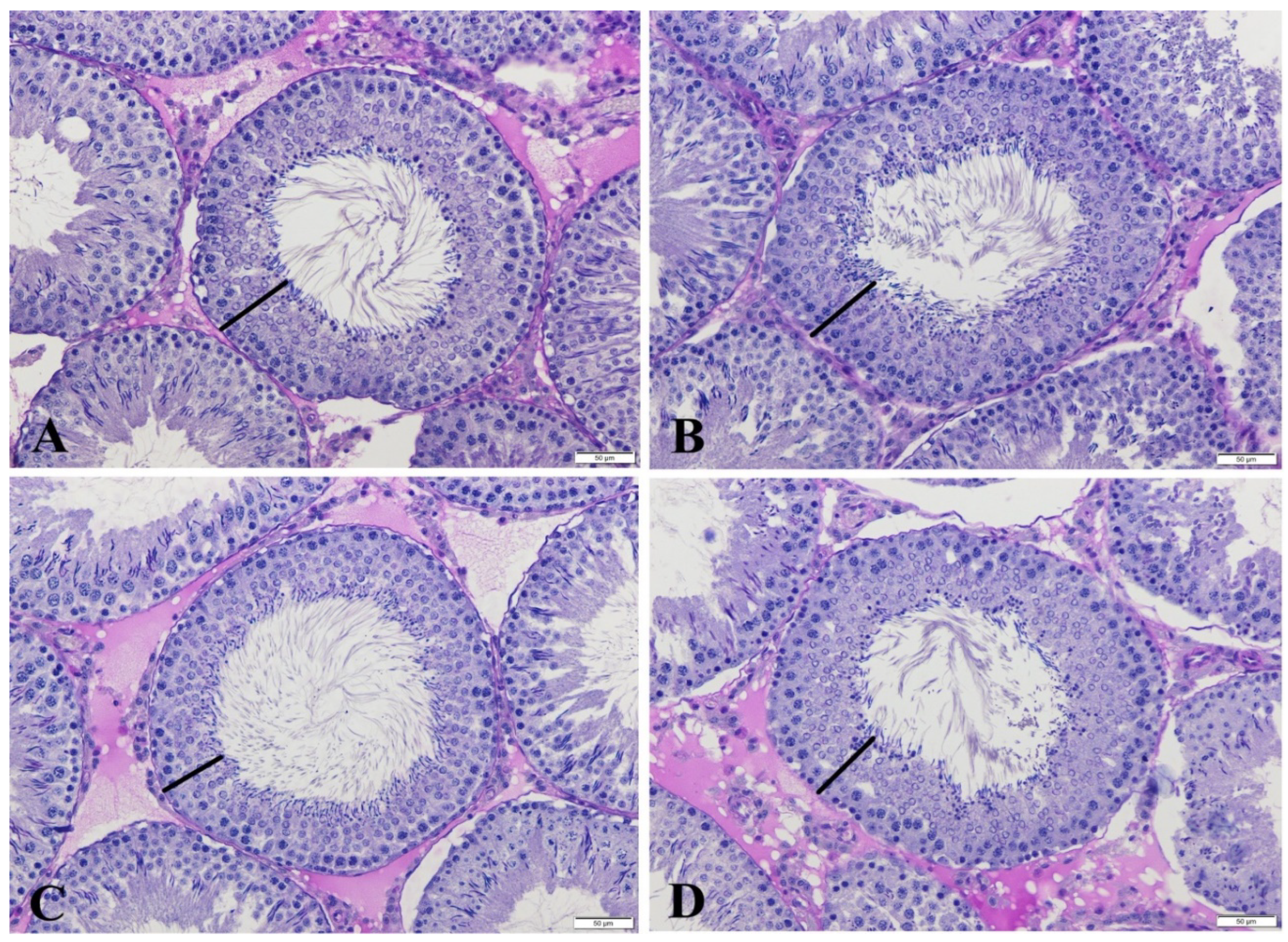
| Groups | Motility % | Testosterone ng/L | Abnormal Sperm Counts | |||
|---|---|---|---|---|---|---|
| Head % | Middle % | Tail % | Total % | |||
| C | 76.42 ± 2.03 a | 433.99 ± 12.03 | 4.07 ± 0.66 b | 5.57 ± 0.74 a | 8.53 ± 1.83 b | 18.64 ± 2.26 b |
| HES | 81.00 ± 2.05 a | 437.76 ± 18.70 | 2.64 ± 0.50 b | 2.21 ± 0.39 b | 6.57 ± 0.39 b | 11.50 ± 0.99 c |
| GLP | 47.57 ± 8.93 b | 470.93 ± 24.79 | 6.35 ± 0.72 a | 6.92 ± 0.81 a | 21.50 ± 1.56 a | 34.92 ± 1.17 a |
| GLP + HES | 75.71 ± 3.56 a | 440.55 ± 11.16 | 7.71 ± 0.82 a | 3.71 ± 0.47 b | 9.50 ± 0.99 b | 21.07 ± 1.61 b |
| p | * | NS | * | * | * | * |
| Groups | H+/E− % | H−/E− % | H+/E+ % | H−/E+ % |
|---|---|---|---|---|
| C | 16.71 ± 2.11 | 26.57 ± 3.37 b | 22.71 ± 2.64 a | 34.00 ± 3.39 |
| HES | 17.42 ± 1.92 | 38.00 ± 2.87 a | 12.42 ± 1.17 b | 31.42 ± 3.28 |
| GLP | 16.42 ± 1.39 | 18.42 ± 1.65 c | 28.71 ± 3.89 a | 36.42 ± 3.83 |
| GLP + HES | 18.00 ± 1.86 | 31.85 ± 2.85 ab | 13.57 ± 1.52 b | 35.14 ± 3.08 |
| p | NS | * | * | NS |
| Groups | Left Testis | Right Testis | Left Epididymis | Right Epididymis |
|---|---|---|---|---|
| C | 6.25 ± 0.11 b | 3.55 ± 0.30 | 6.28 ± 0.12 | 4.07 ± 0.24 ab |
| HES | 6.57 ± 0.19 ab | 3.72 ± 0.33 | 6.61 ± 0.20 | 3.61 ± 0.13 b |
| GLP | 7.07 ± 0.17 a | 3.88 ± 0.16 | 6.40 ± 0.49 | 4.20 ± 0.23 a |
| GLP + HES | 6.86 ± 0.20 a | 4.38 ± 0.41 | 6.90 ± 0.18 | 4.24 ± 0.09 a |
| p | * | NS | NS | * |
| Groups | MDA (nmol/mL) | GSH (nmol/mL) | TAS (mmol/L) | TOS (μmol/L) | OSI (TOS/TAS × 100) |
|---|---|---|---|---|---|
| C | 7.12 ± 0.30 b | 7.12 ± 0.30 a | 0.99 ± 0.03 a | 12.44 ± 0.50 b | 127.36 ± 8.57 b |
| HES | 8.71 ± 0.60 a | 4.35 ± 0.30 b | 0.81 ± 0.02 b | 11.38 ± 0.49 b | 141.60 ± 8.30 b |
| GLP | 9.23 ± 0.49 a | 2.31 ± 0.12 c | 0.77 ± 0.01 b | 17.49 ± 1.44 a | 225.66 ± 17.60 a |
| GLP + HES | 6.94 ± 0.29 b | 2.31 ± 0.09 c | 0.81 ± 0.03 b | 10.83 ± 1.84 b | 134.82 ± 25.83 b |
| p | * | * | * | * | * |
| Groups | Tail Length (μm/s) | Tail DNA (%) | Tail Moment (μm/s) |
|---|---|---|---|
| C | 31.32 ± 0.28 c | 66.73 ± 0.93 | 21.51 ± 0.22 c |
| HES | 34.02 ± 0.34 b | 67.71 ± 0.52 | 23.57 ± 0.23 b |
| GLP | 38.13 ± 0.69 a | 67.46 ± 0.44 | 26.32 ± 0.67 a |
| GLP + HES | 33.69 ± 0.77 b | 68.64 ± 0.62 | 23.28 ± 0.49 b |
| p | * | NS | * |
| Groups | Immature Germinal Cells in Tubular Lumen | Epithelial Degeneration | Vacuolization in Seminiferous Tubules | Atrophy in Seminiferous Tubules | Congestion in Testes |
|---|---|---|---|---|---|
| C | 0.01± 0.01 b | 0.29 ± 0.06 b | 0.49 ± 0.07 b | 0.06 ± 0.03 b | 0.14 ± 0.04 b |
| HES | 0.06 ± 0.03 b | 0.43 ± 0.07 b | 0.54 ± 0.08 b | 0.01 ± 0.01 b | 0.19 ± 0.05 b |
| GLP | 0.46 ± 0.08 a | 1.16 ± 0.10 a | 1.50 ± 0.09 a | 0.27 ± 0.05 a | 0.67 ± 0.09 a |
| GLP + HES | 0.06 ± 0.20 b | 0.31 ± 0.06 b | 0.54 ± 0.08 b | 0.01 ± 0.01 b | 0.24 ± 0.05 b |
| p | *** | *** | *** | *** | *** |
| Groups | Stage VII-VIII STDs (µm) | Stage VII-VIII SEHs (µm) | Stage XIV Tubules (%) |
|---|---|---|---|
| C | 291.91 ± 2.52 | 62.65 ± 0.52 a | 5.57 ± 0.45 |
| HES | 297.42 ± 2.94 | 62.89 ± 0.50 a | 6.64 ± 0.55 |
| GLP | 290.17 ± 2.88 | 59.90 ± 0.56 b | 5.07 ± 0.63 |
| GLP + HES | 294.69 ± 2.86 | 63.66 ± 0.58 a | 5.64 ± 0.68 |
| p | NS | *** | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güngör, Ş.; Kırıkkulak, M.; Denk, B.; Gülhan, M.F.; Güleş, Ö.; Budak, D.; İnanç, M.E.; Avdatek, F.; Yeni, D.; Taşdemir, U. Potential Protective Effect of Hesperidin (Vitamin P) against Glyphosate-Induced Spermatogenesis Damage in Male Rats: Biochemical and Histopathological Findings on Reproductive Parameters. Life 2024, 14, 1190. https://doi.org/10.3390/life14091190
Güngör Ş, Kırıkkulak M, Denk B, Gülhan MF, Güleş Ö, Budak D, İnanç ME, Avdatek F, Yeni D, Taşdemir U. Potential Protective Effect of Hesperidin (Vitamin P) against Glyphosate-Induced Spermatogenesis Damage in Male Rats: Biochemical and Histopathological Findings on Reproductive Parameters. Life. 2024; 14(9):1190. https://doi.org/10.3390/life14091190
Chicago/Turabian StyleGüngör, Şükrü, Murat Kırıkkulak, Barış Denk, Mehmet Fuat Gülhan, Özay Güleş, Duygu Budak, Muhammed Enes İnanç, Fatih Avdatek, Deniz Yeni, and Umut Taşdemir. 2024. "Potential Protective Effect of Hesperidin (Vitamin P) against Glyphosate-Induced Spermatogenesis Damage in Male Rats: Biochemical and Histopathological Findings on Reproductive Parameters" Life 14, no. 9: 1190. https://doi.org/10.3390/life14091190
APA StyleGüngör, Ş., Kırıkkulak, M., Denk, B., Gülhan, M. F., Güleş, Ö., Budak, D., İnanç, M. E., Avdatek, F., Yeni, D., & Taşdemir, U. (2024). Potential Protective Effect of Hesperidin (Vitamin P) against Glyphosate-Induced Spermatogenesis Damage in Male Rats: Biochemical and Histopathological Findings on Reproductive Parameters. Life, 14(9), 1190. https://doi.org/10.3390/life14091190






