Utilizing Invasive Pterygoplichthys pardalis as a Sustainable Fish Meal Substitute and Euphorbia hirta Extract Supplement: Effects on Growth Performance, Organosomatic Indices, Hematological Profiles, and Serum Biochemistry in Chinese Bullfrogs (Hoplobatrachus chinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Preparation of EHE
2.3. Phytochemical Screening of EHE
2.4. Determination of Total Phenolic Content in EHE
2.5. Determination of Total Flavonoid Content in EHE
2.6. Determination of Antioxidant Capacity
2.7. Preparation of Fish Meal from Pterygoplichthys pardalis
2.8. Preparation of EHE-Supplemented PPM
2.9. Biochemical Composition Analysis of PPM and CFM
2.10. Experimental Frogs, Conditions for Acclimatization, and Experimental Design
2.11. Growth Performance and Survival
2.12. Organosomatic Indices
2.13. Study of Hematological Indices
2.14. Serum Biochemistry Study
2.15. Statistical Analysis
3. Results
3.1. Phytochemical Composition, Total Phenolic Content, Total Flavonoid Content, and Antioxidant Capacity of EHE
3.2. Amino Acid and Fatty Acid Profiles of PPM Meal and CFM
3.3. Growth Performance
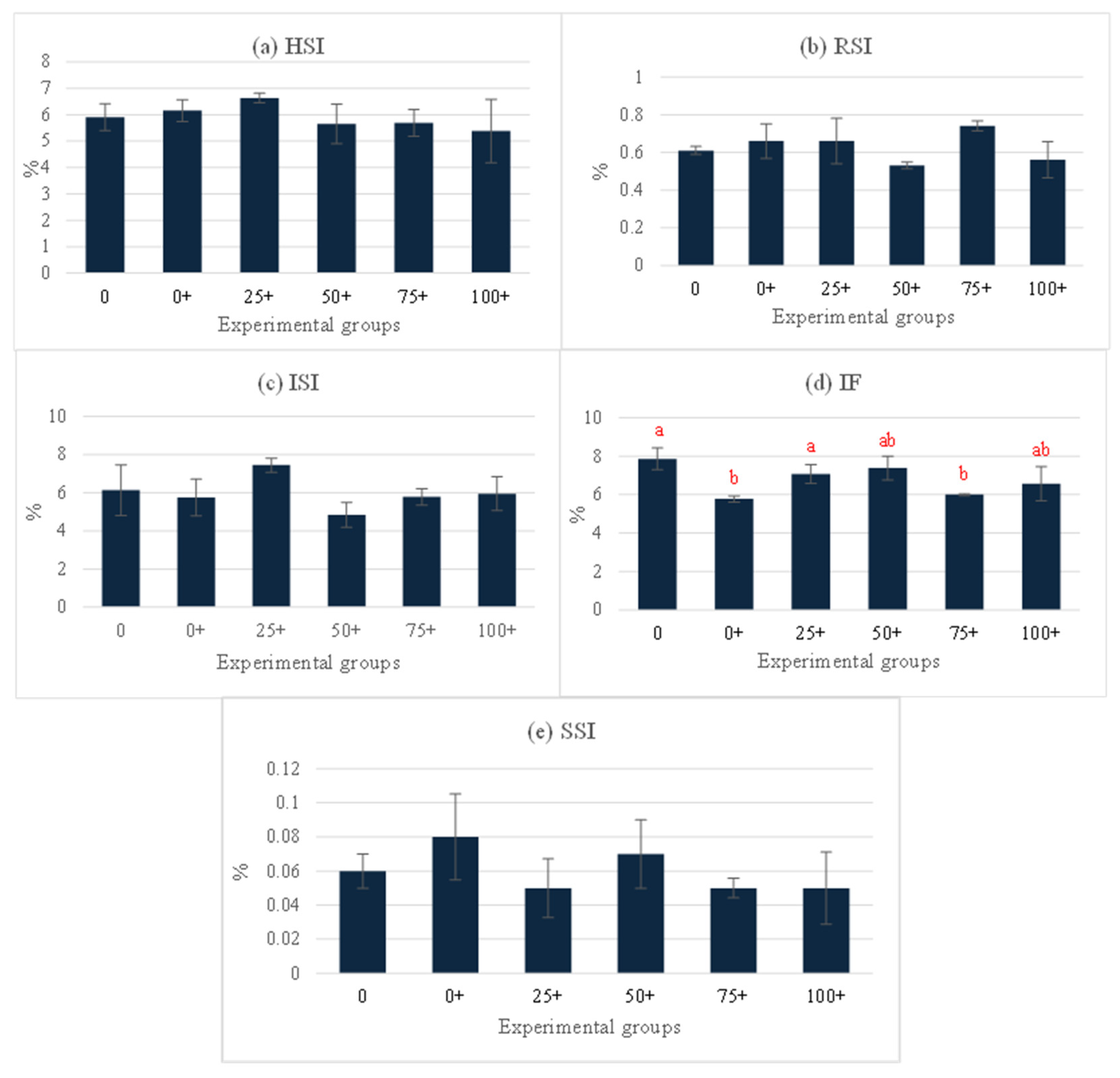
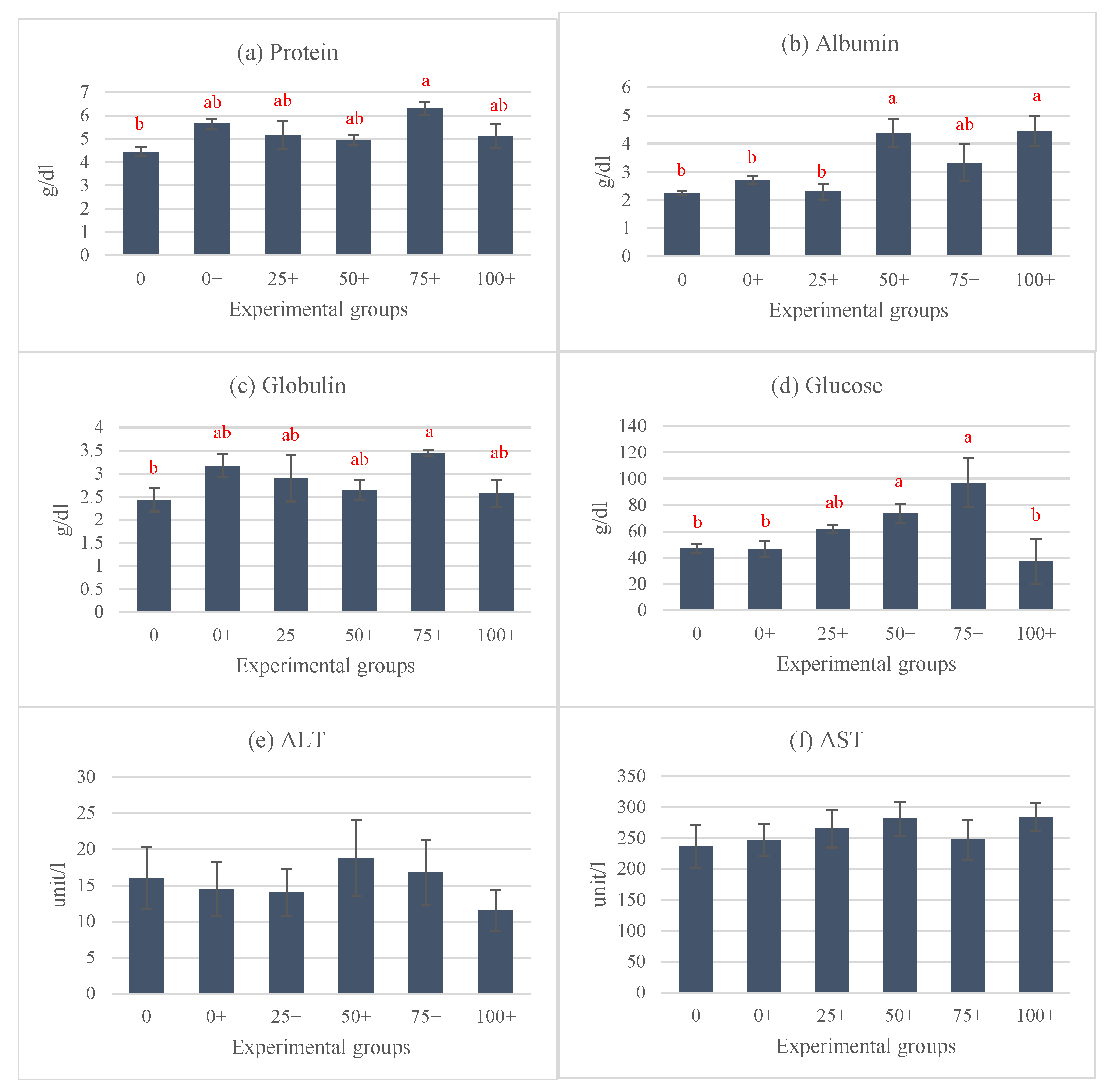
3.4. Organosomatic, Hematological, and Serum Biochemical Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seshagiri, B.; Swain, S.K.; Pillai, B.R.; Satyavati, C.; Sravanti, Y.; Rangacharyulu, P.V.; Rathod, R.; Ratnaprakash, V. Suckermouth Armoured Catfish (Pterygoplichthys spp.) Menace in Freshwater Aquaculture and Natural Aquatic Systems in Andhra Pradesh, India. Int. J. Fish. Aquat. Stud. 2021, 9, 375–384. [Google Scholar] [CrossRef]
- Veena, V.; Sasikala, G.; Raja, S. Occurrence of South American Sucker Armoured Catfish (Pterygoplichthys pardalis) in the Gayathripuzha River, Palakkad, Kerala. Int. J. Fish. Aquat. Stud. 2023, 11, 8–17. [Google Scholar] [CrossRef]
- Saba, A.O.; Rasli, N.F.; Ismail, A.; Zulkifli, S.Z.; Ghani, I.F.A.; Muhammad-Rasul, A.H.; Amal, M.N.A. A Report on Introduced Amazon Sailfin Catfish, Pterygoplichthys pardalis in Gombak Basin, Selangor, with Notes on Two Body Patterns of the Species. Pertanika J. Trop. Agric. Sci. 2020, 43, 693–703. [Google Scholar] [CrossRef]
- Chaichana, R.; Jongphadungkiet, S. Assessment of the Invasive Catfish Pterygoplichthys pardalis (Castelnau,1855) in Thailand: Ecological Impacts and Biological Control Alternatives. Trop. Zool. 2012, 25, 173–182. [Google Scholar] [CrossRef]
- Faulkner, K.T.; Hulme, P.E.; Pagad, S.; Wilson, J.R.U.; Robertson, M.P. Classifying the Introduction Pathways of Alien Species: Are We Moving in the Right Direction? NeoBiota 2020, 62, 143–159. [Google Scholar] [CrossRef]
- Orfinger, A.B.; Gooddingm, D.D. The Global Invasion of the Suckermouth Armored Catfish Genus Pterygoplichthys (Siluriformes: Loricariidae): Annotated List of Species, Distributional Summary, and Assessment of Impacts. Zool. Stud. 2018, 57, e7. [Google Scholar] [CrossRef]
- Hossain, M.S.; Akmal, S.G.; Buřič, M.; Patoka, J. Invasive Amazon Sailfin Catfish in Bangladesh: Wild Distribution, Environmental and Perceived Socioeconomic Consequences. Aquat. Invasions. 2024, 19, 121–136. [Google Scholar] [CrossRef]
- Thalathiah, S.; Palanisamy, V. The Way Forward: Building Capacity to Combat Impacts of Squatic Invasive Alien Species and Associated Transboundary Pathogens in ASEAN Countries. In Proceedings of the Workshop Hosted by the Department of Fisheries, Penang, Malaysia, 12–16 July 2004. [Google Scholar]
- Rodriguez-Santiago, M.A.; Garcia-Prieto, L.; Mendoza-Garfias, B.; Gonzalez-Solis, D.; Grano-Maldonado, M.I. Parasites of Two Coexisting Invasive Sailfin Catfishes (Siluriformes: Loricariidae) in Tropical Region of Mexico. Neotrop. Ichthyol. 2016, 14, e1600021. [Google Scholar] [CrossRef]
- Escalera-Vázquez, L.H.; García-López, J.E.; Sosa-López, A.; Calderón-Cortés, N.; Hinojosa-Garro, D. Impact of the Non-Native Locariid Fish Pterygoplichthys pardalis in Native Fish Community on a Seasonal Tropical Floodplain in Mexico. Aquat. Ecosyst. Health Manag. 2019, 22, 462–472. [Google Scholar] [CrossRef]
- Lai, Q.T.; Orfinger, A.B.; Tran, T.T.; Le, N.K. Distribution of Suckermouth Armoured Catfishes (Siluriformes, Loricariidae) Across the Salinity Gradient of the Mekong Delta, Vietnam. Asian Fish. Sci. 2020, 33, 300–306. [Google Scholar] [CrossRef]
- Intawicha, P.; Tapingkae, W.; Punyatong, M. Suckermouth Armored Catfish (Pterygoplichthys pardalis) as a Replacement for Fish Meal in Thai “Pradu Hang Dam” Native Chicken Diets. Int. J. Poult. Sci. 2020, 19, 380–384. [Google Scholar] [CrossRef]
- Panase, P.; Uppapong, S.; Tuncharoen, S.; Tanitson, J.; Soontornprasit, K.; Intawicha, P. Partial Replacement of Commercial Fish Meal with Amazon Sailfin Catfish Pterygoplichthys pardalis Meal in Diets for Juvenile Mekong Giant Catfish Pangasianodon gigas. Aquac. Res. 2018, 12, 25–29. [Google Scholar] [CrossRef]
- Srinual, O.; Punyatong, M.; Moonmanee, T.; Intawicha, P.; Yachai, M.; Tapingkae, W. Replacement of Fish Meal with Sucker Mouth Armored Catfish and Its Effect on Performance and Intestinal Morphology of Indigenous Thai Chicken. J. Anim. Plant Sci. 2020, 30, 803–810. [Google Scholar] [CrossRef]
- Han, Y.K.; Xu, Y.C.; Luo, Z.; Zhao, T.; Zhen, H.; Tan, X.Y. Fish Meal Replacement by Mixed Plant Protein in the Fiets for Juvenile Yellow Catfish Pelteobagrus fulvidraco: Effects on Growth Performance and Health Status. Aquac. Nutr. 2022, 2022, 2677885. [Google Scholar] [CrossRef] [PubMed]
- Homska, N.; Kowalska, J.; Bogucka, J.; Ziółkowska, E.; Rawski, M.; Kierończyk, B.; Mazurkiewicz, J. Dietary Fish Meal Replacement with Hermetia illucens and Tenebrio molitor Larval Meals Improves the Growth Performance and Nutriphysiological Status of Ide (Leuciscus idus) Juveniles. Animals 2022, 12, 1227. [Google Scholar] [CrossRef]
- De Fonseka, R.; Radampola, K. Feasibility of Using Sailfin Catfish Meal as an Alternative to Commercial Fishmeal in the Diets of Juvenile Guppy (Poecilia reticulata). J. Fish. 2022, 10, 101203. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference, Version 6.2. 2024. Available online: https://amphibiansoftheworld.amnh.org/Amphibia/Anura/Dicroglossidae/Dicroglossinae/Hoplobatrachus/Hoplobatrachus-chinensis (accessed on 26 June 2024).
- Fisheries Department, Ministry of Agriculture and Cooperatives, Thailand. Fisheries Development Policy and Planning Division, Statistics of Freshwater Aquaculture Production. 2022. Available online: https://www4.fisheries.go.th/local/file_document/20230725093333_1_file.pdf (accessed on 26 June 2024). (In Thai).
- Bulfon, C.; Volpatti, D.; Galeotti, M. Current Research on the Use of Plant-Derived Products in Farmed Fish. Aquac. Res. 2015, 46, 513–551. [Google Scholar] [CrossRef]
- Hidayat, Y.; Fuad, F.; Nurhidayati, M. Implementation of economic democracy principle in Islamic banking policies through Financial Services Authority (FSA) in Indonesia. At-Taradhi J. Studi Ekonomi. 2017, 8, 132–154. [Google Scholar] [CrossRef]
- Ear, C.; Sookying, S.; Kannika, K.; Suwannapoom, C.; Panase, P. Positive Effects of Andrographis paniculata Extract on Growth Performance, Haematology, Serum Biochemistry, Organosomatic Indices and Resistance Against Aeromonas hydrophila in Hybrid Catfish (Clarias macrocephalus× Clarias gariepinus). J. Appl. Anim. Res. 2024, 52, 2320230. [Google Scholar] [CrossRef]
- Gabriel, N.N. Review on the Progress in the Role of Herbal Extracts in Tilapia Culture. Cogent Food Agric. 2019, 5, 1619651. [Google Scholar] [CrossRef]
- Sookying, S.; Panase, A.; Srisuttha, P.; Chaophothun, A.; Panase, P. Devil’s Tree Flower (Alstonia scholaris) Extract: Positive Effects on Growth Performance and Serum Biochemical Indices in Channa striata (Bloch, 1793). J. Appl. Anim. Res. 2023, 51, 677–683. [Google Scholar] [CrossRef]
- Wigraiboon, S.; Panchan, R.; Luang-In, V.; Ounjit, W.; Panase, P.; Sookying, S.; Sutthi, N. Effects of Dietary Tuber Ethanolic Extract of Nut Grass (Cyperus rotundus Linn.) on Growth, Immune Response, and Disease Resistance in Nile Tilapia (Oreochromis niloticus). Animals 2024, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Asha, S.; Thirunavukkarasu, P.; Mohamad, S.A. Phytochemical Screening of Euphorbia hirta Linn Leaf Extracts. World J. Pharm. Res. 2015, 3, 1104–1112. [Google Scholar]
- Panase, P.; Kamee, B.; Moungmor, S.; Tipdacho, P.; Matidtor, J.; Sutthi, N. Effects of Euphorbia hirta Plant Leaf Extract on Growth Performance, Hematological and Organosomatic Indices of Hybrid Catfish, Clarias macrocephalus × C. gariepinus. Fish. Sci. 2018, 84, 1025–1036. [Google Scholar] [CrossRef]
- Pratheepa, V.; Sukumaran, N. Effect of Euphorbia hirta Plant Leaf Extract on Immunostimulant Response of Aeromonas hydrophila Infected Cyprinus carpio. PeerJ 2014, 2, e671. [Google Scholar] [CrossRef]
- Hartanti, D.; Cahyani, A.N. Plant Cyanogenic Glycosides: An Overview. Farmasains J. Farm. Dan Ilmu Kesehat. 2020, 5, 1–6. [Google Scholar]
- Pant, D.R.; Pant, N.D.; Saru, D.B.; Yadav, U.N.; Khanal, D.P. Phytochemical Screening and Study of Antioxidant, Antimicrobial, Antidiabetic, Anti-Inflammatory and Analgesic Activities of Extracts from Stem Wood of Pterocarpus marsupium Roxburgh. J. Intercult. Ethnopharmacol. 2017, 6, 170–176. [Google Scholar] [CrossRef]
- Shaikh, J.R.; Patil, M.K. Qualitative Tests for Preliminary Phytochemical Screening: An Overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid. Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef]
- Bello, A.A.; Katta, A.; Obaydo, R.H.; Jazmati, A. Phytochemical Analysis and Antioxidant Efficacy of Chrysojasminum fruticans (L.) Banfi in Syrian Flora. Heliyon 2024, 10, e37322. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2006; pp. 38–46. [Google Scholar]
- Determination of amino acids. Off. J. Eur. J. Communities 1998. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1998:257:0014:0028:En:PDF (accessed on 13 January 2025).
- AOAC Official Method of Analysis of AOAC Internation, 21st ed.; AOAC International: Rockville, MD, USA, 2019.
- Thip-uten, T.; Tippayawat, P.; Yuangsoi, B.; Wongmaneeprateep, S. Dietary Spirulina (Arthrospira platensis) Supplementation on Growth Performance, Haematology, Immune Response and Disease Resistance of Rugose Frog (Hoplobatrachus rugulosus). J. Pure Appl. Microbiol. 2021, 15, 1139–1149. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y.; Wang, G.; Zhang, L.; Zhang, G.; Huo, Z.; Ge, H. Heritability Estimates for Growth Traits and Correlation Analysis between Weight and Metamorphosis Rate in the Bullfrog Rana (Aquarana) catesbeiana. Fishes 2024, 9, 105. [Google Scholar] [CrossRef]
- Bagenal, T. Methods for Assessment of Fish Production in Fresh Waters, 3rd ed.; Blackwell Scientific Publication: Oxford, UK, 1978; ISBN 0632001259. [Google Scholar]
- Smith, J.M.; Stump, K.C. Isoflurane Anesthesia in the African Clawed Frog (Xenopus laevis). Contemp. Top. Lab. Anim. Sci. 2000, 39, 39–42. [Google Scholar] [PubMed]
- Hadidi, S.; Gavin, W.G.; Timothy, J.W.; Jefrey, T.S.; Gregory, D.W. Spleen Size Predicts Resistance of Rainbow Trout to Flavobacterium psychrophilum Challenge. J. Immun. 2008, 180, 4156–4165. [Google Scholar] [CrossRef] [PubMed]
- Heatley, J.J.; Johnson, M. Clinical Technique: Amphibian Hematology: A Practitioner’s Guide. J. Exot. Pet Med. 2009, 18, 14–19. [Google Scholar] [CrossRef]
- Rehulka, J. Haematological Analyses in Rainbow Trout Oncorhynchus mykiss Affected by Viral Haemorrhagic Septicaemia (VHS). Dis. Aquat. Org. 2003, 56, 185–193. [Google Scholar] [CrossRef]
- Nghia, V.D.; Lan, P.T.P.; Tram, N.D.Q. Using Black Soldier Fly Larvae as Feed for Thai Frog (Rana rugosa Temminck and Schlegel, 1838)—Preliminary Study of the Effect on Production Parameters. Isr. J. Aquac. Bamidgeh 2023, 75, 1–8. [Google Scholar] [CrossRef]
- Klahan, R.; Kaithong, S.; Ounsawat, S. Compensatory Growth Response with Switching Dietary Protein Levels in Common Lowland Frog, Rana rugulosa. Int. J. Agric. Technol. 2022, 18, 1587–1600. [Google Scholar]
- Millamena, O.M. Replacement of Fish Meal by Animal By-Product Meals in a Practical Diet for Grow-Out Culture of Grouper Epinephelus coioides. Aquaculture 2002, 204, 75–84. [Google Scholar] [CrossRef]
- Ibrahim, D.; Arisha, A.H.; Khater, S.I.; Gad, W.M.; Hassan, Z.; Abou-Khadra, S.H.; Mohamed, D.I.; Ahmed Ismail, T.; Gad, S.A.; Eid, S.A.M.; et al. Impact of Omega-3 Fatty Acids Nano-Formulation on Growth, Antioxidant Potential, Fillet Quality, Immunity, AutophagyRelated Genes and Aeromonas hydrophila Resistance in Nile Tilapia (Oreochromis niloticus). Antioxidants 2022, 11, 1523. [Google Scholar] [CrossRef]
- Kareem, Z.H.; Abdelhadi, Y.M.; Christianus, A.; Karim, M.; Romano, N. Effects of Some Dietary Crude Plant Extracts on the Growth and Fonadal Maturity of Nile Tilapia (Oreochromis niloticus) and Their Resistance to Streptococcus agalactiae Infection. Fish Physiol. Biochem. 2016, 42, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, Q.; Dong, S.; Dong, K.; Xu, Y.; Mei, Y.; Hou, Z. Effects of Chronic Stress from High Stocking Density in Mariculture: Evaluations of Growth Performance and Lipid Metabolism of Rainbow Trout (Oncorhychus mykiss). Biology 2024, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Xun, P.; Zhuang, S.; Yao, H.; Su, J.; Yang, Y.; Shu, H.; Yu, W.; Lin, H. Effects of Sodium Acetate Supplementation on Growth, Hematologic and Plasma Biochemical Parameter, Lipid Deposition, and Intestinal Health of Juvenile Golden Pompano Trachinotus Ovatus Fed High-Lipid Diets. Aquac. Nutr. 2024, 2024, 7904141. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yin, P.; Tian, L.; Yu, Y.; Liu, Y.; Niu, J. Dietary Supplementation of Astaxanthin Improved the Growth Performance, Antioxidant Ability and Immune Response of Juvenile Largemouth Bass (Micropterus salmoides) Fed High-Fat Diet. Mar. Drugs 2020, 18, 642. [Google Scholar] [CrossRef]
- Li, M.; Qiang, J.; Zhu, X.; Bao, J.; Tao, Y.; Zhu, H. Effect of Siberian Ginseng Water Extract as a Dietary Additive on Growth Performance, Blood Biochemical Indexes, Lipid Metabolism, and Expression of PPARs Pathway-Related Genes in Denetically Improved Farmed Tilapia (Oreochromis niloticus). Fishes 2022, 7, 149. [Google Scholar] [CrossRef]
- Yao, J.; Hu, P.; Zhu, Y.; Xu, Y.; Tan, Q.; Liang, X. Lipid-Lowering Effects of Lotus Leaf Alcoholic Extract on Serum, Hepatopancreas, and Muscle of Juvenile Grass Carp via Gene Expression. Front. Physiol. 2020, 11, 584782. [Google Scholar] [CrossRef]
- Mekam, P.N.; Martini, S.; Nguefack, J.; Tagliazucchi, G.; Stefani, E. Phenolic Compounds Profile of Water and Ethanol Extracts of Euphorbia hirta L. Leaves Showing Antioxidant and Antifungal Properties. S. Afr. J. Bot. 2019, 127, 319–332. [Google Scholar] [CrossRef]
- Nhu, T.Q.; Hang, B.T.B.; Bach, L.T.; Hue, B.T.B.; Quetin-Leclercq, J.; Scippo, M.L.; Phuong, N.T.; Kestemont, P. Plant Extract-Based Diets Differently Modulate Immune Responses and Eesistance to Bacterial Infection in Striped Catfish (Pangasianodon hypophthalmus). Fish Shellfish Immunol. 2019, 20, 913–924. [Google Scholar] [CrossRef]
- Goda, A.M.A.S. Effect of Dietary Ginseng Herb (Ginsana ® G115) Supplementation on Growth, Feed Utilization, and Hematological Indices of Nile Tilapia, Oreochromis niloticus (L.), Fingerlings. J. World Aquacult. Soc. 2008, 39, 205–214. [Google Scholar] [CrossRef]
- Latha, C.; Krishnakumar, V.; Munuswam, N. Growth, Survival and Haemato-Biochemical Profiles of the Freshwater Catfish, Pangasius sutchi (Fowler, 1937) Fingerlings Fed with Tinospora cordifolia Leaf Extract Supplemented Diet. Egypt. J. Aquat. Biol. Fish. 2020, 24, 249–262. [Google Scholar] [CrossRef]
- Bhagavan, N.V.; Ha, C.E. Chapter 14—Carbohydrate Metabolism II: Gluconeogenesis, Glycogen Synthesis and Breakdown, and Alternative Pathways. In Essentials of Medical Biochemistry, 2nd ed.; Bhagavan, N.V., Ha, C.E., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 205–225. ISBN 9780124166974. [Google Scholar]
- Liu, S.; Zang, X.; Liu, B.; Zhang, X.; Arunakumara, K.K.U.; Zhang, X.; Liang, B. Effect of Growth Hormone Transgenic Synechocystis on Growth, Feed efficiency, Muscle Composition, Haematology and Histology of Turbot (Scophthalmus maximus L.). Aquac. Res. 2007, 38, 1283–1292. [Google Scholar] [CrossRef]
- Shahsavani, D.; Mohri, M.; Kanani, H.G. Determination of Normal Values of Some Blood Serum Enzymes in Acipenser stellatus. Pallas. Fish Physiol. Biochem. 2010, 36, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, J.; Qin, C.; Yu, C.; Wang, S.; Jia, J. Growth Performance, Serum Biochemical Parameters, Immune Parameters and Hepatic Antioxidant Status of Yellow Catfish Pelteobagrus fulvidraco Supplemented with Sargassum horneri Hot-Water Extract. Aquac. Rep. 2021, 21, 100839. [Google Scholar] [CrossRef]


| Proximate Composition | P. pardalis Meal (% in Dry Weight) | Commercial Fish Meal (% in Dry Weight) |
|---|---|---|
| Moisture | 4.43 ± 0.52 | 4.99 ± 1.13 |
| Ash | 16.64 ± 0.43 | 28.43 ± 0.01 |
| Protein | 62.13 ± 0.25 | 59.07 ± 1.00 |
| Lipid | 6.40 ± 0.53 | 7.40 ± 0.05 |
| Fiber | 0.507 ± 0.002 | 0.507 ± 0.003 |
| Nitrogen-free extract | 9.89 ± 0.58 | 9.6 ± 0.73 |
| Ingredient Composition (%) | Experimental Diet (Dry Weight) | |||||
|---|---|---|---|---|---|---|
| 0 | 0+ | 25+ | 50+ | 75+ | 100+ | |
| Commercial fish meal | 25.02 | 25.02 | 18.765 | 12.51 | 6.255 | 0 |
| P. pardalis meal | 0 | 0 | 6.255 | 12.51 | 18.765 | 25.02 |
| Soybean meal | 25.03 | 25.03 | 25.03 | 25.03 | 25.03 | 25.03 |
| Corn starch | 16.55 | 16.55 | 16.55 | 16.55 | 16.55 | 16.55 |
| Rice bran | 16.55 | 16.55 | 16.55 | 16.55 | 16.55 | 16.55 |
| Broken rice | 16.55 | 16.55 | 16.55 | 16.55 | 16.55 | 16.55 |
| Premix | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| 300 mg/kg Euphorbia hirta extract | − | + | + | + | + | + |
| Total (%) | 100 | 100 | 100 | 100 | 100 | 100 |
| Proximate Composition (% in Dry Weight) | ||||||
| Moisture | 3.67 ± 2.07 | 2.28 ± 0.17 | 1.79 ± 0.01 | 2.39 ± 0.24 | 2.13 ± 0.03 | 2.48 ± 0.22 |
| Ash | 10.52 ± 2.78 | 12.32 ± 2.38 | 14.63 ± 0.06 | 10.36 ± 0.16 | 13.92 ± 0.58 | 12.46 ± 2.16 |
| Protein | 31.38 ± 0.66 | 31.13 ± 0.89 | 32.76 ± 1.96 | 32.83 ± 3.09 | 33.82 ± 1.69 | 34.07 ± 1.67 |
| Lipid | 11.67 ± 1.04 | 14.25 ± 0.35 | 13.33 ± 2.25 | 11.75 ± 1.06 | 11.57 ± 0.38 | 11.23 ± 0.31 |
| Fiber | 2.48 ± 0.22 | 5.16 ± 1.00 | 5.20 ± 0.37 | 5.33 ± 0.34 | 5.44 ± 0.26 | 5.62 ± 0.13 |
| Nitrogen-free extract | 40.28 ±0.23 | 34.85 ± 2.14 | 32.28 ± 1.32 | 37.34 ± 0.25 | 33.12 ± 1.14 | 34.13 ±1.56 |
| Phytochemical | Result | Phytochemical | Result |
|---|---|---|---|
| Alkaloids | − | Phenolics | + |
| Steroids | − | Flavonoids | − |
| Triterpenoids | + | Anthraquinones | − |
| Saponins | − | Cyanogenic glycosides | − |
| Cardiac glycosides | − | Carbohydrates | + |
| Coumarins | + |
| Analysis | Total Phenolics (mg GAE/g Extract) | Total Flavonoids (mg QE/g Extract) | Antioxidant Capacity (IC50) (μg/mL) |
|---|---|---|---|
| E. hirta extract | 573.58 ± 29.83 | 39.17 ± 2.90 | 11.06 ± 6.54 |
| Ascorbic acid | - | - | 4.73 ± 1.85 |
| Amino Acid Composition | Ingredient (mg/100 g) | |
|---|---|---|
| P. pardalis Meal | Commercial Fish Meal | |
| Essential amino acids (EAA) | ||
| Arginine | 6184.61 | 3859.79 |
| Histidine | 2430.52 | 1359.29 |
| Isoleucine | 4444.77 | 2400.27 |
| Leucine | 7943.66 | 4196.22 |
| Lysine | 9114.96 | 4316.96 |
| Phenylalanine | 4210.00 | 2491.27 |
| Threonine | 4585.82 | 2562.71 |
| Tryptophan | 777.76 | 402.73 |
| Valine | 5269.75 | 3044.43 |
| Methionine | 2953.96 | 1623.08 |
| ΣEAA | 47,915.81 | 26,256.75 |
| Non-essential amino acids (NEAA) | ||
| Alanine | 5716.19 | 4209.99 |
| Aspartic acid | 10,089.22 | 5522.03 |
| Glutamic acid | 16,280.00 | 9027.22 |
| Glycine | 4955.22 | 4944.43 |
| Hydroxyproline | 604.04 | 1204.32 |
| Proline | 3693.46 | 2961.60 |
| Serine | 4117.72 | 2399.07 |
| Tyrosine | 3496.56 | 1870.37 |
| Cystine | 978.82 | 541.9 |
| ΣNEAA | 49,931.23 | 32,680.93 |
| Fatty Acid Composition | Ingredient (g/100 g) | |
|---|---|---|
| P. pardalis Meal | Commercial Fish Meal | |
| Saturated fatty acid (SFA) | ||
| Lauric acid (C12:0) | 0.02 | 0.02 |
| Myristic acid (C14:0) | 0.07 | 0.41 |
| Pentadecanoic acid (C15:0) | 0.02 | 0.09 |
| Palmitic acid (C16:0) | 0.41 | 1.95 |
| Heptadecanoic acid (C17:0) | 0.01 | 0.12 |
| Stearic acid (C18:0) | 0.18 | 0.75 |
| Arachidic acid (C20:0) | Not detected | 0.06 |
| Heneicosanoic acid (C21:0) | Not detected | 0.01 |
| Behenic acid (C22:0) | 0.01 | 0.04 |
| Lignoceric acid (C24:0) | Not detected | 0.04 |
| ΣSFA | 0.72 | 3.49 |
| Unsaturated fatty acid (UFA) | ||
| Trans-fatty acid | Not detected | 0.17 |
| Omega 3 | 0.06 | 0.84 |
| Omega 6 | 0.21 | 0.36 |
| Omega 9 | 0.35 | 0.83 |
| ΣUFA | 0.62 | 2.20 |
| Monounsaturated fatty acid (MUFA) | ||
| Palmitoleic acid (C16:1n-7) | 0.01 | 0.53 |
| Cis-9-oleic acid (C18:1n-9c) | 0.35 | 0.01 |
| Cis-10-heptadecenoic acid (C17:1n10) | Not detected | 0.04 |
| Trans-9-elaidic acid (C18:1n9t) | Not detected | 0.02 |
| Cis-9-oleic acid (C18:1n9c) | Not detected | 0.77 |
| Cis-11-eicosenoic acid (C20:1n11) | Not detected | 0.05 |
| Erucic acid (C12:1n9) | Not detected | 0.01 |
| Nervonic acid (C24:1n9) | Not detected | 0.05 |
| ΣMUFA | 0.36 | 1.48 |
| Polyunsaturated fatty acid (PUFA) | ||
| Trans-linolelaidic acid (C18:2n6t) | Not detected | 0.15 |
| Cis-9-12-linoleic acid (C18:2n6) | 0.11 | 0.12 |
| Gamma-linolenic acid (C18:3n6) | 0.02 | 0.01 |
| Alpha-linoleic acid (C18:3n3) | 0.01 | 0.07 |
| Cis-11,14-eicosatrienoic acid (C20:2n6) | Not detected | 0.03 |
| Cis-8,11,14-eicosatrienoic acid (C20:3n6) | 0.03 | 0.04 |
| Arachidonic acid (C20:4n6) | 0.06 | 0.19 |
| Cis-5,8,11,14,17-eicosapentaenoic acid (C20:5n3) | 0.01 | 0.28 |
| 4,7,10,13,16,19-Docosahexaenoic acid (C22:6n3) | 0.04 | 0.49 |
| ΣPUFA | 0.28 | 1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sookying, S.; Srisuttha, P.; Rodprasert, V.; Chaodon, C.; Phinrub, W.; Sutthi, N.; Panase, P. Utilizing Invasive Pterygoplichthys pardalis as a Sustainable Fish Meal Substitute and Euphorbia hirta Extract Supplement: Effects on Growth Performance, Organosomatic Indices, Hematological Profiles, and Serum Biochemistry in Chinese Bullfrogs (Hoplobatrachus chinensis). Life 2025, 15, 115. https://doi.org/10.3390/life15010115
Sookying S, Srisuttha P, Rodprasert V, Chaodon C, Phinrub W, Sutthi N, Panase P. Utilizing Invasive Pterygoplichthys pardalis as a Sustainable Fish Meal Substitute and Euphorbia hirta Extract Supplement: Effects on Growth Performance, Organosomatic Indices, Hematological Profiles, and Serum Biochemistry in Chinese Bullfrogs (Hoplobatrachus chinensis). Life. 2025; 15(1):115. https://doi.org/10.3390/life15010115
Chicago/Turabian StyleSookying, Sontaya, Phanit Srisuttha, Vipada Rodprasert, Chanthima Chaodon, Wikit Phinrub, Nantaporn Sutthi, and Paiboon Panase. 2025. "Utilizing Invasive Pterygoplichthys pardalis as a Sustainable Fish Meal Substitute and Euphorbia hirta Extract Supplement: Effects on Growth Performance, Organosomatic Indices, Hematological Profiles, and Serum Biochemistry in Chinese Bullfrogs (Hoplobatrachus chinensis)" Life 15, no. 1: 115. https://doi.org/10.3390/life15010115
APA StyleSookying, S., Srisuttha, P., Rodprasert, V., Chaodon, C., Phinrub, W., Sutthi, N., & Panase, P. (2025). Utilizing Invasive Pterygoplichthys pardalis as a Sustainable Fish Meal Substitute and Euphorbia hirta Extract Supplement: Effects on Growth Performance, Organosomatic Indices, Hematological Profiles, and Serum Biochemistry in Chinese Bullfrogs (Hoplobatrachus chinensis). Life, 15(1), 115. https://doi.org/10.3390/life15010115








