Abstract
The presence of Demodex spp. mites on the skin is a common phenomenon in the human population. In most people, it is an asymptomatic infestation, but in some patients, it can contribute to the occurrence of diseases such as demodicosis, rosacea, or blepharitis, as well as non-specific symptoms. In this study, the results of tests assessing the presence of Demodex spp. by direct microscopic examination (DME) in 2508 patients from northern Poland with the suspicion of demodicosis were retrospectively analyzed. A total of 3684 tests were performed. Among them, only 1585 (43.02%) revealed the presence of Demodex spp., while 2099 (56.98%) were negative. It was shown that in the analyzed population of patients with clinical suspicion of demodicosis, the degree of confirmation of the presence of Demodex spp. positively correlated with the patient’s age (p = 0.001) and the mite was mainly found on the edges of eyelids and on the facial skin. Additionally, in men, the presence of Demodex was more often confirmed than in women (p = 0.004). In conclusion, the proper diagnosis of demodicosis still constitutes an important clinical problem due to the non-specificity of symptoms and the low confirmation of clinical suspicions of infestation by DME, especially in lower age groups.
1. Introduction
Demodex folliculorum (DF) and Demodex brevis (DB) are ectoparasites often found on human skin [1]. They live mainly in the sebaceous glands and hair follicles, where they feed on sebum and epidermis [1]. For this reason, the presence of these mites is mostly detected in areas rich in sebaceous glands, mainly on the face—on the cheeks, chin, nose, and on the eyelids, inhabiting eyelash follicles [1,2,3]. Additionally, Demodex brevis may occur outside these areas, spreading to the skin of the entire body [2,3]. The prevalence of Demodex spp. in the population varies depending on age—the highest density of Demodex mites is observed among elderly people, and the lowest in children [2,3,4,5,6]. It is estimated that Demodex spp. may occur in up to 100% of the adult population [2,4,7]. In most cases, it is an asymptomatic infestation, but an increased density of these parasites may lead to the development of various skin disorders, especially demodicosis [1,2,3,4,5,6,7,8,9,10]. Some of the most common diseases associated with Demodex infestation are folliculitis, rosacea, and ophthalmological complications such as dry eye syndrome, blepharitis, or chalazion [1,2,3,4,5,6,8]. It is believed that this may be related to the colonization of the mites’ bodies by numerous microorganisms, as well as the induction of an inflammatory reaction by the dead bodies of Demodex mites within the hair follicles [2,8]. The diagnosis of demodicosis is made by correlating clinical symptoms with the results of direct microscopic examination (DME) or standardized skin surface biopsy (SSSB) [2,5,11,12]. These methods differ in the way they obtain the material, but in both cases, acquired samples are assessed under a light microscope [11]. DME does not visualize hair follicles, which is important for detecting Demodex spp. [12]. SSSB, however, has limitations related to the small surface and reduced depth of the collected sample, as well as poor adherence of mites to the microscope slide, potentially resulting in false-negative outcomes [11,13]. DME is considered a simple, effective, and time-efficient method for mite detection; it detects both Demodex folliculorum and Demodex brevis, while SSSB only identifies Demodex brevis [11,14]. The confirmation of demodicosis through DME requires a density of mites above 5 D/cm2 [2]. Once the diagnosis is established, treatment is implemented to reduce the amount of Demodex spp. on the patient’s skin and to eliminate clinical symptoms. The most commonly used topical drugs are metronidazole, permethrin, benzoyl benzoate, and ivermectin 1% [2,3].
The aim of this study was to determine the prevalence of Demodex spp. infestation in a population of patients in northern Poland with clinical suspicion of demodicosis, along with an analysis of the relationship between Demodex infestation and sociodemographic factors, based on the results of tests conducted in the Mycological Laboratory of the University Clinical Centre in Gdańsk. So far, there is no study concerning Demodex spp. infestation on such a large population in the Pomeranian region of Poland.
2. Materials and Methods
The histories of patients diagnosed in the Mycology Outpatient Clinic of the University Clinical Centre in Gdańsk, obtained from paper patient records, were retrospectively analyzed. The study included patients with at least one visit to the Mycology Outpatient Clinic of the University Clinical Center in Gdańsk in the years 2019–2024, referred by dermatologists with clinical suspicion of demodicosis. All patients were exclusively of Polish descent (the population of northern Poland). The inclusion criteria were met by 2508 people. People suspected of Demodex spp. infestation were tested in specific locations, depending on the symptoms presented. A direct microscopic examination was conducted using an MB-100 microscope (OPTA-TECH, Warsaw, Poland) equipped with a camera. After clinical evaluation, six eyelashes from both the upper and lower eyelids and/or six eyebrow hairs were collected from patients using tweezers. Skin scrapings from the face were obtained using a sterile scalpel. Hair samples were collected in the amount of 20 strands using tweezers. The obtained material was placed on the microscope slide with a mixture of dimethyl sulfoxide and 20% potassium hydroxide. Then, each sample was evaluated under the microscope at 100× magnification by qualified personnel, who looked for adult specimens and larvae to confirm infestation. A criterion for a positive result was a mite density above 5 D/cm2. Each visit of a qualified patient was analyzed in terms of the following information: the gender and age of the patient, the place of residence of the patient (urban/rural), the location of lesions (facial skin/eyelids edges/eyebrows/hair), and the result of direct examination in a light microscope. The obtained data were subjected to statistical analysis using Statistica 13.3 (StatSoft Polska Sp. z o.o. Inc., 2017, Cracow, Poland) software. An analysis of qualitative features was conducted with the χ2 test in the Pearson method. Independent variables fulfilling the assumptions for parametric tests were analyzed with the Student’s t test. Independent variables that did not meet the parametric test assumptions were analyzed with non-parametric tests (ANOVA equivalents): the U Mann–Whitney test (the comparison of two tests) or the Kruskal–Wallis test (the comparison of many samples). In all tests, p < 0.05 was considered a significant level of statistical significance.
3. Results
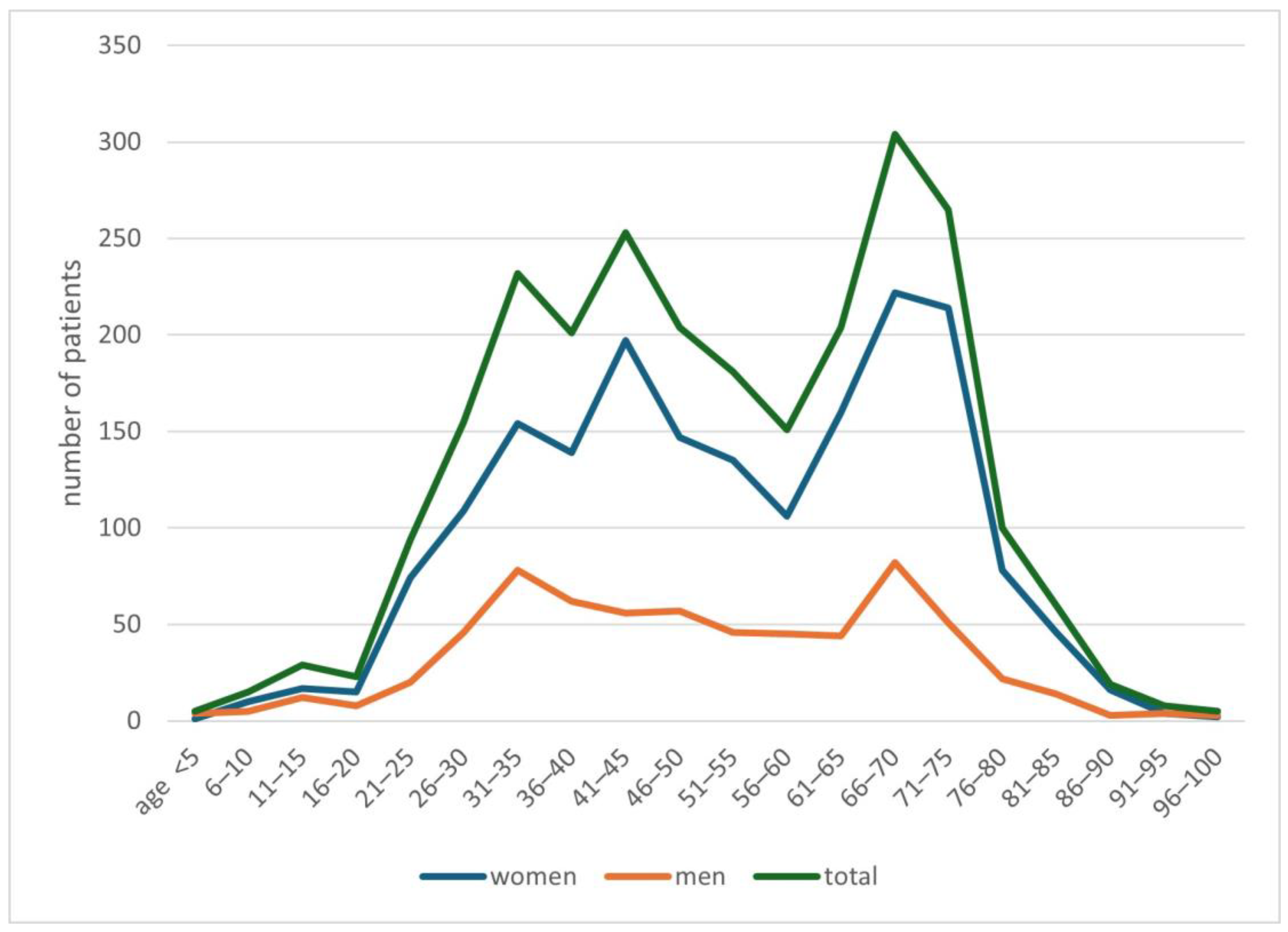
In the years 2019–2024, 2508 people with a suspicion of demodicosis were admitted to the Mycology Outpatient Clinic of the University Clinical Centre in Gdańsk. Of this group, 1846 (74%) people were women, while 662 (26%) were men. The average age of patients was 51.9 years, with an average of 52.6 years for women, and for men 50.0 years. Detailed data on the age of patients are presented in Figure 1.
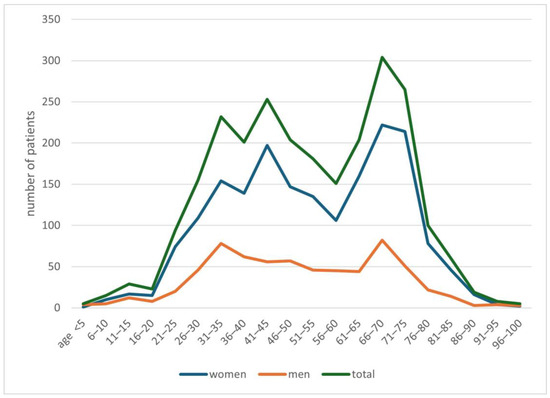
Figure 1.
Characteristics of the study population.
Moreover, 2212 (88.2%) of people suspected of Demodex spp. infestation lived in the city, and 296 (11.8%) people in the countryside. Both urban and rural women were statistically more likely to be tested for Demodex infestation (p < 0.0001). A total of 3684 tests were performed. Among them, 1585 (43.02%) showed the presence of Demodex spp., while 2099 (56.98%) had a negative result. It was shown that there is a statistically significant higher probability that at least one of the tests performed during the visit will have a result confirming the presence of Demodex spp. if the patient is male (p = 0.004) (Table 1).

Table 1.
The frequency of confirmation of the presence of Demodex spp. in a given patient.
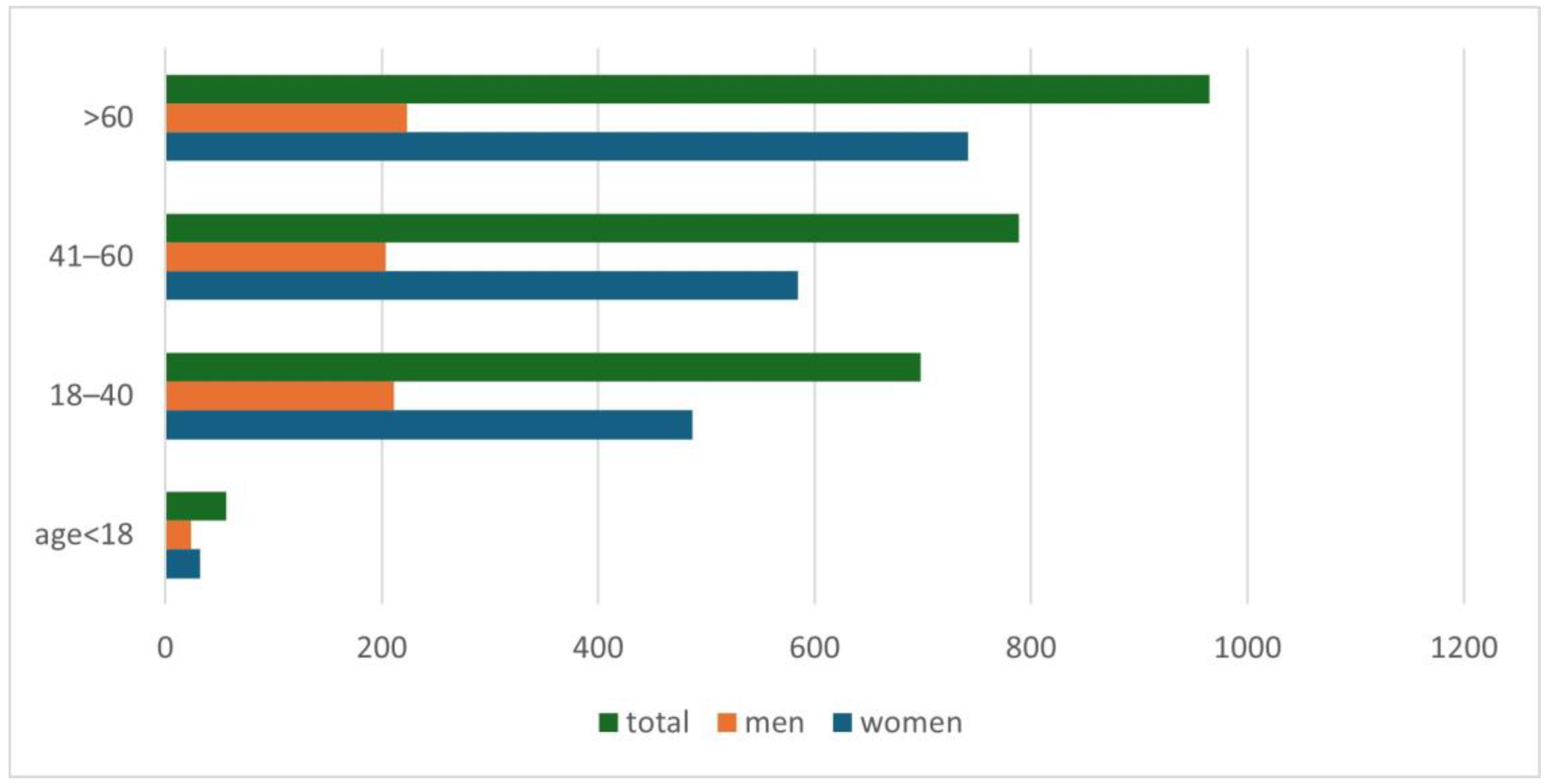
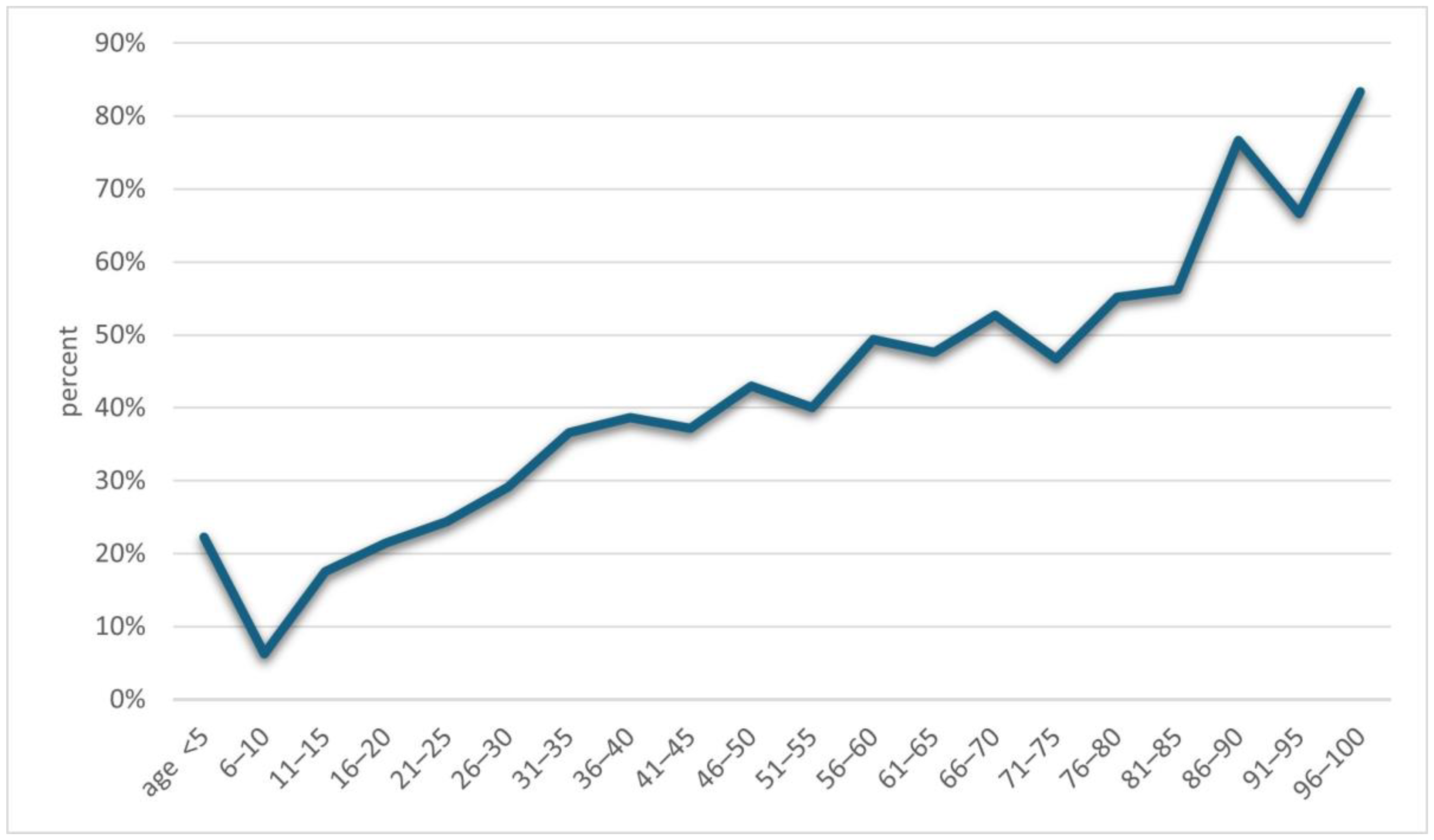
An increase in positive results for Demodex spp. was observed along with the age of the examined patients (p = 0.001) (Figure 2). A detailed list of results in each age group is provided in Table 2. It was shown that in the analyzed population of patients with clinical suspicion of demodicosis, the degree of confirmation of the presence of Demodex spp. using DME positively correlated with the patient’s age (p = 0.0001) (Figure 3).
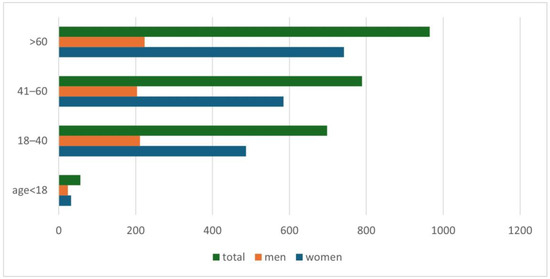
Figure 2.
The division of patients with a positive test result for Demodex spp., taking into account patients’ age and gender.

Table 2.
The frequency of confirmation of the presence of Demodex spp. in given age groups of patients.
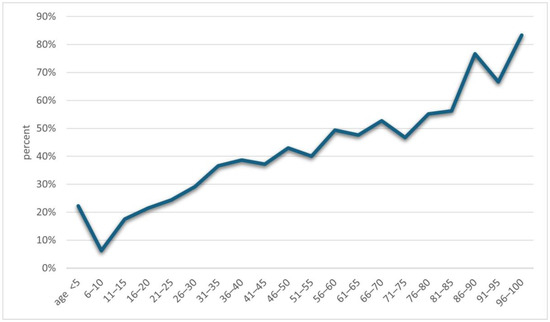
Figure 3.
The percentages of results confirming the presence of Demodex depending on the patients’ age.
The most frequently assessed area was the skin of the face (p = 0.00001) (Table 3). However, locations in which DME showed the presence of Demodex mites most commonly were the edges of eyelids, then the facial skin, eyebrows, and hair (Table 3). When analyzing the relationship between the most commonly assessed location of demodicosis lesions and the place of residence in patients by gender, no statistical significance of the obtained results was demonstrated (p = 0.18 for women and p = 0.95 for men).

Table 3.
Division of tests for Demodex spp. in terms of location.
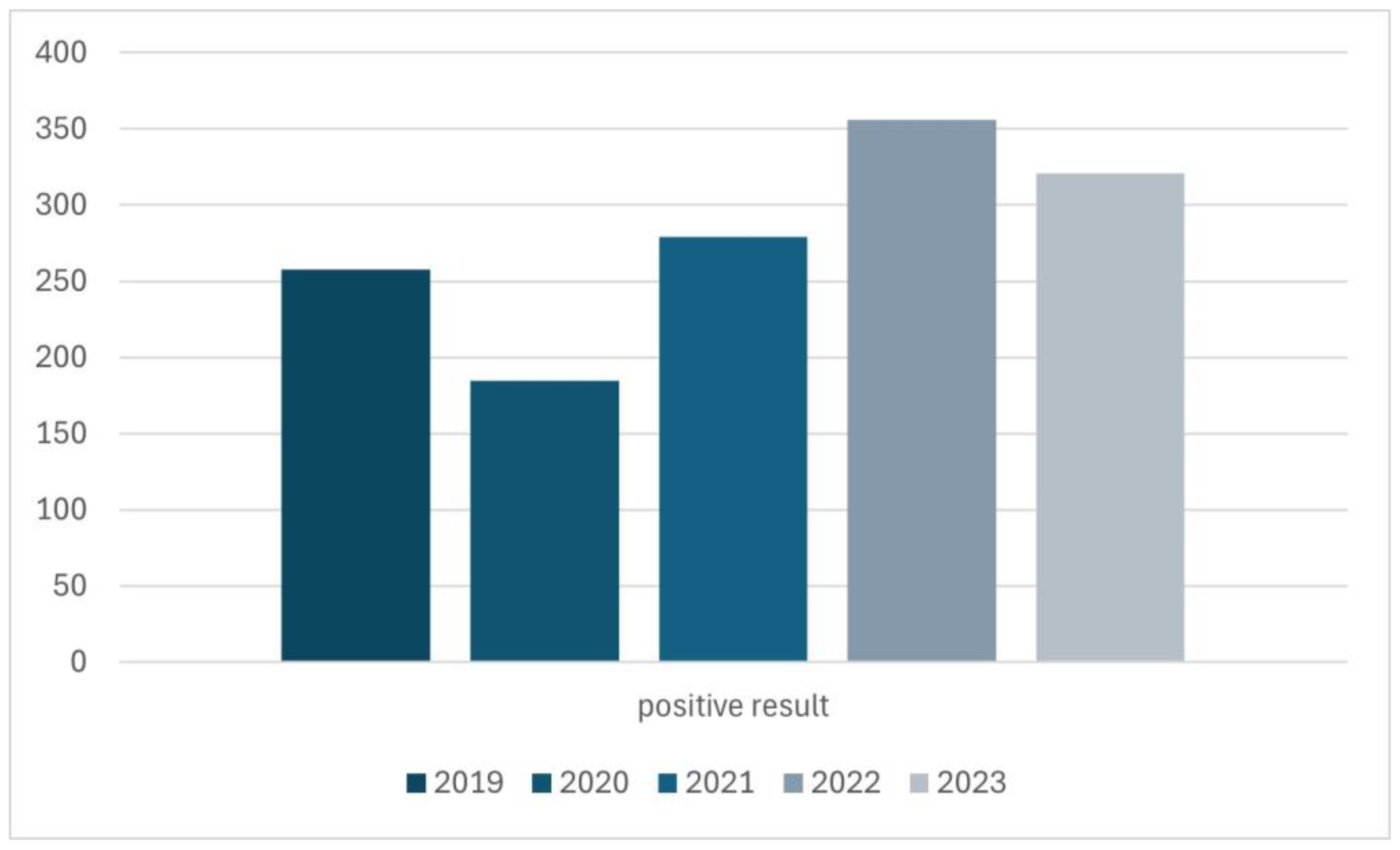
The quantitative distribution of results over the years was also investigated (Figure 4). The year 2024 was excluded from the assessment in Figure 4 because only visits from 6 months of this year were analyzed, which would make it impossible to reliably evaluate these data.
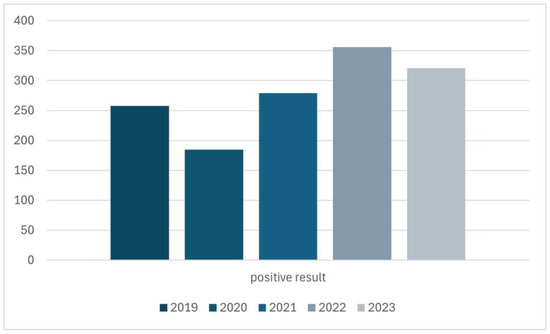
Figure 4.
The distribution of positive results for Demodex spp. infestation in the years 2019–2023.
4. Discussion
The presence of Demodex infestation is very common, especially in the adult population. Previous studies in Poland have analyzed the prevalence of this mite in small groups of people, showing its presence in over 40% of respondents [15,16,17]. This study confirms the above data, with the prevalence out of the analyzed group being 46.57% (Table 2). However, it should be noted that this study was conducted with patients whose clinical symptoms prompted specialists to refer them for diagnostic tests. Therefore, these results should not be treated as an adequate illustration of the prevalence of Demodex infestation in the general Polish population, but only in the population of people showing symptoms that may suggest excessive proliferation of mites in their skin, so a dermatologist’s patients. The presented statistics also expose that despite the fact that the decision to conduct the test was made by a dermatologist, less than half tests confirmed the presence of Demodex spp. in cases of clinical suspicion of infestation, and this rate was particularly low in younger age groups, which well reflects the problem of Demodex spp. invasion. Although it is a common problem, the skin lesions caused by this invasion are so non-specific that they can often be confused with other dermatoses, even by experienced dermatologists [18,19]. Another reason for the aforementioned relationship is the fact that the patients included in this study were examined using the DME method. It is not certain whether this type of testing for the presence of Demodex spp. is the most effective. In a publication by Ü. Aşkın and D. Seçkin, the higher sensitivity of another method of testing—standardized skin surface biopsy (SSSB)—was demonstrated [12]. Conversely, Chul Hyun Yun et al. reached a conclusion in their research that the DME method exhibited greater value than the SSSB technique [11]. In addition, there are also other, more modern methods of detecting Demodex spp. They include reflectance confocal microscopy (RCM) and high-definition optical coherence tomography (HD-OCT). These techniques offer rapid, visual results but have limited application due to low availability and high costs [20]. Despite the lack of consensus on the best method for testing for Demodex spp., it may be worthwhile to re-evaluate the patient using a different detection method if there is strong clinical evidence of demodicosis in the patient.
Another issue that has already appeared in previous publications and which is confirmed by this study is the positive correlation of the occurrence of Demodex spp. with age—the higher the age of the patient, the higher the probability of being infected with this mite [2,3,4,5,6,15,16,17]. During the analysis, it was shown that the age group in which patients are most at risk for the presence of Demodex spp. is people over 60 years of age (Figure 2), among whom 58.65% received a positive test result (Table 2). In turn, in the group of minors, i.e., under 18 years of age, the occurrence of infestation was confirmed in only 14.29% of people. The correlation between age and the frequency of Demodex spp. presence confirmation in a given patient is shown in Figure 3. As can be seen, this relationship is especially true for age groups above 15 years of age. This may be related to the small number of patients below this age, which makes it difficult to statistically adequately represent a given population.
According to current knowledge, there is no relationship between gender and excessive proliferation of ectoparasites [9,15,16,17]. The study showed that this is not necessarily true for the patient population in northern Poland and also enabled the observation of a different correlation. Women were statistically more often referred to testing for demodicosis than men. This association had no connection with place of residence—it was true for both urban and rural residents. Despite the fact that women were more likely to be examined, the group in which statistically at least one test for demodicosis turned out to be positive was men (Table 1). The nature of this scientific study did not allow for determining the cause of this difference in results between the sexes. We propose a hypothesis considering differences in preparation for the test—women are more likely to use cosmetic products, such as creams or make-up, which can cause false negative results for Demodex spp. [21]. In addition, women have a greater cultural emphasis on meeting certain appearance requirements than men, so minor skin changes can prompt them to visit a dermatologist, which in turn may lead to an increased likelihood of testing for Demodex spp. despite less pronounced symptoms [22]. These may be some of the factors conditioning the observed trend.
The prevalence of Demodex spp. varies in terms of location. In the population of dermatological patients in northern Poland, the presence of this mite was most often confirmed in the hair follicles of the eyelashes, i.e., on the edges of the eyelids, then on the facial skin, eyebrows, and hair. This coincides with the results of other studies, which have shown an increased occurrence of Demodex spp. in areas with increased sebum production, mainly on the facial skin and around the eyelashes [1,2].
This study did not assess the incidence of specific dermatoses depending on the absence or presence of Demodex. However, it should be noted that this infestation is a significant clinical problem because it can lead to various skin symptoms. The most common is folliculitis, which is considered to be the initial symptom of increased multiplication of mites on the patient’s skin. This may be accompanied by a burning sensation, itching, and facial erythema [2,4]. The clinical picture of demodicosis may also resemble perioral inflammation, seborrheic dermatitis, and rosacea [2,3,23,24,25]. In turn, research conducted by Zhao YE, et al. proved that Demodex spp. infestation is one of the risk factors for the development of acne vulgaris [26]. This was confirmed inter alia by Akçınar UG et al. in. “Demodex spp. as a possible aetiopathogenic factor of acne and relation with acne severity and type” [27]. In 2023, another analysis was published showing an increased incidence of Demodex spp. in people with blepharitis and chalazion [28]. Different publications have shown that blepharitis, which is associated with Demodex spp. infestation, has a strongly negative impact on the well-being of patients suffering from it [29]. This highlights how important the clinical problem of demodicosis is, and that its correct diagnosis and treatment are necessary to maintain a high quality of life for patients.
5. Conclusions
In conclusion, the proper diagnosis of demodicosis still constitutes an important clinical problem due to the non-specificity of symptoms and the low confirmation of clinical suspicions of the presence of Demodex spp. in direct microscopic examination, especially in lower age groups. In a population of northern Poland patients, Demodex spp. most often occurs on the edges of eyelids and on the facial skin but is much less common on eyebrows and hair. Mite infestation shows a positive correlation with the age of patients and, in the Pomeranian region, with the patient’s sex being male. Effective treatment leading to the disappearance of disease symptoms can improve the quality of life of patients, which should be the goal of every diagnostic and therapeutic path.
Author Contributions
Conceptualization, K.R. and D.P.-B.; methodology, K.R.; software, K.R.; validation, K.R.; formal analysis, K.R., J.S., M.Z., R.J.N., L.B., and D.P.-B.; investigation, K.R.; resources, K.R.; data curation, K.R., J.S., M.Z., and D.P.-B.; writing—original draft preparation, K.R. and J.S.; writing—review and editing, D.P.-B., R.J.N., and L.B.; visualization, K.R.; supervision, D.P.-B.; project administration, K.R. and D.P.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Independent Bioethics Committee for Scientific Research of Medical University of Gdańsk (KB/330/2024, approval date: 10 July 2024) for studies involving humans.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the research.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Murphy, O.; O’Dwyer, V.; Lloyd-McKernan, A. Ocular Demodex folliculorum: Prevalence and associated symptoms in an Irish population. Int. Ophthalmol. 2019, 39, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Paichitrojjana, A. Demodex: The worst enemies are the ones that used to be friends. Dermatol. Rep. 2022, 14, 9339. [Google Scholar] [CrossRef] [PubMed]
- Elston, C.A.; Elston, D.M. Demodex mites. Clin. Dermatol. 2014, 32, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.; Kelly, P.; Gatault, S.; Powell, F. Demodex: A skin resident in man and his best friend. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Lacey, N.; Russell-Hallinan, A.; Powell, F.C. Study of Demodex mites: Challenges and Solutions. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 764–775. [Google Scholar] [CrossRef]
- Wei, F.; Li, L.; Kong, Y.; Yan, X.; Varghese, K.J.; Zhang, S.; Jiang, J.; Chai, B.; Chen, H. Evidence for the Clinical Association between Demodex and Rosacea: A Review. Dermatology 2024, 240, 95–102. [Google Scholar] [CrossRef]
- Thoemmes, M.S.; Fergus, D.J.; Urban, J.; Trautwein, M.; Dunn, R.R. Ubiquity and diversity of human-associated Demodex mites. PLoS ONE 2014, 9, e106265. [Google Scholar] [CrossRef]
- Lacey, N.; Delaney, S.; Kavanagh, K.; Powell, F.C. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br. J. Dermatol. 2007, 157, 474–481. [Google Scholar] [CrossRef]
- Aktaş Karabay, E.; Aksu Çerman, A. Demodex folliculorum infestations in common facial dermatoses: Acne vulgaris, rosacea, seborrheic dermatitis. An. Bras. Dermatol. 2020, 95, 187–193. [Google Scholar] [CrossRef]
- Lacey, N.; Ní Raghallaigh, S.; Powell, F.C. Demodex mites-commensals, parasites or mutualistic organisms? Dermatology 2011, 222, 128–130. [Google Scholar] [CrossRef]
- Yun, C.H.; Yun, J.H.; Baek, J.O.; Roh, J.Y.; Lee, J.R. Demodex Mite Density Determinations by Standardized Skin Surface Biopsy and Direct Microscopic Examination and Their Relations with Clinical Types and Distribution Patterns. Ann. Dermatol. 2017, 29, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Aşkin, U.; Seçkin, D. Comparison of the two techniques for measurement of the density of Demodex folliculorum: Standardized skin surface biopsy and direct microscopic examination. Br. J. Dermatol. 2010, 162, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Forton, F. Standardized skin surface biopsy: Method to estimate the Demodex folliculorum density, not to study the Demodex folliculorum prevalence. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1301–1302. [Google Scholar] [CrossRef] [PubMed]
- Bunyaratavej, S.; Rujitharanawong, C.; Kasemsarn, P.; Boonchai, W.; Muanprasert, C.; Matthapan, L.; Leeyaphan, C. Skin scrapings versus standardized skin surface biopsy to detect Demodex mites in patients with facial erythema of uncertain cause—A comparative study. Indian. J. Dermatol. Venereol. Leprol. 2016, 82, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Sędzikowska, A.; Osęka, M.; Skopiński, P. The impact of age, sex, blepharitis, rosacea and rheumatoid arthritis on Demodex mite infection. Arch. Med. Sci. 2018, 14, 353–356. [Google Scholar] [CrossRef]
- Wesolowska, M.; Knysz, B.; Reich, A.; Blazejewska, D.; Czarnecki, M.; Gladysz, A.; Pozowski, A.; Misiuk-Hojlo, M. Prevalence of Demodex spp. in eyelash follicles in different populations. Arch. Med. Sci. 2014, 10, 319–324. [Google Scholar] [CrossRef]
- Garbacewicz, A.; Udziela, M.; Grytner-Ziecina, B.; Szaflik, J.P.; Szaflik, J. Demodex infections in general Polish population, in patients suffering from blepharitis, and among people who work with microscopes. Klin. Ocz. 2010, 112, 307–310. [Google Scholar]
- Karincaoglu, Y.; Bayram, N.; Aycan, O.; Esrefoglu, M. The clinical importance of demodex folliculorum presenting with nonspecific facial signs and symptoms. J. Dermatol. 2004, 31, 618–626. [Google Scholar] [CrossRef]
- Chen, W.; Plewig, G. Human demodicosis: Revisit and a proposed classification. Br. J. Dermatol. 2014, 170, 1219–1225. [Google Scholar] [CrossRef]
- Dopytalska, K.; Lipa, K.; Sobolewski, P.; Szymańska, E.; Walecka, I. Role of Demodex folliculorum in dermatology. Dermatol. Rev./Przegląd Dermatol. 2019, 106, 507–514. [Google Scholar] [CrossRef]
- Kim, S.; Min, H.S.; Lee, W.J.; Choe, S.A. Occupational differences in personal care product use and urinary concentration of endocrine disrupting chemicals by gender. J. Expo Sci. Environ. Epidemiol. 2023, 33, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Åberg, E.; Kukkonen, I.; Sarpila, O. From double to triple standards of ageing. Perceptions of physical appearance at the intersections of age, gender and class. J. Aging Stud. 2020, 55, 100876. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.K.; Hsu, M.M.; Lee, J.Y. Demodicosis: A clinicopathological study. J. Am. Acad. Dermatol. 2009, 60, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Huang, Y.C. Role of Demodex mite infestation in rosacea: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 441–447. [Google Scholar] [CrossRef]
- Veraldi, S.; Pisapia, A.; Nazzaro, G.; Boneschi, V. Unilateral rosacea, unilateral demodicidosis, unitaleral Demodex sp. folliculitis: Three names for the same disease. J. Cosmet. Dermatol. 2023, 22, 335–336. [Google Scholar] [CrossRef]
- Zhao, Y.E.; Hu, L.; Wu, L.P.; Ma, J.X. A meta-analysis of association between acne vulgaris and Demodex infestation. J. Zhejiang Univ. Sci. B 2012, 13, 192–202. [Google Scholar] [CrossRef]
- Akçınar, U.G.; Ünal, E.; Al, F.D. Demodex spp. as a possible aetiopathogenic factor of acne and relation with acne severity and type. Postepy Dermatol. Alergol. 2018, 35, 174–181. [Google Scholar] [CrossRef]
- Akkucuk, S.; Kaya, O.M.; Aslan, L.; Ozdemir, T.; Uslu, U. Prevalence of Demodex folliculorum and Demodex brevis in patients with blepharitis and chalazion. Int. Ophthalmol. 2023, 43, 1249–1259. [Google Scholar] [CrossRef]
- O’Dell, L.; Dierker, D.S.; Devries, D.K.; Garlich, J.; Whitley, W.O.; Holdbrook, M.; Baba, S.N.; Yeu, E. Psychosocial Impact of Demodex Blepharitis. Clin. Ophthalmol. 2022, 16, 2979–2987. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).