Impact of the Ketogenic Diet on Neurological Diseases: A Review
Abstract
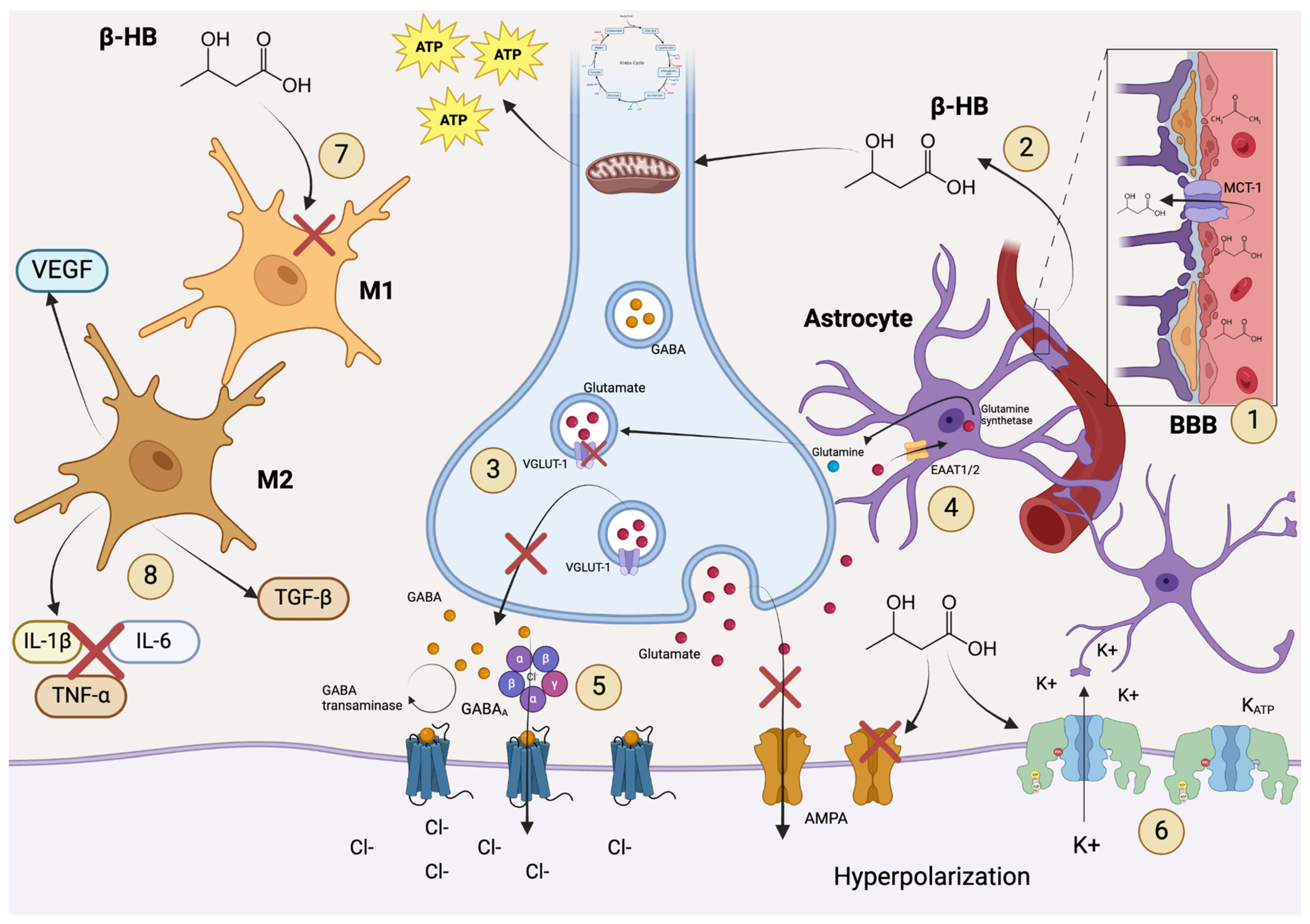
1. Introduction
2. Ketogenic Diet in Epilepsy
3. Ketogenic Diet in Alzheimer’s Disease
4. Ketogenic Diet in Parkinson’s Disease
5. Ketogenic Diet in Multiple Sclerosis
6. Ketogenic Diet in Huntington’s Disease
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavón, S.; Lázaro, E.; Martínez, O.; Amayra, I.; López-Paz, J.F.; Caballero, P.; Al-Rashaida, M.; Luna, P.M.; García, M.; Pérez, M.; et al. Ketogenic diet and cognition in neurological diseases: A systematic review. Nutr. Rev. 2021, 79, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A. Ketogenic diet for obesity: Friend or foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.M.; Mathew, T.C.; Al-Zaid, N.S. Efficacy of low-carbohydrate ketogenic diet in the treatment of type 2 diabetes. Med. Princ. Pract. 2021, 30, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Nasser, S.; Vialichka, V.; Biesiekierska, M.; Balcerczyk, A.; Pirola, L. Effects of ketogenic diet and ketone bodies on the cardiovascular system: Concentration matters. World J. Diabetes 2020, 11, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Verde, L.; Camajani, E.; Cernea, S.; Frias-Toral, E.; Lamabadusuriya, D.; Ceriani, F.; Savastano, S.; Colao, A.; Muscogiuri, G. Ketogenic diet as medical prescription in women with polycystic ovary syndrome (PCOS). Curr. Nutr. Rep. 2023, 12, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic diet in the treatment of cancer—Where do we stand? Mol. Metab. 2020, 33, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.L.; Mattingly, S.; Schirrmacher, R.; Sawyer, M.B.; Fine, E.J.; Prado, C.M. A nutritional perspective of ketogenic diet in cancer: A narrative review. J. Acad. Nutr. Diet. 2018, 118, 668–688. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.; Brown, N.I.; Williams, S.; Plaisance, E.P.; Fontaine, K.R. Ketogenic diet for cancer: Critical assessment and research recommendations. Nutrients 2021, 13, 3562. [Google Scholar] [CrossRef]
- Włodarek, D. Role of ketogenic diets in neurodegenerative diseases (Alzheimer’s disease and Parkinson’s disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef]
- Phillips, M.C.; Murtagh, D.K.; Gilbertson, L.J.; Asztely, F.J.; Lynch, C.D. Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov. Disord. 2018, 33, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Broom, G.M.; Shaw, I.C.; Rucklidge, J.J. The ketogenic diet as a potential treatment and prevention strategy for Alzheimer’s disease. Nutrition 2019, 60, 118–121. [Google Scholar] [CrossRef]
- Taylor, M.K.; Sullivan, D.K.; Mahnken, J.D.; Burns, J.M.; Swerdlow, R.H. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Rusek, M.; Pluta, R.; Ułamek-Kozioł, M.; Czuczwar, S.J. Ketogenic diet in Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 3892. [Google Scholar] [CrossRef]
- Meira, I.D.; Romão, T.T.; Prado, H.J.P.D.; Krüger, L.T.; Pires, M.E.P.; da Conceição, P.O. Ketogenic Diet and epilepsy: What we know so far. Front. Neurosci. 2019, 13, 5. [Google Scholar] [CrossRef]
- Malinowska, D.; Żendzian-Piotrowska, M. Ketogenic Diet: A review of composition diversity, mechanism of action and clinical application. J. Nutr. Metab. 2024, 2024, 6666171. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, D.; Kasperek, K.; Rękawek, P.; Piątkowska-Chmiel, I. The therapeutic role of ketogenic diet in neurological disorders. Nutrients 2022, 14, 1952. [Google Scholar] [CrossRef]
- Barañano, K.W.; Hartman, A.L. The ketogenic diet: Uses in epilepsy and other neurologic illnesses. Curr. Treat. Options Neurol. 2008, 10, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.S.; Kim, Y.S.; Choi, W.S. Neuroprotective effects of the ketogenic diet. Epilepsia 2008, 49, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Gough, S.M.; Casella, A.; Ortega, K.J.; Hackam, A.S. Neuroprotection by the ketogenic diet: Evidence and controversies. Front. Nutr. 2021, 8, 782657. [Google Scholar] [CrossRef] [PubMed]
- Gano, L.B.; Patel, M.; Rho, J.M. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 2014, 55, 2211–2228. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, M.; Rho, J.M.; Mattson, M.P. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Rev. 2009, 59, 293–315. [Google Scholar] [CrossRef]
- Youngson, N.A.; Morris, M.J.; Ballard, J.W.O. The mechanisms mediating the antiepileptic effects of the ketogenic diet, and potential opportunities for improvement with metabolism-altering drugs. Seizure 2017, 52, 15–19. [Google Scholar] [CrossRef]
- Kim, D.Y.; Abdelwahab, M.G.; Lee, S.H.; O’neill, D.; Thompson, R.J.; Duff, H.J.; Sullivan, P.G.; Rho, J.M. Ketones prevent oxidative impairment of hippocampal synaptic integrity through KATP channels. PLoS ONE 2015, 10, e0119316. [Google Scholar] [CrossRef] [PubMed]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The role of ketogenic diet in the treatment of neurological diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef] [PubMed]
- Rudy, L.; Carmen, R.; Daniel, R.; Artemio, R.; Moisés, R.-O. Anticonvulsant mechanisms of the ketogenic diet and caloric restriction. Epilepsy Res. 2020, 168, 106499. [Google Scholar] [CrossRef] [PubMed]
- Shaafi, S.; Najmi, S.; Aliasgharpour, H.; Mahmoudi, J.; Sadigh-Etemad, S.; Farhoudi, M.; Baniasadi, N. The efficacy of the ketogenic diet on motor functions in Parkinson’s disease: A rat model. Iran. J. Neurol. 2016, 15, 63–69. [Google Scholar]
- Sun, W.; Wang, Q.; Zhang, R.; Zhang, N. Ketogenic diet attenuates neuroinflammation and induces conversion of M1 microglia to M2 in an EAE model of multiple sclerosis by regulating the NF-κB/NLRP3 pathway and inhibiting HDAC3 and P2X7R activation. Food Funct. 2023, 14, 7247–7269. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Löscher, W.; Rho, J.M. Mechanisms of action of antiseizure drugs and the ketogenic diet. Cold Spring Harb. Perspect. Med. 2016, 6, a022780. [Google Scholar] [CrossRef] [PubMed]
- Yudkoff, M.; Daikhin, Y.; Nissim, I.; Horyn, O.; Lazarow, A.; Luhovyy, B.; Wehrli, S. Response of brain amino acid metabolism to ketosis. Neurochem. Int. 2005, 47, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.R.; Ribeiro, L.C.; Hagenn, M.; Siqueira, L.R.; Araújo, E.; Torres, I.L.S.; Gottfried, C.; Netto, C.A.; Gonçalves, C. Ketogenic diet increases glutathione peroxidase activity in rat hippocampus. Neurochem. Res. 2003, 28, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, H.; Andlauer, T.F.M.; Korn, T.; Mühlau, M.; Henningsen, P.; Hemmer, B.; Ploner, M. Fatigue, depression, and pain in multiple sclerosis: How neuroinflammation translates into dysfunctional reward processing and anhedonic symptoms. Mult. Scler. J. 2022, 28, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Yang, X.; An, L.; Gao, B.; Liu, X.; Liu, S. Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res. 2009, 1286, 25–31. [Google Scholar] [CrossRef]
- Masino, S.A.; Li, T.; Theofilas, P.; Sandau, U.S.; Ruskin, D.N.; Fredholm, B.B.; Geiger, J.D.; Aronica, E.; Boison, D. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J. Clin. Investig. 2011, 121, 2679–2683. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Gille, C.; Göktas, Ö.; Reißhauer, A.; Neuhaus, J.; Weylandt, K.-H.; Guschin, A.; Bock, M. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front. Microbiol. 2017, 8, 1141. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, D.S.; Tamai, T.K.; Bains, R.S.; Villanueva, S.A.M.; Luk, S.H.C.; Dell’angelica, D.; Block, G.D.; Ghiani, C.A.; Colwell, C.S. Dietary ketosis improves circadian dysfunction as well as motor symptoms in the BACHD mouse model of Huntington’s disease. Front. Nutr. 2022, 9, 1034743. [Google Scholar] [CrossRef]
- Giménez-Cassina, A.; Martínez-François, J.R.; Fisher, J.K.; Szlyk, B.; Polak, K.; Wiwczar, J.; Tanner, G.R.; Lutas, A.; Yellen, G.; Danial, N.N. BAD-dependent regulation of fuel metabolism and KATP channel activity confers resistance to epileptic seizures. Neuron 2012, 74, 719–730. [Google Scholar] [CrossRef]
- Rubio, C.; Rosiles-Abonce, A.; Trejo-Solis, C.; Rubio-Osornio, M.; Mendoza, C.; Custodio, V.; Martinez-Lazcano, J.C.; Gonzalez, E.; Paz, C. Increase Signaling of Wnt/β-Catenin Pathway and Presence of Apoptosis in Cerebellum of Kindled Rats. CNS Neurol. Disord. Drug Targets 2017, 16, 772–780. [Google Scholar] [CrossRef]
- McDaniel, S.S.; Rensing, N.R.; Thio, L.L.; Yamada, K.A.; Wong, M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 2011, 52, e7–e11. [Google Scholar] [CrossRef]
- Michael-Titus, A.T.; Priestley, J.V. Omega-3 fatty acids and traumatic neurological injury: From neuroprotection to neuroplasticity? Trends Neurosci. 2014, 37, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Nolte, R.T.; Wisely, G.B.; Westin, S.; Cobb, J.E.; Lambert, M.H.; Kurokawa, R.; Rosenfeld, M.G.; Willson, T.M.; Glass, C.K.; Milburn, M.V. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ. Nature 1998, 395, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, J.; Liu, H.; Li, F.; Peng, Q.; Liu, C. Effects of ketogenic diet containing medium-chain fatty acids on serum inflammatory factor and mTOR signaling pathway in rats. Chem. Biol. Technol. Agric. 2020, 7, 27. [Google Scholar] [CrossRef]
- Jayasinghe, M.; Prathiraja, O.; Alam Kayani, A.M.; Jena, R.; Caldera, D.; Silva, M.S.; Singhal, M.; Pierre, J. The role of diet and gut microbiome in multiple sclerosis. Cureus 2022, 14, e28975. [Google Scholar] [CrossRef] [PubMed]
- Simeone, T.A.; Simeone, K.A.; Stafstrom, C.E.; Rho, J.M. Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology 2018, 133, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Galindo, J.G.; Vigil-Martínez, A.; Ochoa, E. The role of the ketogenic diet on parkinson’s disease symptoms. In The Ketogenic Diet Reexamined: Myth vs. Reality; Nova Science Publishers: Hauppauge, NY, USA, 2024; pp. 23–39. [Google Scholar] [CrossRef]
- Düking, T.; Spieth, L.; Berghoff, S.A.; Piepkorn, L.; Schmidke, A.M.; Mitkovski, M.; Kannaiyan, N.; Hosang, L.; Scholz, P.; Shaib, A.H.; et al. Ketogenic diet uncovers differential metabolic plasticity of brain cells. Sci. Adv. 2022, 8, eabo7639. [Google Scholar] [CrossRef] [PubMed]
- Chorley, B.N.; Campbell, M.R.; Wang, X.; Karaca, M.; Sambandan, D.; Bangura, F.; Xue, P.; Pi, J.; Kleeberger, S.R.; Bell, D.A. Identification of novel NRF2-regulated genes by ChIP-Seq: Influence on retinoid X receptor alpha. Nucleic Acids Res. 2012, 40, 7416–7429. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Zhou, Y.; Yu, L.; Zhang, L.; Wang, Y. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res. 2018, 145, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, R. [AM-01]: Genetic basis of cerebral amyloidosis. Alzheimer’s Dement. 2005, 1, S1. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, X.; Zhang, H.; Yin, J.; Zhao, P.; Yin, Q.; Wang, Z. Ketogenic diet protects MPTP-induced mouse model of Parkinson’s disease via altering gut microbiota and metabolites. Medcomm 2023, 4, e268. [Google Scholar] [CrossRef] [PubMed]
- Auvin, S. New developments for dietary treatment of epilepsy after a century of history for the ketogenic diet. Brain Commun. 2021, 3, fcab234. [Google Scholar] [CrossRef] [PubMed]
- Höhn, S.; Dozières-Puyravel, B.; Auvin, S. History of dietary treatment from Wilder’s hypothesis to the first open studies in the 1920s. Epilepsy Behav. 2019, 101 Pt A, 106588. [Google Scholar] [CrossRef]
- Peterman, M.G. The ketogenic diet in the treatment of epilepsy: A preliminary report. Am. J. Dis. Child. 1924, 28, 28–33. [Google Scholar] [CrossRef]
- Greene, A.E.; Todorova, M.T.; McGowan, R.; Seyfried, T.N. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia 2001, 42, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Lennox, W.G.; Cobb, S. Epilepsy: From the standpoint of physiology and treatment. Medicine 1928, 7, 105–290. [Google Scholar] [CrossRef]
- Wheless, J.W. History of the ketogenic diet. Epilepsia 2008, 49, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.M.; Kossoff, E.H. Ketosis and the ketogenic diet, 2010: Advances in treating epilepsy and other disorders. Adv. Pediatr. 2010, 57, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Gasior, M.; Rogawski, M.A.; Hartman, A.L. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav. Pharmacol. 2006, 17, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. The ketogenic diet for the treatment of childhood epilepsy: A randomised controlled trial. Lancet Neurol. 2008, 7, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Stafstrom, C.E.; Rho, J.M. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharmacol. 2012, 3, 59. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Ketogenic diet and epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef]
- Yum, M.-S.; Lee, M.; Woo, D.-C.; Kim, D.W.; Ko, T.-S.; Velíšek, L. β-Hydroxybutyrate attenuates NMDA-induced spasms in rats with evidence of neuronal stabilization on MR spectroscopy. Epilepsy Res. 2015, 117, 125–132. [Google Scholar] [CrossRef]
- Weinshenker, D. The contribution of norepinephrine and orexigenic neuropeptides to the anticonvulsant effect of the ketogenic diet. Epilepsia 2008, 49, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, M.; Månsson, J.-E.; Åmark, P. CSF levels of dopamine and serotonin, but not norepinephrine, metabolites are influenced by the ketogenic diet in children with epilepsy. Epilepsy Res. 2012, 99, 132–138. [Google Scholar] [CrossRef]
- Calderón, N.; Betancourt, L.; Hernández, L.; Rada, P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: A microdialysis study. Neurosci. Lett. 2017, 642, 158–162. [Google Scholar] [CrossRef]
- Kawamura, M.; Ruskin, D.N.; Masino, S.A. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J. Neurosci. 2010, 30, 3886–3895. [Google Scholar] [CrossRef]
- Cheng, M.; Li, T.; Hu, E.; Yan, Q.; Li, H.; Wang, Y.; Luo, J.; Tang, T. A novel strategy of integrating network pharmacology and transcriptome reveals antiapoptotic mechanisms of Buyang Huanwu Decoction in treating intracerebral hemorrhage. J. Ethnopharmacol. 2024, 319, 117123. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Berg, J.; Yellen, G. Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. J. Neurosci. 2007, 27, 3618–3625. [Google Scholar] [CrossRef]
- Koene, L.M.C.; van Grondelle, S.E.; Onori, M.P.; Wallaard, I.; Kooijman, N.H.R.M.; van Oort, A.; Schreiber, J.; Elgersma, Y. Effects of antiepileptic drugs in a new TSC/mTOR-dependent epilepsy mouse model. Ann. Clin. Transl. Neurol. 2019, 6, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Warren, E.C.; Dooves, S.; Lugarà, E.; Damstra-Oddy, J.; Schaf, J.; Heine, V.M.; Walker, M.C.; Williams, R.S.B. Decanoic acid inhibits mTORC1 activity independent of glucose and insulin signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 23617–23625. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.G.; Padwad, Y.S.; Singh, D. Anticancer mammalian target of rapamycin (mTOR) signaling pathway inhibitors: Current status, challenges and future prospects in management of epilepsy. CNS Neurol. Disord. Drug Targets 2016, 15, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Marsan, E.; Baulac, S. Review: Mechanistic target of rapamycin (mTOR) pathway, focal cortical dysplasia and epilepsy. Neuropathol. Appl. Neurobiol. 2018, 44, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Milder, J.B.; Liang, L.-P.; Patel, M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol. Dis. 2010, 40, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Zhang, K.; Yang, W.; Li, B. The anticonvulsant effects of ketogenic diet on epileptic seizures and potential mechanisms. Curr. Neuropharmacol. 2018, 16, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Vamecq, J.; Vallée, L.; Lesage, F.; Gressens, P.; Stables, J.P. Antiepileptic popular ketogenic diet: Emerging twists in an ancient story. Prog. Neurobiol. 2005, 75, 1–28. [Google Scholar] [CrossRef]
- Hughes, S.; Foster, R.G.; Peirson, S.N.; Hankins, M.W. Expression and localisation of two-pore domain (K2P) background leak potassium ion channels in the mouse retina. Sci. Rep. 2017, 7, srep46085. [Google Scholar] [CrossRef]
- Barzegar, M.; Afghan, M.; Tarmahi, V.; Behtari, M.; Khamaneh, S.R.; Raeisi, S. Ketogenic diet: Overview, types, and possible anti-seizure mechanisms. Nutr. Neurosci. 2021, 24, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.S.; Kang, S.S.; Kim, D.W.; Kim, Y.H.; Park, C.H.; Han, J.Y.; Cho, G.J.; Choi, W.S. Ketogenic diet increases calbindin-D28k in the hippocampi of male ICR mice with kainic acid seizures. Epilepsy Res. 2005, 65, 153–159. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Campbell, I.; Campbell, H. Mechanisms of insulin resistance, mitochondrial dysfunction and the action of the ketogenic diet in bipolar disorder. Focus on the PI3K/AKT/HIF1-a pathway. Med. Hypotheses 2020, 145, 110299. [Google Scholar] [CrossRef]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterström, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. Npj Biofilms Microbiomes 2019, 5, 5. [Google Scholar] [CrossRef]
- Xie, G.; Zhou, Q.; Qiu, C.-Z.; Dai, W.-K.; Wang, H.-P.; Li, Y.-H.; Liao, J.-X.; Lu, X.-G.; Lin, S.-F.; Ye, J.-H.; et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J. Gastroenterol. 2017, 23, 6164–6171. [Google Scholar] [CrossRef]
- El-Shafie, A.M.; Bahbah, W.A.; El Naby, S.A.A.; Omar, Z.A.; Basma, E.M.; Hegazy, A.A.A.; El Zefzaf, H.M.S. Impact of two ketogenic diet types in refractory childhood epilepsy. Pediatr. Res. 2023, 94, 1978–1989. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, D.A.J.E.; de Kinderen, R.J.A.; Vles, J.S.H.; de Louw, A.J.A.; Aldenkamp, A.P.; Majoie, H.J.M. A randomized controlled trial of the ketogenic diet in refractory childhood epilepsy. Acta Neurol. Scand. 2017, 135, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.R.; Hosseini, S.A.; Zamani, G.R.; Mohammadi, M.; Tavassoli, A.; Badv, R.S.; Heidari, M.; Karimi, P.; Malamiri, R.A. The efficacy of the ketogenic diet in infants and young children with refractory epilepsies using a formula-based powder. Acta Neurol. Belg. 2017, 117, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dabla, S.; Kaushik, J.S. Modified Atkins diet vs. Ketogenic diet in the management of children with epileptic spasms refractory to first line treatment: An open labelled, randomized controlled trial. Indian J. Pediatr. 2023, 90, 969–973. [Google Scholar] [CrossRef]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 2009, 50, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Chen, L.; She, D.; Chung, Y.; Ge, L.; Han, L. Ketogenic diet for epilepsy: An overview of systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022, 76, 1234–1244. [Google Scholar] [CrossRef]
- Sourbron, J.; Klinkenberg, S.; van Kuijk, S.M.J.; Lagae, L.; Lambrechts, D.; Braakman, H.M.H.; Majoie, M. Ketogenic diet for the treatment of pediatric epilepsy: Review and meta-analysis. Child’s Nerv. Syst. 2020, 36, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, F.; Collotta, A.D.; Di Nora, A.; Costanza, G.; Ruggieri, M.; Falsaperla, R. Ketogenic diet in pediatric seizures: A randomized controlled trial review and meta-analysis. Expert Rev. Neurother. 2022, 22, 169–177. [Google Scholar] [CrossRef]
- Mustafa, M.S.; Shafique, M.A.; Aheed, B.; Ashraf, F.; Ali, S.M.S.; Iqbal, M.F.; Haseeb, A. The impact of ketogenic diet on drug-resistant epilepsy in children: A comprehensive review and meta-analysis. Ir. J. Med. Sci. 2024, 193, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Díez-Arroyo, C.; García-García, M.; Soto-Méndez, M.J.; Molina-Montes, E.; Gil-Campos, M.; Gil, Á.; Gutiérrez-Jimeno, M.; Hernández-Ruiz, Á. Effect of the ketogenic diet as a treatment for refractory epilepsy in children and adolescents: A systematic review of reviews. Nutr. Rev. 2024, 82, 487–502. [Google Scholar] [CrossRef] [PubMed]
- van Berkel, A.A.; Ijff, D.M.; Verkuyl, J.M. Cognitive benefits of the ketogenic diet in patients with epilepsy: A systematic overview. Epilepsy Behav. 2018, 87, 69–77. [Google Scholar] [CrossRef]
- Neri, L.d.C.L.; Guglielmetti, M.; Fiorini, S.; Pasca, L.; Zanaboni, M.P.; de Giorgis, V.; Tagliabue, A.; Ferraris, C. Adherence to ketogenic dietary therapies in epilepsy: A systematic review of literature. Nutr. Res. 2024, 126, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Desli, E.; Spilioti, M.; Evangeliou, A.; Styllas, F.; Magkos, F.; Dalamaga, M. The efficacy and safety of ketogenic diets in drug-resistant epilepsy in children and adolescents: A systematic review of randomized controlled trials. Curr. Nutr. Rep. 2022, 11, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Cochrane. Cochrane Database of Systematic Reviews; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Rezaei, S.; Abdurahman, A.A.; Saghazadeh, A.; Badv, R.S.; Mahmoudi, M. Short-term and long-term efficacy of classical ketogenic diet and modified Atkins diet in children and adolescents with epilepsy: A systematic review and meta-analysis. Nutr. Neurosci. 2017, 22, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Swerdlow, R.H. Amyloid precursor protein processing and bioenergetics. Brain Res. Bull. 2017, 133, 71–79. [Google Scholar] [CrossRef]
- Takahashi, R.H.; Nagao, T.; Gouras, G.K. Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol. Int. 2017, 67, 185–193. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Brain aging, Alzheimer’s disease, and mitochondria. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M. Insulin resistance and neurodegeneration: Progress towards the development of new therapeutics for Alzheimer’s disease. Drugs 2017, 77, 47–65. [Google Scholar] [CrossRef]
- Koppel, S.J.; Swerdlow, R.H. Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem. Int. 2018, 117, 114–125. [Google Scholar] [CrossRef]
- Castellano, C.-A.; Nugent, S.; Paquet, N.; Tremblay, S.; Bocti, C.; Lacombe, G.; Imbeault, H.; Turcotte, É.; Fulop, T.; Cunnane, S.C. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer’s disease dementia. J. Alzheimer’s Dis. 2015, 43, 1343–1353. [Google Scholar] [CrossRef]
- Van der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr. Metab. 2005, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Bergman, C.; Lee, J.-H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, F.; Graziano, M.; Hagnäs, M.; Frittita, L.; Tumminia, A. Influence of the Mediterranean and ketogenic diets on cognitive status and decline: A narrative review. Nutrients 2020, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Campion, D.; Dumanchin, C.; Hannequin, D.; Dubois, B.; Belliard, S.; Puel, M.; Thomas-Anterion, C.; Michon, A.; Martin, C.; Charbonnier, F.; et al. Early-onset autosomal dominant Alzheimer disease: Prevalence, genetic heterogeneity, and mutation spectrum. Am. J. Hum. Genet. 1999, 65, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.T. Parkinson’s disease and parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Sheard, J.M.; Ash, S.; Mellick, G.D.; A Silburn, P.; Kerr, G.K. Improved nutritional status is related to improved quality of life in Parkinson’s disease. BMC Neurol. 2014, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Summer, S.S.; Sullivan, P.G.; Duker, A.P.; Isaacson, R.S.; Espay, A.J. Nutritional ketosis for mild cognitive impairment in Parkinson’s disease: A controlled pilot trial. Clin. Park. Relat. Disord. 2019, 1, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tang, X.; Cheng, Z.; Dong, Q.; Ruan, G. The anti-inflammatory effect of preventive intervention with ketogenic diet mediated by the histone acetylation of mGluR5 promotor region in rat Parkinson’s disease model: A dual-tracer PET study. Park. Dis. 2022, 2022, 3506213. [Google Scholar] [CrossRef] [PubMed]
- Dong-Chen, X.; Yong, C.; Yang, X.; Chen-Yu, S.; Li-Hua, P. Signaling pathways in Parkinson’s disease: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 73. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Takeshima, T.; Mori, N.; Nakashima, K.; Clarke, K.; Veech, R.L. d-β-Hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 5440–5444. [Google Scholar] [CrossRef]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.-C.; Yan, S.-D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-P.; Wang, J.-F.; Xue, W.-J.; Liu, H.-M.; Liu, B.-R.; Zeng, Y.-L.; Li, S.-N.; Huang, B.-X.; Lv, Q.-K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of diet and nutritional supplements in Parkinson’s disease progression. Oxidative Med. Cell. Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef]
- Koyuncu, H.; Fidan, V.; Toktas, H.; Binay, O.; Celik, H. Effect of ketogenic diet versus regular diet on voice quality of patients with Parkinson’s disease. Acta Neurol. Belg. 2021, 121, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- VanItallie, T.B.; Nonas, C.; Di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S.B. Treatment of Parkinson disease with diet-induced hyperketonemia: A feasibility study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Tidman, M.M.; White, D.; White, T. Effects of an low carbohydrate/healthy fat/ketogenic diet on biomarkers of health and symptoms, anxiety and depression in Parkinson’s disease: A pilot study. Neurodegener. Dis. Manag. 2022, 12, 57–66. [Google Scholar] [CrossRef]
- Tidman, M.M.; White, D.R.; A White, T. Impact of a keto diet on symptoms of Parkinson’s disease, biomarkers, depression, anxiety and quality of life: A longitudinal study. Neurodegener. Dis. Manag. 2024, 14, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; Ruppar, T.M. Ketogenic therapies in Parkinson’s disease, Alzheimer’s disease, and mild cognitive impairment: An integrative review. Appl. Nurs. Res. 2023, 74, 151745. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Tousinas, G.; Balodimou, C.; Anastasilakis, D.A.; Gkiouras, K.; Dardiotis, E.; Evangeliou, A.E.; Bogdanos, D.P.; Goulis, D.G. Ketogenic therapy for Parkinson’s disease: A systematic review and synthesis without meta-analysis of animal and human trials. Maturitas 2022, 163, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.G.; Alves-Silva, J.; de Souza, J.M.; Real, A.L.; Doria, J.G.; Vieira, E.L.; Gomes, G.F.; de Oliveira, A.C.; Miranda, A.S.; Ribeiro, F.M. Metabotropic glutamate receptor 5 ablation accelerates age-related neurodegeneration and neuroinflammation. Neurochem. Int. 2019, 126, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Kuter, K.Z.; Olech, Ł.; Głowacka, U.; Paleczna, M. Increased beta-hydroxybutyrate level is not sufficient for the neuroprotective effect of long-term ketogenic diet in an animal model of early Parkinson’s disease. Exploration of Brain and Liver Energy Metabolism Markers. Int. J. Mol. Sci. 2021, 22, 7556. [Google Scholar] [CrossRef]
- Di Majo, D.; Cacciabaudo, F.; Accardi, G.; Gambino, G.; Giglia, G.; Ferraro, G.; Candore, G.; Sardo, P. Ketogenic and modified Mediterranean diet as a tool to counteract neuroinflammation in multiple sclerosis: Nutritional suggestions. Nutrients 2022, 14, 2384. [Google Scholar] [CrossRef]
- Tafti, D.; Ehsan, M.; Xixis, K.L. Multiple sclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Availale online: https://www.ncbi.nlm.nih.gov/pubmed/29763024 (accessed on 7 January 2025).
- Esposito, S.; Bonavita, S.; Sparaco, M.; Gallo, A.; Tedeschi, G. The role of diet in multiple sclerosis: A review. Nutr. Neurosci. 2018, 21, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.L.; Ribot, J.; Galmés, S.; Serra, F.; Palou, A. Carotenoids and carotenoid conversion products in adipose tissue biology and obesity: Pre-clinical and human studies. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158676. [Google Scholar] [CrossRef]
- Giammanco, M.; Aiello, S.; Casuccio, A.; La Guardia, M.; Cicero, L.; Puleio, R.; Vazzana, I.; Tomasello, G.; Cassata, G.; Leto, G.; et al. Effects of 3,5-diiodo-L-thyronine on the liver of high fat diet fed rats. J. Biol. Res. 2016, 89, 5667. [Google Scholar] [CrossRef]
- Swindell, W.R. Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genom. 2009, 10, 585. [Google Scholar] [CrossRef]
- Jung, K.J.; Lee, E.K.; Kim, J.Y.; Zou, Y.; Sung, B.; Heo, H.S.; Kim, M.K.; Lee, J.; Kim, N.D.; Yu, B.P.; et al. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm. Res. 2009, 58, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Linden, J. Molecular approach to adenosine receptors: Receptor-mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 775–787. [Google Scholar] [CrossRef]
- Ngamsri, K.-C.; Wagner, R.; Vollmer, I.; Stark, S.; Reutershan, J. Adenosine receptor A1 regulates polymorphonuclear cell trafficking and microvascular permeability in lipopolysaccharide-induced lung injury. J. Immunol. 2010, 185, 4374–4384. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Cenci, L.; Pompei, P.; Sahin, N.; Bianco, A.; Neri, M.; Caprio, M.; Moro, T. Effects of two months of very low carbohydrate ketogenic diet on body composition, muscle strength, muscle area, and blood parameters in competitive natural body builders. Nutrients 2021, 13, 374. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Deyama, S.; Fogaça, M.V. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 2021, 53, 126–139. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef]
- Genzer, Y.; Dadon, M.; Burg, C.; Chapnik, N.; Froy, O. Effect of dietary fat and the circadian clock on the expression of brain-derived neurotrophic factor (BDNF). Mol. Cell. Endocrinol. 2016, 430, 49–55. [Google Scholar] [CrossRef]
- Veer, A.V.; Du, Y.; Fischer, T.Z.; Boetig, D.R.; Wood, M.R.; Dreyfus, C.F. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J. Neurosci. Res. 2009, 87, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Geyer, E.; Flach, A.-C.; Jung, K.; Gold, R.; Flügel, A.; Linker, R.A.; Lühder, F. Central nervous system rather than immune cell-derived BDNF mediates axonal protective effects early in autoimmune demyelination. Acta Neuropathol. 2012, 123, 247–258. [Google Scholar] [CrossRef]
- Hu, E.; Li, T.; Li, Z.; Su, H.; Yan, Q.; Wang, L.; Li, H.; Zhang, W.; Tang, T.; Wang, Y. Metabolomics reveals the effects of hydroxysafflor yellow A on neurogenesis and axon regeneration after experimental traumatic brain injury. Pharm. Biol. 2023, 61, 1054–1064. [Google Scholar] [CrossRef]
- De Santi, L.; Annunziata, P.; Sessa, E.; Bramanti, P. Brain-derived neurotrophic factor and TrkB receptor in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Neurol. Sci. 2009, 287, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zyla-Jackson, K.; Walton, D.A.; Plafker, K.S.; Kovats, S.; Georgescu, C.; Brush, R.S.; Tytanic, M.; Agbaga, M.-P.; Plafker, S.M. Dietary protection against the visual and motor deficits induced by experimental autoimmune encephalomyelitis. Front. Neurol. 2023, 14, 1113954. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Hao, J.; Liu, R.; Turner, G.; Shi, F.-D.; Rho, J.M. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS ONE 2012, 7, e35476. [Google Scholar] [CrossRef]
- Sun, W.; Wen, M.; Liu, M.; Wang, Q.; Liu, Q.; Li, L.; Siebert, H.-C.; Loers, G.; Zhang, R.; Zhang, N. Effect of β-hydroxybutyrate on behavioral alterations, molecular and morphological changes in CNS of multiple sclerosis mouse model. Front. Aging Neurosci. 2022, 14, 1075161. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, N.; Zhang, R.; Jin, L.; Petridis, A.K.; Loers, G.; Zheng, X.; Wang, Z.; Siebert, H.-C. Cuprizone-induced demyelination in mouse hippocampus is alleviated by ketogenic diet. J. Agric. Food Chem. 2020, 68, 11215–11228. [Google Scholar] [CrossRef] [PubMed]
- Nathan, J.; Kale, D.K.; Naik, V.; Thakker, F.; Bailur, S. Dietary therapy in secondary progressive multiple sclerosis: A case report. Cureus 2019, 11, e5341. [Google Scholar] [CrossRef]
- Brenton, J.N.; Banwell, B.; Bergqvist, A.C.; Lehner-Gulotta, D.; Gampper, L.; Leytham, E.; Coleman, R.; Goldman, M.D. Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e565. [Google Scholar] [CrossRef] [PubMed]
- Brenton, J.N.; Lehner-Gulotta, D.; Woolbright, E.; Banwell, B.; Bergqvist, A.G.C.; Chen, S.; Coleman, R.; Conaway, M.; Goldman, M.D. Phase II study of ketogenic diets in relapsing multiple sclerosis: Safety, tolerability and potential clinical benefits. J. Neurol. Neurosurg. Psychiatry 2022, 93, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Titcomb, T.J.; Bisht, B.; Rubenstein, L.M.; Louison, R.; Wahls, T.L. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with Multiple Sclerosis compared to a modified Paleolithic diet: A waitlist-controlled, randomized pilot study. J. Am. Coll. Nutr. 2021, 40, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Perlman, J.; Wetmore, E.; Lehner-Gulotta, D.; Banwell, B.; Bergqvist, A.C.; Coleman, R.; Chen, S.; Conaway, M.; Goldman, M.D.; Morse, A.M.; et al. Impact of a ketogenic diet on sleep quality in people with relapsing multiple sclerosis. Sleep Med. 2024, 122, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Merlino, G.; Garbo, R.; Bello, S.D.; Del Negro, I.; Lamon, E.; Filippi, F.; Bernardini, A.; Lorenzut, S.; Ceccarelli, L.; Cella, A.; et al. Ketogenic diet may improve sleep quality and daytime somnolence in patients affected by multiple sclerosis. Results of an exploratory study. Sleep Med. 2023, 112, 181–187. [Google Scholar] [CrossRef]
- Wetmore, E.; Lehner-Gulotta, D.; Florenzo, B.; Banwell, B.; Bergqvist, A.C.; Coleman, R.; Conaway, M.; Goldman, M.D.; Brenton, J.N. Ketogenic diet in relapsing multiple sclerosis: Patient perceptions, post-trial diet adherence & outcomes. Clin. Nutr. 2023, 42, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Ortí, J.E.d.l.R.; Cuerda-Ballester, M.; Sanchis-Sanchis, C.E.; Romance, J.M.L.; Navarro-Illana, E.; Pardo, M.P.G. Exploring the impact of ketogenic diet on multiple sclerosis: Obesity, anxiety, depression, and the glutamate system. Front. Nutr. 2023, 10, 1227431. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch. Eur. J. Physiol. 2010, 460, 525–542. [Google Scholar] [CrossRef]
- Korn, T.; Magnus, T.; Jung, S. Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: A mechanism mediated by tumor necrosis factor-α. FASEB J. 2005, 19, 1878–1880. [Google Scholar] [CrossRef]
- Vonsattel, J.P.G.; DiFiglia, M. Huntington disease. J. Neuropathol. Exp. Neurol. 1998, 57, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, S. Huntington’s disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a007476. [Google Scholar] [CrossRef]
- Jakel, R.J.; Maragos, W.F. Neuronal cell death in Huntington’s disease: A potential role for dopamine. Trends Neurosci. 2000, 23, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Gash, M.T.; Mann, V.M.; Javoy-Agid, F.; Cooper, J.M.; Schapira, A.H.V. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann. Neurol. 1996, 39, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Björkqvist, M.; Fex, M.; Renström, E.; Wierup, N.; Petersén, Å.; Gil, J.; Bacos, K.; Popovic, N.; Li, J.-Y.; Sundler, F.; et al. The R6/2 transgenic mouse model of Huntington’s disease develops diabetes due to deficient β-cell mass and exocytosis. Hum. Mol. Genet. 2005, 14, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Hurlbert, M.S.; Zhou, W.; Wasmeier, C.; Kaddis, F.G.; Hutton, J.C.; Freed, C.R. Mice transgenic for an expanded CAG repeat in the Huntington’s disease gene develop diabetes. Diabetes 1999, 48, 649–651. [Google Scholar] [CrossRef]
- Hult, S.; Soylu, R.; Björklund, T.; Belgardt, B.F.; Mauer, J.; Brüning, J.C.; Kirik, D.; Petersén, Å. Mutant huntingtin causes metabolic imbalance by disruption of hypothalamic neurocircuits. Cell Metab. 2011, 13, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Lalić, N.M.; Marić, J.; Svetel, M.; Jotić, A.; Stefanova, E.; Lalić, K.; Dragašević, N.; Miličić, T.; Lukić, L.; Kostić, V.S. Glucose homeostasis in Huntington disease. Arch. Neurol. 2008, 65, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Valenza, M.; Cattaneo, E. Emerging roles for cholesterol in Huntington’s disease. Trends Neurosci. 2011, 34, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Turley, S.D. Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004, 45, 1375–1397. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Tran, C.; Hwang, L.; Deng, G.; Jung, M.E.; Faull, K.F.; Levine, M.S.; Cepeda, C. Partial amelioration of peripheral and central symptoms of Huntington’s disease via modulation of lipid metabolism. J. Huntington’s Dis. 2016, 5, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.L.; McManus, E.J.; Brinkhuis, M.; Romero-Ferrando, B. Time-restricted ketogenic diet in Huntington’s disease: A case study. Front. Behav. Neurosci. 2022, 16, 931636. [Google Scholar] [CrossRef] [PubMed]
- Boron, W.F.; Boulpaep, E.L. (Eds.) Fisiología Médica, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017; p. 1312. Available online: https://books.google.com/books?hl=es&lr=&id=1J_HDgAAQBAJ&oi=fnd&pg=PP1&dq=Boron,+W.F.,+%26+Boulpaep,+E.L.+(2017).+&ots=R_8txr09sg&sig=AJeyhr9PWbu9qGudFTUVqbc73WE (accessed on 7 January 2025).
- Bordone, M.P.; Salman, M.M.; Titus, H.E.; Amini, E.; Andersen, J.V.; Chakraborti, B.; Diuba, A.V.; Dubouskaya, T.G.; Ehrke, E.; de Freitas, A.E.; et al. The energetic brain—A review from students to students. J. Neurochem. 2019, 151, 139–165. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Maes, M.; Berk, M.; Carvalho, A.F.; Puri, B.K. Nutritional ketosis as an intervention to relieve astrogliosis: Possible therapeutic applications in the treatment of neurodegenerative and neuroprogressive disorders. Eur. Psychiatry 2020, 63, e8. [Google Scholar] [CrossRef]
- Lan, Z.; Tan, F.; He, J.; Liu, J.; Lu, M.; Hu, Z.; Zhuo, Y.; Liu, J.; Tang, X.; Jiang, Z.; et al. Curcumin-primed olfactory mucosa-derived mesenchymal stem cells mitigate cerebral ischemia/reperfusion injury-induced neuronal PANoptosis by modulating microglial polarization. Phytomedicine 2024, 129, 155635. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Castillo, E.; Frias, E.S.; Swanson, R.A. Bioenergetic regulation of microglia. Glia 2018, 66, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Lu, Y.; Huang, M.-J.; Yang, Y.-Y.; Xing, H.-Y.; Liu, X.-X.; Zhou, M.-W. Ketogenic diet-mediated steroid metabolism reprogramming improves the immune microenvironment and myelin growth in spinal cord injury rats according to gene and co-expression network analyses. Aging 2021, 13, 12973–12995. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Muhammad, S.; Khan, M.A.; Chen, H.; Ridder, D.A.; Müller-Fielitz, H.; Pokorná, B.; Vollbrandt, T.; Stölting, I.; Nadrowitz, R.; et al. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014, 5, 3944. [Google Scholar] [CrossRef]

| Epilepsy | Alzheimer’s Disease (AD) | Parkinson’s Disease (PD) | Multiple Sclerosis (MS) | Huntington’s Disease (HD) | |
|---|---|---|---|---|---|
| Mechanism of action | Slows down energy availability, stabilizes, and reduces neuronal excitability [25]. | Compensates for glucose deficiency by providing alternative energy with KBs [11]. | Eluding energy failure and Complex I activity [26]. | Modulates inflammatory regulators such as Nfkbia, TNFα, IL-6, COX-2, iNOS, VCAM-1, and ICAM-1 [27]. | Compensates for glucose deficiency by providing alternative energy with KBs [11]. |
| Excitatory–inhibitory regulation of key neurotransmitters such as glutamate and GABA [28]. | Decreased levels of Aβ and Tau [29]. | Reducing free radicals by improving mitochondrial respiratory efficiency by increasing NADH [30]. | Anti-inflammatory effects by adenosine reducing systemic inflammation by modulating LPS and reducing CXCL2/3 [31]. | Antioxidant effects due to increased glutathione and glutathione peroxidase activity [32]. | |
| Increase in ATP, activating KATP, leading to hyperpolarization of the neuronal membrane [33]. | Increased angiogenesis and capillary density by the M2 phenotype of microglia secreting IL-10 and VEGF [34]. | Antioxidant effects due to increased glutathione and glutathione peroxidase activity [32]. | Beta-hydroxybutyrate induces NF-κβ upregulation, activating histone acetyltransferase p300/EP300 and BDNF synthesis [35]. | Regulation of inflammation and stress genes such as Il6ra, Nos1, and Npy [36]. | |
| Modifications of the Bad protein, Wnt/β-catenin signaling pathway, mTOR, and Nrf2 [37,38,39]. | Influence on antioxidant defense and mitochondrial function genes such as SOD2, PINK1, and DJ-1 [34]. | Increase in neurotrophins such as BDNF, NT-3, GDNF, and chaperones [23]. | BDNF enables synaptic plasticity, neuronal function, neurodevelopment, glucose homeostasis, and neuroprotection through myelin integrity [31]. | Expression of the sleep-regulated gene Homer1 [36]. | |
| Activates K2P channels, leading to a continuous flow of potassium ions across the cell membrane, causing hyperpolarization [40]. | Regulation of inflammatory genes in microglia, such as IL-1β and TNF-α, helping to reduce neuroinflammation [41]. | Modify cell signaling pathways, such as the mTOR pathway [42]. | BDNF upregulation allows remyelination of MS-induced lesions [43]. | Increase in neurotrophins such as BDNF, NT-3, GDNF, and chaperones [23]. | |
| Allows inhibition of caspase 3 and regulation of the NLRP3 inflammasome [44]. | Regulation of the NLRP3 inflammasome [41]. | Decreased GABAergic activity from striatal neurons to GPi and SNpr [45]. | Increased CD4+, CD8+, and CD45+ immune cells [46]. | Allows inhibition of caspase 3 and regulation of the NLRP3 inflammasome [44]. | |
| Increases fatty acid oxidation by increasing PUFA levels [40]. | Beta-hydroxybutyrate activates PPARγ, thereby inhibiting nuclear NF-κβ expression. It also upregulates Nrf2 [47]. | Increases activity of pro-apoptotic factors and prevention of inflammatory mediators such as interleukins and TNFα [21]. | Decrease in pro-inflammatory cytokines such as IL-1β and IL-6, and increase in anti-inflammatory cytokines such as TGF-β [27]. | Changes in gut microbiota [36]. | |
| Changes in gut microbiota [48]. | Regulation of genes that cause the production of excessive amounts of Aβ, such as APP, PSEN1, and PSEN2 [49]. | Changes in gut microbiota [50]. | Changes in gut microbiota [35]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio, C.; López-Landa, A.; Romo-Parra, H.; Rubio-Osornio, M. Impact of the Ketogenic Diet on Neurological Diseases: A Review. Life 2025, 15, 71. https://doi.org/10.3390/life15010071
Rubio C, López-Landa A, Romo-Parra H, Rubio-Osornio M. Impact of the Ketogenic Diet on Neurological Diseases: A Review. Life. 2025; 15(1):71. https://doi.org/10.3390/life15010071
Chicago/Turabian StyleRubio, Carmen, Alejandro López-Landa, Hector Romo-Parra, and Moisés Rubio-Osornio. 2025. "Impact of the Ketogenic Diet on Neurological Diseases: A Review" Life 15, no. 1: 71. https://doi.org/10.3390/life15010071
APA StyleRubio, C., López-Landa, A., Romo-Parra, H., & Rubio-Osornio, M. (2025). Impact of the Ketogenic Diet on Neurological Diseases: A Review. Life, 15(1), 71. https://doi.org/10.3390/life15010071








