The Effect of Audible Joint Manipulation Sounds in the Upper Cervical Spine on Brain Wave and Autonomic Nervous System Activity
Abstract
:1. Introduction
2. Materials and Methods
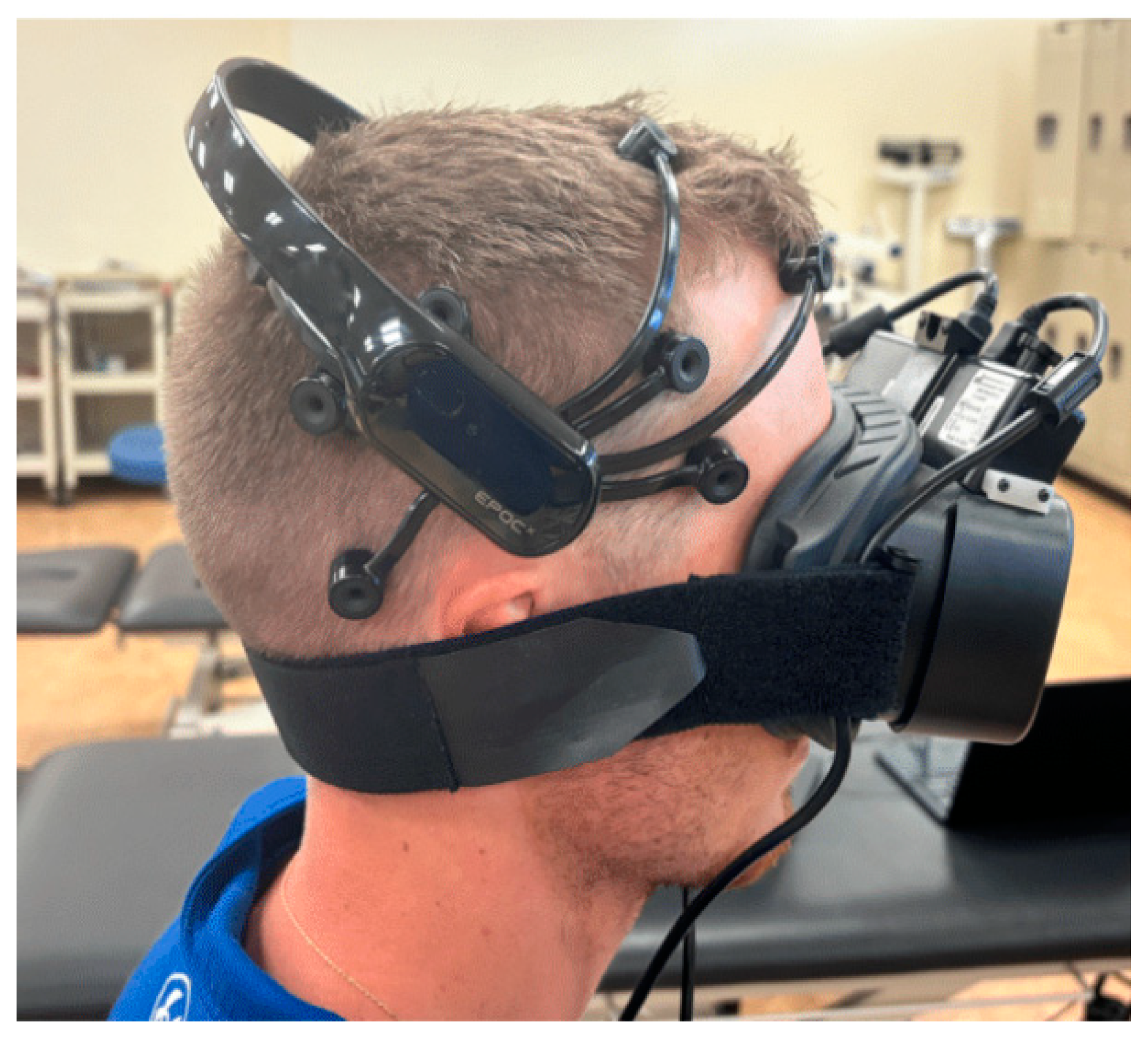
2.1. Study Protocol
2.2. Statistical Analysis
3. Results
3.1. Electroencephalography Analysis
3.2. Pupillometry Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alvarez, G.; Solà, I.; Sitjà-Rabert, M.; Fort-Vanmeerhaeghe, A.; Gich, I.; Fernández, C.; Bonfill, X.; Urrútia, G. A Methodological Review Revealed That Reporting of Trials in Manual Therapy Has Not Improved over Time. J. Clin. Epidemiol. 2020, 121, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Mintken, P.E.; DeRosa, C.; Little, T.; Smith, B. A Model for Standardizing Manipulation Terminology in Physical Therapy Practice. J. Man. Manip. Ther. 2008, 16, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Schiller, L. Effectiveness of Spinal Manipulative Therapy in the Treatment of Mechanical Thoracic Spine Pain: A Pilot Randomized Clinical Trial. J. Manip. Physiol. Ther. 2001, 24, 394–401. [Google Scholar] [CrossRef]
- Sillevis, R. Autonomic Dysfunction: A Conceptual Model the Effects of a Physical Therapeutic Manipulation Targeting the T3-T4 Segment on the Autonomic Nervous System; Nova Southeastern University: Fort Lauderdale, FL, USA, 2009. [Google Scholar]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; George, S.Z. The Mechanisms of Manual Therapy in the Treatment of Musculoskeletal Pain: A Comprehensive Model. Man. Ther. 2009, 14, 531–538. [Google Scholar] [CrossRef]
- Bishop, M.D.; Torres-Cueco, R.; Gay, C.W.; Lluch-Girbés, E.; Beneciuk, J.M.; Bialosky, J.E. What Effect Can Manual Therapy Have on a Patient’s Pain Experience? Pain Manag. 2015, 5, 455–464. [Google Scholar] [CrossRef]
- Clar, C.; Tsertsvadze, A.; Court, R.; Hundt, G.L.; Clarke, A.; Sutcliffe, P. Clinical Effectiveness of Manual Therapy for the Management of Musculoskeletal and Non-Musculoskeletal Conditions: Systematic Review and Update of UK Evidence Report. Chiropr. Man. Ther. 2014, 22, 12. [Google Scholar] [CrossRef]
- Colloca, C.J.; Keller, T.S.; Harrison, D.E.; Moore, R.J.; Gunzburg, R.; Harrison, D.D. Spinal Manipulation Force and Duration Affect Vertebral Movement and Neuromuscular Responses. Clin. Biomech. 2006, 21, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, B.F.; Darmani, N.A.; Johnson, J.C.; Towns, L.C.; Rhodes, D.C.J.; Trinh, C.; McClanahan, B.; DiMarzo, V. Role of Osteopathic Manipulative Treatment in Altering Pain Biomarkers: A Pilot Study. J. Am. Osteopath. Assoc. 2007, 107, 387–400. [Google Scholar]
- King, H.H.; Janig, W.; Patterson, M.M. The Science and Clinical Application of Manual Therapy; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010; ISBN 978-0-7020-4995-8. [Google Scholar]
- LaPelusa, A.; Bordoni, B. High Velocity Low Amplitude Manipulation Techniques; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Protapapas, M.G.; Cymet, T.C. Joint Cracking and Popping: Understanding Noises That Accompany Articular Release. J. Am. Osteopath. Assoc. 2002, 102, 283–287. [Google Scholar]
- Roura, S.; Álvarez, G.; Solà, I.; Cerritelli, F. Do Manual Therapies Have a Specific Autonomic Effect? An Overview of Systematic Reviews. PLoS ONE 2021, 16, e0260642. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sáez, M.; Fernández-de-las-Peñas, C.; Blanco, C.R.; Martínez-Segura, R.; García-León, R. Changes in Pressure Pain Sensitivity in Latent Myofascial Trigger Points in the Upper Trapezius Muscle After a Cervical Spine Manipulation in Pain-Free Subjects. J. Manip. Physiol. Ther. 2007, 30, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.H.; Hendry, M.; Lewis, R.; Russell, I.; Westmoreland, A.; Wilkinson, C. Psychological Response in Spinal Manipulation (PRISM): A Systematic Review of Psychological Outcomes in Randomised Controlled Trials. Complement. Ther. Med. 2007, 15, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.W.; Breen, A.C. A Biomechanical Model for Mechanically Efficient Cavitation Production During Spinal Manipulation: Prethrust Position and the Neutral Zone. J. Manip. Physiol. Ther. 2006, 29, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Reggars, J.W. The Therapeutic Benefit of the Audible Release Associated with Spinal Manipulative Therapy. A Critical Review of the Literature. Australas. Chiropr. Osteopat. 1998, 7, 80–85. [Google Scholar]
- Bialosky, J.E.; Bishop, M.D.; Robinson, M.E.; George, S.Z. The Relationship of the Audible Pop to Hypoalgesia Associated With High-Velocity, Low-Amplitude Thrust Manipulation: A Secondary Analysis of an Experimental Study in Pain-Free Participants. J. Manip. Physiol. Ther. 2010, 33, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sillevis, R.; Cleland, J. Immediate Effects of the Audible Pop from a Thoracic Spine Thrust Manipulation on the Autonomic Nervous System and Pain: A Secondary Analysis of a Randomized Clinical Trial. J. Manip. Physiol. Ther. 2011, 34, 37–45. [Google Scholar] [CrossRef]
- Flynn, T.W.; Fritz, J.M.; Wainner, R.S.; Whitman, J.M. The Audible Pop Is Not Necessary for Successful Spinal High-Velocity Thrust Manipulation in Individuals with Low Back pain11The Opinions and Assertions Contained Herein Are the Private Views of the Authors and Are Not to Be Construed as Official or as Reflecting the Views of the US Department of the Army or the US Department of Defense.No Commercial Party Having a Direct Financial Interest in the Results of the Research Supporting This Article Has or Will Confer a Benefit upon the Authors(s) or upon Any Organization with Which the Author(s) Is/Are Associated. Arch. Phys. Med. Rehabil. 2003, 84, 1057–1060. [Google Scholar] [CrossRef]
- Light, G.A.; Williams, L.E.; Minow, F.; Sprock, J.; Rissling, A.; Sharp, R.; Swerdlow, N.R.; Braff, D.L. Electroencephalography (EEG) and Event-Related Potentials (ERPs) with Human Participants. Curr. Protoc. Neurosci. 2010, 52, 6.25.1–6.25.24. [Google Scholar] [CrossRef]
- Singh, H.; Bauer, M.; Chowanski, W.; Sui, Y.; Atkinson, D.; Baurley, S.; Fry, M.; Evans, J.; Bianchi-Berthouze, N. The Brain’s Response to Pleasant Touch: An EEG Investigation of Tactile Caressing. Front. Hum. Neurosci. 2014, 8, 893. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.S.; Bhuvaneswari, P. Analysis of Electroencephalography (EEG) Signals and Its Categorization–A Study. Procedia Eng. 2012, 38, 2525–2536. [Google Scholar] [CrossRef]
- Niedermeyer, E.; Silva, F.H.L. da Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; ISBN 978-0-7817-5126-1. [Google Scholar]
- Desai, R.; Tailor, A.; Bhatt, T. Effects of Yoga on Brain Waves and Structural Activation: A Review. Complement. Ther. Clin. Pract. 2015, 21, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, R.N.S.; Gaspard, N.; Gerrard, J.L.; Hirsch, L.J.; Spencer, D.D.; Zaveri, H.P. Delta Rhythm in Wakefulness: Evidence from Intracranial Recordings in Human Beings. J. Neurophysiol. 2015, 114, 1248–1254. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Clifford, W.; Markham, C.; Ward, T.E.; Deegan, C. Validation of Low-Cost Wireless EEG System for Measuring Event-Related Potentials. In Proceedings of the 2018 29th Irish Signals and Systems Conference (ISSC), Belfast, UK, 21–22 June 2018; pp. 1–6. [Google Scholar]
- Williams, N.S.; McArthur, G.M.; de Wit, B.; Ibrahim, G.; Badcock, N.A. A Validation of Emotiv EPOC Flex Saline for EEG and ERP Research. PeerJ 2020, 8, e9713. [Google Scholar] [CrossRef]
- Buijs, R.M. Chapter 1-The Autonomic Nervous System: A Balancing Act. In Handbook of Clinical Neurology; Buijs, R.M., Swaab, D.F., Eds.; Autonomic Nervous System; Elsevier: Amsterdam, The Netherlands, 2013; Volume 117, pp. 1–11. [Google Scholar]
- Sgoifo, A.; Carnevali, L.; Pico Alfonso, M.D.L.A.; Amore, M. Autonomic Dysfunction and Heart Rate Variability in Depression. Stress 2015, 18, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.; Lucero-Wagoner, B. Handbook of Psychophysiology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Kıylıoğlu, N.; Kılıç, M.A.; Kocatürk, T.; Özkan, S.B.; Bilgen, M. A Custom-Made Pupillometer System for Characterizing Pupillary Light Response. Turk. J. Ophthalmol. 2018, 48, 185–189. [Google Scholar] [CrossRef]
- Meeker, M.; Du, R.; Bacchetti, P.; Privitera, C.M.; Larson, M.D.; Holland, M.C.; Manley, G. Pupil Examination: Validity and Clinical Utility of an Automated Pupillometer. J. Neurosci. Nurs. 2005, 37, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sillevis, R.; Cleland, J.; Hellman, M.; Beekhuizen, K. Immediate Effects of a Thoracic Spine Thrust Manipulation on the Autonomic Nervous System: A Randomized Clinical Trial. J. Man. Manip. Ther. 2010, 18, 181–190. [Google Scholar] [CrossRef]
- Menck, J.Y.; Requejo, S.M.; Kulig, K. Thoracic Spine Dysfunction in Upper Extremity Complex Regional Pain Syndrome Type I. J. Orthop. Sports Phys. Ther. 2000, 30, 401–409. [Google Scholar] [CrossRef]
- Welch, A.; Boone, R. Sympathetic and Parasympathetic Responses to Specific Diversified Adjustments to Chiropractic Vertebral Subluxations of the Cervical and Thoracic Spine. J. Chiropr. Med. 2008, 7, 86–93. [Google Scholar] [CrossRef]
- Gyer, G.; Michael, J.; Inklebarger, J.; Tedla, J.S. Spinal Manipulation Therapy: Is It All about the Brain? A Current Review of the Neurophysiological Effects of Manipulation. J. Integr. Med. 2019, 17, 328–337. [Google Scholar] [CrossRef]
- Picchiottino, M.; Leboeuf-Yde, C.; Gagey, O.; Hallman, D.M. The Acute Effects of Joint Manipulative Techniques on Markers of Autonomic Nervous System Activity: A Systematic Review and Meta-Analysis of Randomized Sham-Controlled Trials. Chiropr. Man. Ther. 2019, 27, 17. [Google Scholar] [CrossRef]
- Sparks, C.; Cleland, J.A.; Elliott, J.M.; Zagardo, M.; Liu, W.-C. Using Functional Magnetic Resonance Imaging to Determine If Cerebral Hemodynamic Responses to Pain Change Following Thoracic Spine Thrust Manipulation in Healthy Individuals. J. Orthop. Sports Phys. Ther. 2013, 43, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Tashiro, M.; Masud, M.; Watanuki, S.; Shibuya, K.; Yamaguchi, K.; Itoh, M.; Fukuda, H.; Yanai, K. Cerebral Metabolic Changes in Men After Chiropractic Spinal Manipulation for Neck Pain. Altern. Ther. Health Med. 2011, 17, 12–17. [Google Scholar] [PubMed]
- Kovanur Sampath, K.; Mani, R.; Cotter, J.D.; Tumilty, S. Measureable Changes in the Neuro-Endocrinal Mechanism Following Spinal Manipulation. Med. Hypotheses 2015, 85, 819–824. [Google Scholar] [CrossRef]
- Daligadu, J.; Haavik, H.; Yielder, P.C.; Baarbe, J.; Murphy, B. Alterations in Cortical and Cerebellar Motor Processing in Subclinical Neck Pain Patients Following Spinal Manipulation. J. Manip. Physiol. Ther. 2013, 36, 527–537. [Google Scholar] [CrossRef]
- Taylor, H.H.; Murphy, B. Altered Central Integration of Dual Somatosensory Input after Cervical Spine Manipulation. J. Manip. Physiol. Ther. 2010, 33, 178–188. [Google Scholar] [CrossRef]
- Sillevis, R.; Hansen, A.W. Could the Suboccipital Release Technique Result in a Generalized Relaxation and Self-Perceived Improvement? A Repeated Measure Study Design. J. Clin. Med. 2024, 13, 5898. [Google Scholar] [CrossRef] [PubMed]
- Homan, R.W.; Herman, J.; Purdy, P. Cerebral Location of International 10-20 System Electrode Placement. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 376–382. [Google Scholar] [CrossRef]
- Ossebaard, H.C. Stress Reduction by Technology? An Experimental Study into the Effects of Brainmachines on Burnout and State Anxiety. Appl. Psychophysiol. Biofeedback 2000, 25, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Mathôt, S. Pupillometry: Psychology, Physiology, and Function. J. Cogn. 2018, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; Hakerem, G.; Sutton, S.; Fleiss, J.L. Effect of Stimulus Uncertainty on the Pupillary Dilation Response and the Vertex Evoked Potential. Electroencephalogr. Clin. Neurophysiol. 1973, 34, 475–484. [Google Scholar] [CrossRef]
- Al-Chalabi, M.; Reddy, V.; Gupta, S. Neuroanatomy, Spinothalamic Tract. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gibbons, P.F.; Gosling, C.M.; Holmes, M. Short-Term Effects of Cervical Manipulation on Edge Light Pupil Cycle Time: A Pilot Study. J. Manip. Physiol. Ther. 2000, 23, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.A.; Cook, D.; Brodsky, J.; Tice, D.; Parrish, D.; Reenan, A.; Halter, J.B.; Porte, D. Quantitative Evaluation of Sympathetic and Parasympathetic Control of Iris Function. Diabetes Care 1982, 5, 518–528. [Google Scholar] [CrossRef]
- Filipe, J.A.C.; Falcão-Reis, F.; Castro-Correia, J.; Barros, H. Assessment of Autonomic Function in High Level Athletes by Pupillometry. Auton. Neurosci. Basic Clin. 2003, 104, 66–72. [Google Scholar] [CrossRef]
- Rickmann, A.; Waizel, M.; Kazerounian, S.; Szurman, P.; Wilhelm, H.; Boden, K.T. Digital Pupillometry in Normal Subjects. Neuro-Ophthalmol. 2017, 41, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Boev, A.N.; Fountas, K.N.; Karampelas, I.; Boev, C.; Machinis, T.G.; Feltes, C.; Okosun, I.; Dimopoulos, V.; Troup, C. Quantitative Pupillometry: Normative Data in Healthy Pediatric Volunteers. J. Neurosurg. 2005, 103, 496–500. [Google Scholar] [CrossRef]

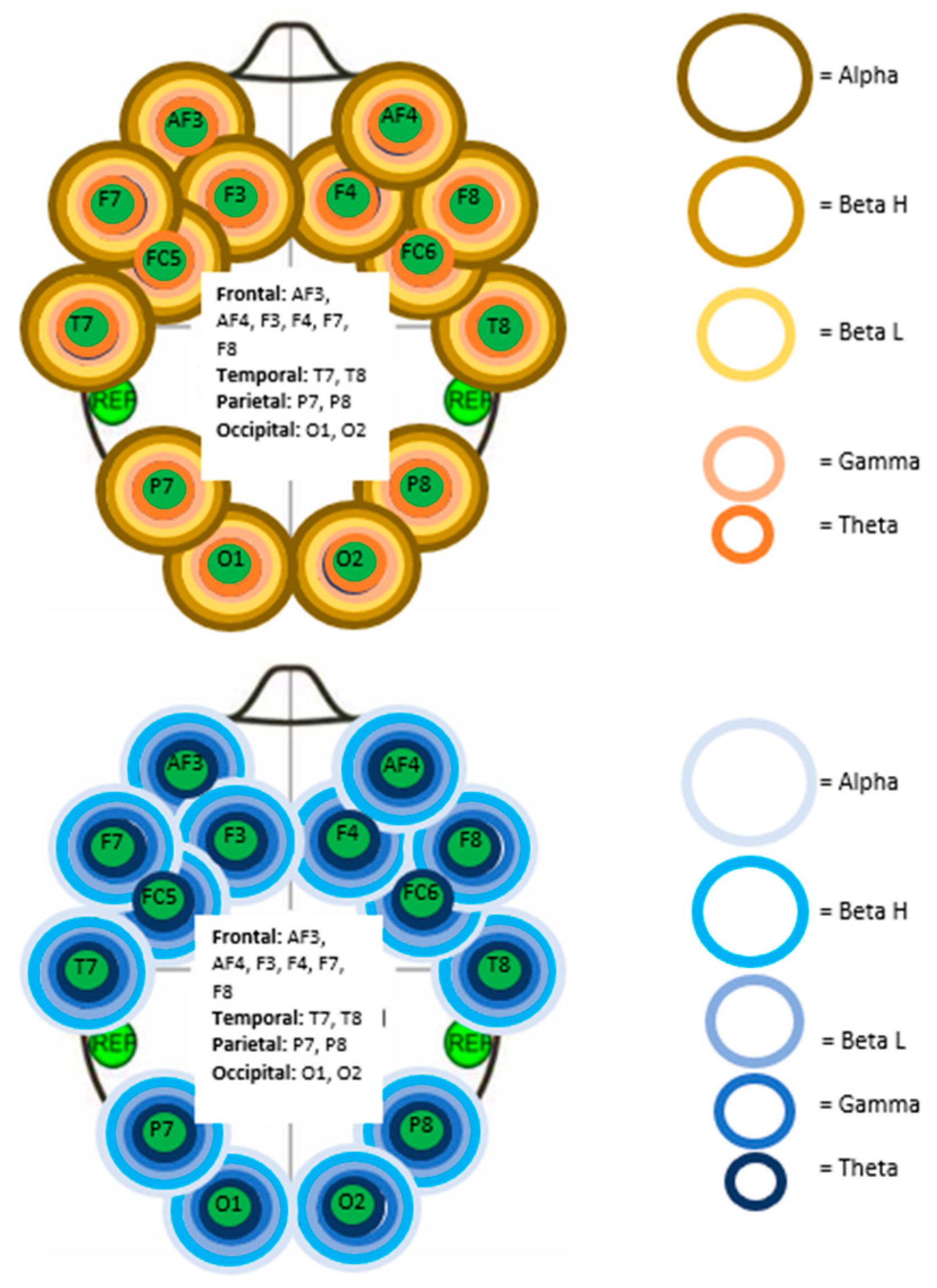
| Right Frontal | Right Parietal |
|---|---|
| AF4 gamma | P8 gamma |
| F4 gamma | |
| F8 gamma | |
| FC6 gamma |
| Left Parietal | Left Occipital | Right Frontal | Right Temporal | Right Parietal | Right Occipital |
|---|---|---|---|---|---|
| P7 gamma | O1 beta O1 gamma | F4 gamma F4 theta F8 theta | T8 beta H T8 gamma T8 theta | P8 gamma | O2 gamma |
| AS Left Eye | AS Right Eye | NAS Left Eye | NAS Right Eye | |
|---|---|---|---|---|
| N | 20 | 20 | 20 | 20 |
| Chi-Square | 6.700 | 6.100 | 7.600 | 5.700 |
| Degrees of Freedom | 2 | 2 | 2 | 2 |
| p-value | 0.035 | 0.047 | 0.022 | 0.058 |
| Group | Left Pre-M to IA-M | Left IA-M to Post-M | Left Pre-M to Post-M | Right Pre-M to IA-M | Right IA-M to Post-M | Right Pre-M to Post-M | |
|---|---|---|---|---|---|---|---|
| Z | NAS | −1.643n | −0.859n | −0.149n | −1.829n | −0.597n | −1.904p |
| p-value | NAS | 0.100 | 0.391 | 0.881 | 0.067 | 0.550 | 0.057 |
| Z | AS | −0.971n | −2.315p | −0.523n | −1.419n | −0.709p | −0.821n |
| p-value | AS | 0.332 | 0.021 | 0.601 | 0.156 | 0.478 | 0.411 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitman, D.; Sillevis, R.; Frommelt, M. The Effect of Audible Joint Manipulation Sounds in the Upper Cervical Spine on Brain Wave and Autonomic Nervous System Activity. Life 2025, 15, 103. https://doi.org/10.3390/life15010103
Whitman D, Sillevis R, Frommelt M. The Effect of Audible Joint Manipulation Sounds in the Upper Cervical Spine on Brain Wave and Autonomic Nervous System Activity. Life. 2025; 15(1):103. https://doi.org/10.3390/life15010103
Chicago/Turabian StyleWhitman, Dalton, Rob Sillevis, and Matthew Frommelt. 2025. "The Effect of Audible Joint Manipulation Sounds in the Upper Cervical Spine on Brain Wave and Autonomic Nervous System Activity" Life 15, no. 1: 103. https://doi.org/10.3390/life15010103
APA StyleWhitman, D., Sillevis, R., & Frommelt, M. (2025). The Effect of Audible Joint Manipulation Sounds in the Upper Cervical Spine on Brain Wave and Autonomic Nervous System Activity. Life, 15(1), 103. https://doi.org/10.3390/life15010103






