Abstract
Carl Woese’s thesis of cellular evolution emphasized that the last universal common/cellular ancestor (LUCA) must have evolved by drawing from “global inventions”. Yet, existing theories regarding the origin(s) of LUCA have mostly centered upon scenarios that LUCA had evolved mostly independently. In an earlier paper, we advanced a new theory regarding the origin(s) of LUCA that extends Woese’s original insights. Our theory centers upon the possibility that different vesicles and protocells can merge with and acquire each other as a form of variation, selection, and retention, driven by wet-and-dry cycles and other similar cyclical processes. In this paper, we use computer simulation to show that under a variety of simulated conditions, LUCA can indeed be produced by our proposed processes. We hope that our study can stimulate laboratory testing of some key hypotheses that vesicles’ absorption, acquisition, and merger has indeed been a central force in driving the evolution of LUCA.
1. Introduction
The coming of the Last Universal Common (Cellular) Ancestor (LUCA) was a major transition in the making of the biotic world [1,2,3,4] (Although LUCA has been conventionally taken to be the “Last Universal Common Ancestor”, it is now generally accepted that LUCA must have been a fairly complete cell [3,4]). Recently, Tang advanced a new theory regarding the origin of LUCA [5]. His core thesis is that vesicle absorption, acquisition, and fusion via breaking and repacking, proto-endocytosis, proto-endosymbiosis, and other similar processes, driven by “wet-and-dry” cycles or other similar thermochemical cycles within Darwin’s “warm little pond(s)” [6,7,8,9], has been a central mechanism that propelled the origin(s) of the First Universal Cellular Ancestors (FUCAs) and then the evolution of LUCA from FUCAs. Hence, our position critically differs from Prosdocimi and his colleagues [10,11,12], who also used the abbreviation FUCA for “the First Universal Common Ancestor”. Prosdocimi et al. insist that FUCA is not a cell but an RNA-dependent peptide synthesis machinery or peptidyl transferase center (PTC). We believe it is more reasonable to assume that FUCAs were already proto-cells (vesicles), with a more or less functional cell membrane [13,14]. Indeed, according to our theory [5], only if FUCA is a proto-cell can LUCA emerge, partly because vesicles are necessary for concentration, crowding, and extensive molecular interaction.
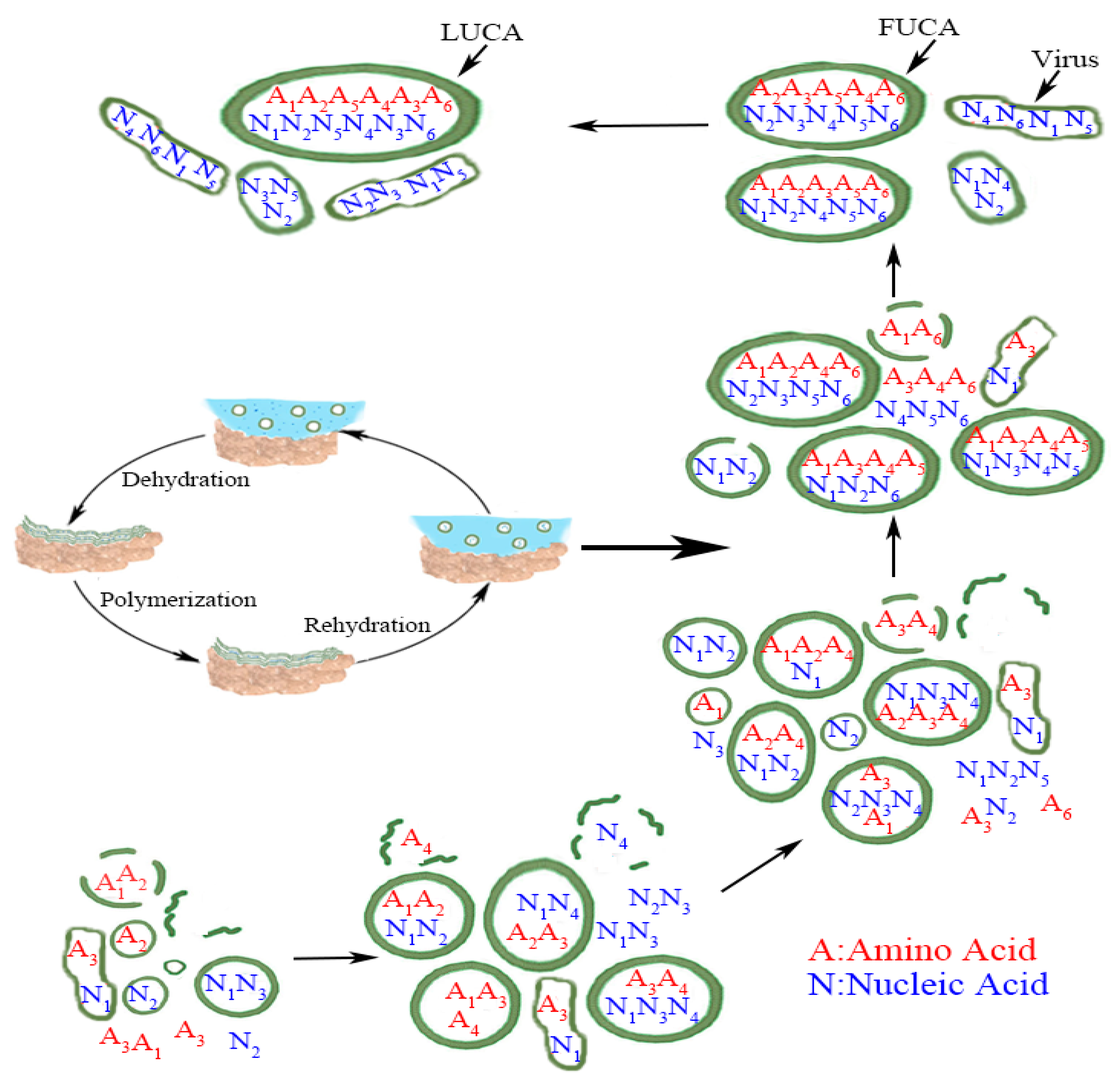
Tang further hypothesized that the evolution of LUCA came in two major stages: the evolution of FUCAs, and then evolution of FUCAs to LUCA [5]. What is common to both stages has been that both involve the same process of absorption, acquisition, and merger by these protocells (or vesicles), and this process had been the key behind Carl Woese’s thesis that LUCA must have evolved by drawing from “global inventions” [15,16,17]. The whole evolutionary process from simple vesicles to FUCAs and then LUCA is summarized in Figure 1 below (reproduced from [5]).
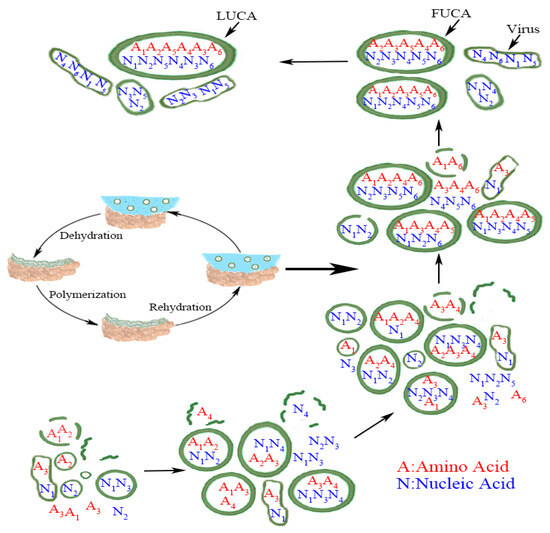
Figure 1.
From vesicles to LUCA (reproduced from [5] with permission.) Numbers in subscript denote different amino acids and nucleic acids. The exact matching between amino acid and nucleic acid within LUCA, in a metaphorical sense, implies that the standard genetic code (SGC) had evolved most completely by the time of LUCA. The less than exact matching amino acid and nucleic acid within FUCA and vesicles before FUCA denotes the evolutionary path of SGC from a rudimentary form to a mature form in LUCA. The wet-and-dry cycle part within the figure is adapted from [7] with their kind permission. Figure 1 retains virus to make the whole picture complete, but we do not simulate the evolution of virus here. Credit: author.
Tang has provided some chemical and biological evidence that supports his hypothesis [5]. Ultimately, however, chemical and biological evidence can only show that the hypothesis is possible but not necessarily viable for the making of LUCA because recreating LUCA from scratch in a lab is highly unlikely.
We therefore turn to the next best thing: to “visualize” the viability of the proposed hypothesis with computer simulation, following similar efforts with different focuses [18,19,20,21]. More specifically, we aim to show that merger and acquisition by vesicles and other similar processes can indeed produce FUCAs, and then inevitably, LUCA.
Before proceeding further, three caveats are in order.
First, we are mostly interested in simulating the hypothesis that FUCAs and LUCA had most likely evolved by drawing from “global innovation” via vesicles’ merger and acquisition [5,15,16,17,22]. As such, we do not simulate the chemical synthesis of polymers from nucleotides or amino acids [23,24], the evolution of autocatalytic cycles, the evolution of an RNA self-replication system [25,26,27], the formation of an RNA–peptide world [28,29,30], the forming of vesicles [18,19,20], the division of vesicles driven by absorption of ingredients from the environment or merging with other vesicles [9], the coupling of an RNA replication system to vesicles [31], or the coupling of cellularity to metabolism [21,32,33], partly because they have been performed earlier, either via simulation or via wet experiments. Also, simulating the whole process from chemical evolution to the coming of LUCA will be a daunting, if not an impossible, task, without necessarily shedding much light on the central mechanism we highlight here (for instance, Yin et al. [31] simulated how an RNA replication system can evolve and then spread, and their “cellular” evolution is really about vesicles engulfing an RNA replication system). Thus, their simulation does not deal with the evolution of LUCA, or even the evolution of FUCAs. Our simulation (based on our theory) subsumes vesicles engulfing ingredients such as amino acids, nucleotides, peptides, and RNAs. Most critically, Yin et al. [31] based their simulation on the “hydrothermal vent” scenario, which many others and we believe to be rather unrealistic [5,6,7,8]). We therefore focus on the late stage of the evolution of LUCA, somewhat similar to Takagi et al. [21] and Nunes Palmeira et al. [32].
Second, we concur with several earlier studies that LUCA has at least three hallmarks: (1) a fully functioning membrane; (2) a fully evolved standard genetic code (SGC); (3) about one hundred proteins and several hundred genes [34,35,36,37,38,39] (most likely, these genes had been linked into a chromosome [40]). More recent estimation has put the number of proteins within LUCA at 2600, comparable to modern prokaryotes [41]. Here, we use the more conservative estimates. In our simulation, we focus on the coming together of one hundred peptides (plus several hundred genes) and the coming of the SGC while assuming that LUCA has a fully functioning membrane.
Third, we do not engage in the ongoing debates on more specific puzzles of the origins of life (e.g., which comes first? RNA world, RNA–peptide world, metabolism, membrane, etc.), mostly because these puzzles cannot possibly be resolved any time soon (for an excellent survey of the debates, see [42]).
2. Materials and Methods
Computer simulations are performed with the GAMA simulation platform, version 1.8.1 (https://gama-platform.org/ (accessed on 20 October 2022)), on local servers hosted by our center. All computer codes and experimental data are available online (https://github.com/xymbgm/2023-05-Tang-and-Gao-Simulation-of-from-FUCA-to-LUCA-codes (accessed on 1 October 2024)).
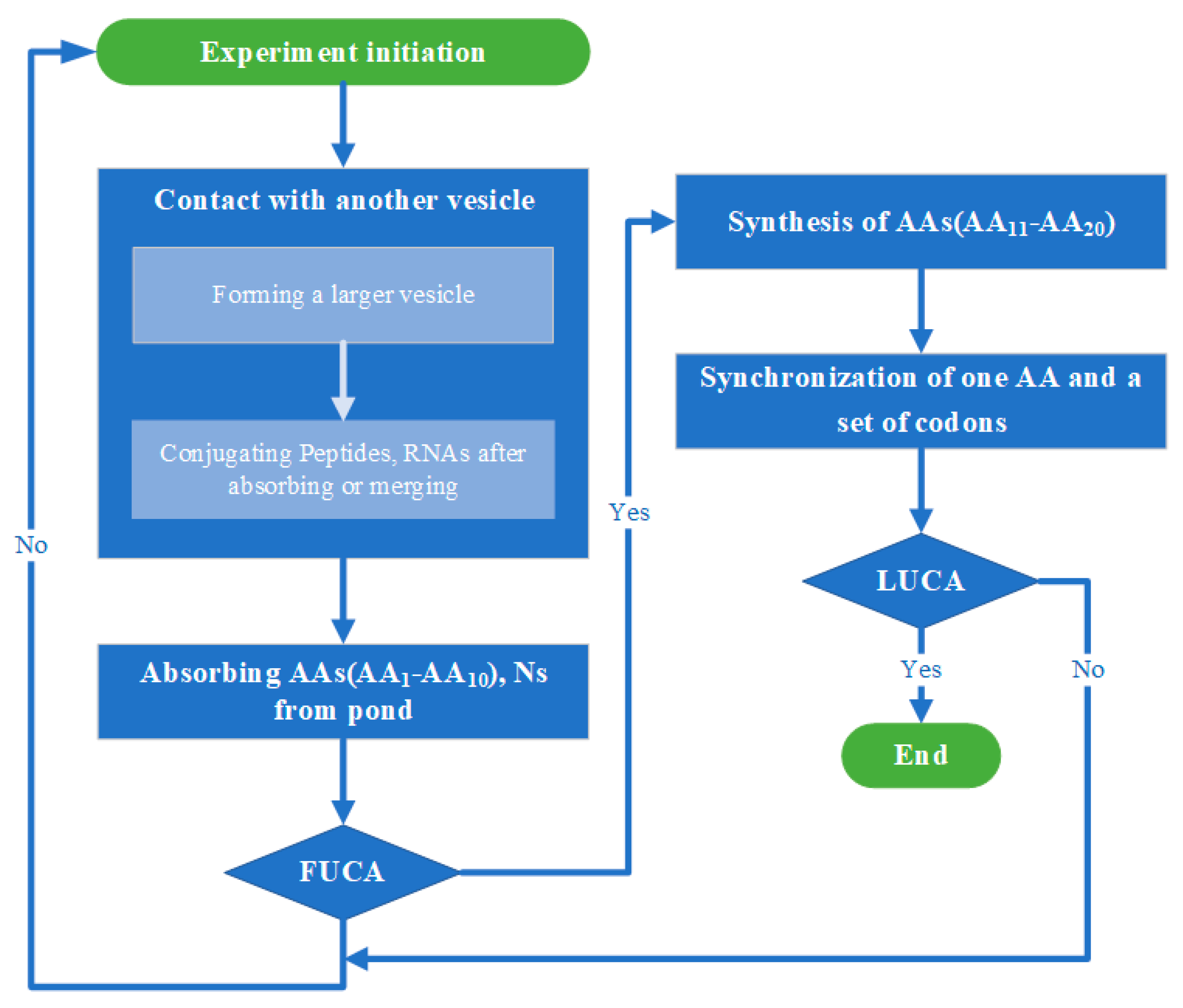
Each of the simulations described below (i.e., whether the two stages of evolution from simple vesicles to FUCAs and then LUCA are simulated separately or together) starts with one single “warm little pond” (see below for details). All simulations follow a similar flow (see Figure 2 for the flowchart), with one “cycle” in a simulation run being one wet phase or one dry phase, alternated. Table 1 summarizes the parameters in the simulation.
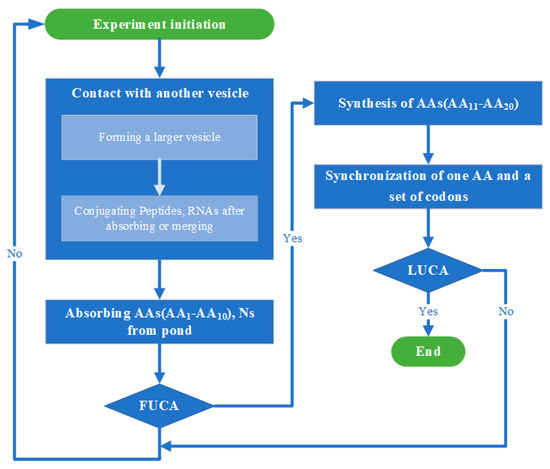
Figure 2.
Flowchart of simulation. Credit: author.

Table 1.
Parameters in the simulations.

Table 4.
(A). Rules for joining peptides after absorbing or merging. (B) Rules for joining RNAs after absorbing or merging.

Table 3.
Probabilities of absorbing another vesicle vs. being absorbed (the first number for a vesicle in rows; the second for a vesicle in columns).

Table 2.
Types of vesicles (V1 to V6).
Table 1.
Parameters in the simulations.
| Parameters | Explanation | Value |
|---|---|---|
| tickCount | count of cycles within a simulation | 1 cycle = 1 wet phase or one dry phase, alternated |
| currentVolume | units of volume of a pond | dry phase: (50, 80), wet phase: (80, 100); initiation: 100 |
| numPeptide | number of peptide | see Table 2 for specific values |
| numRNA | number of RNA | see Table 2 for specific values |
| numAA | number of AAs in a peptide | see Table 2 for specific values |
| numNBase | number of nucleotides in an RNA | 3 × numAA |
| V1InitNum | initial number of V1 | 3000 |
| V2InitNum | initial number of V2 | 2000 |
| totalnum | external supply of V1 and V2 in every cycle | dry phase: (2000, 3000), wet phase: (4000, 5000) |
| pContact | probability of contact with another vesicle | (10−5, 10−6) × (100/currentVolume)2 |
| mergeP | probability of absorbing another vesicle | See Table 3 for details |
| conjugateP | probability of joining peptides or RNAs after absorbing or merging | See Table 4 for details |
| fitnessScore | jointly determined by the number of AAs assigned (NA) and the total number of peptides (NP) within the vesicle | FS = NA × NP |
| pSurvival | vesicles’ probability of survival | pSurvival = ln(fitnessScore)/10 |
| rndIndexAA | type of AAs within a vesicle | V1–V5: AA1–AA10, V6: AA1–AA20 |
| pABS | probability of absorbing more AAs and NNs from the pond | 5 × 10−3 |
| pSynchronize | probability of assigning one AA to one set of codons within a cycle | 0.999 |
Following Klein et al. [18], every entity (i.e., amino acid, lipid, polypeptide, nucleic acid, or vesicle) is normalized to a ball-like entity, with a radius of 1 and a mass of 1. Vesicles can absorb amino acids, nucleotides, lipids, and other useful ingredients or components, which are assumed to have an unlimited supply within each pond. The ratio between peptides and RNAs within a vesicle is set to be 2.5–3.5 (e.g., if a vesicle has 2–4 peptides, then it has 5–10 RNAs). Moreover, a vesicle must contain at least 2–3 peptides and 5–10 RNAs in order to qualify as a vesicle (i.e., V1 is the minimum threshold of being a vesicle). Finally, different vesicles have different numbers of AAs being assigned a proper set of codons.
There are six types of vesicles in our simulation (Table 2), and FUCAs are protocells with at least 41–50 peptides and 120–160 RNAs. In other words, only type V6 vesicles are counted as FUCAs. FUCAs, however, have yet to possess a fully evolved SGC [5].
LUCA is a fully functional (proto-)cell with at least one hundred proteins (peptides) and several hundred RNAs (as genes). Moreover, LUCA has a fully evolved SGC, symbolized by the tight coupling of nucleotides (within RNAs) and amino acids (within peptides). In other words, only FUCAs that have evolved the tight linkage of RNA and peptide can become LUCA. Most likely, RNA and proteins co-evolved with each other [43].
Vesicles’ encounters and interactions are driven by wet-and-dry or other similar cycles [7,9,44,45], which is captured by the increased-and-decreased volume of the “warm little pond”. The pond’s changing volume in turn drives changes in the probability of vesicles coming into contact with each other. When in contact, a vesicle can acquire another vesicle according to the probabilities dictated in Table 2.
When two vesicles are merged or one absorbs the other, the new vesicle gains all the peptides and RNAs from its two ancestral vesicles. For simplicity, we assume that peptides and RNAs within the vesicles are fully functional but are agonistic about their specific functions. Moreover, the peptides and RNAs within the new vesicle can be joined to form a longer molecule (i.e., two peptides forming a longer peptide, or two RNAs forming a longer RNA). The probabilities of joining different peptides and RNAs are set in Table 4A and Table 4B, respectively.
Consistent with the thesis that there were two phases of SGC evolution [38,46,47,48,49,50,51,52], we dictate that a vesicle or protocell must first get the 10 early AAs assigned with their respective codons, and only after can a vesicle or protocell get the 10 late AAs assigned with their codons (The ten early AAs are: Gly, Ala, Ser, Asp, Glu, Val, Leu, Ile, Pro, and Thr. The ten late AAs are Phe, Tyr, Arg, His, Trp, Asn, Gln, Lys, Cys, and Met). Consistent with the co-evolutionary theory of codons, proteins, and cellular functions, the more AAs are assigned to their codons, the more functions a protocell will gain from more complex peptides or proteins. Accordingly, a vesicle or protocell should gain a bit of fitness with each “optimal assignment” of AA to its proper codons.
The net fitness score (FS) of a vesicle is jointly determined by the number of AAs assigned (NA) and the total number of peptides (NP) within the vesicle. That is, FS = NA × NP.
For vesicles that do not go through the processes of merger and acquisition during the wet-and-dry cycles, those vesicles with higher fitness scores will have a greater probability of surviving in the pond than those with lower fitness scores. The probability of survival () is determined by the simple equation below:
Finally, when a FUCA has assigned all the 20 AAs (i.e., ) and has more than 100 peptides (i.e., ) and 300 RNAs, it becomes a LUCA, and the simulation ends.
2.1. The First Stage: The Origin(s) of FUCAs
For the first stage, we start all simulations with only V1 and V2 vesicles.
In the initial state, there were anywhere between 2000 and 3000 V1 and V2 vesicles within a pond of 100 units of volume. For each “cycle” within a simulation, the pond also produces or gains more V1 and V2 vesicles. During the wet phase, anywhere between 4000 and 5000 new V1 and V2 vesicles will be added to the pond, whereas during the dry phase, anywhere between 2000 and 3000 new V1 and V2 vesicles will be added. Vesicles can also absorb other biochemical components and ingredients (e.g., amino acids, nucleotides) and thus become larger vesicles. Biochemical components and ingredients such as amino acids, nucleotides, and lipids are exogenously generated and added to the pond with unlimited supply to speed up the simulations.
Within each cycle, a vesicle has a certain probability of coming in contact with another vesicle. The probability of being in contact is regulated by the total volume of the pond, dictated by the following function: , with X being the unit of volume of the whole pond at a given cycle. We further assume that in the dry phase, the total volume of the pond decreases from 100 units of volume to 50–80 units of volume, thus increasing the probability of contact by vesicles. The dry phase also breaks some vesicles. Reversely, in the wet phase, the total volume of the pond increases back to 80–100 units of volume, thus decreasing the rate of contact by vesicles but also allowing new vesicles to form.
When two vesicles come in contact with each other, they can merge with or acquire each other with certain probabilities specified in Table 3. And when smaller vesicles (e.g., V1, V2, and V3) merge with or acquire each other, they form larger vesicles (e.g., V3, V4, and V5).
2.2. The Second Stage: From FUCAs to LUCA
It is now widely accepted that LUCAs possessed about several hundred genes, about half of which were in RNA metabolism and translation, with the SGC fully in place. LUCAs also possessed about 140 proteins or domains [36,37,38,48,52,53,54]. We thus assume that LUCA must have about 100–120 proteins and 300–360 RNAs, with each protein being more than 50 AAs and each RNA being more than 150 nucleotide bases long. We also require that LUCA have assigned all twenty AAs to the full genetic code.
For this second stage, we assume that only FUCAs (i.e., V6) can perform the task of assigning the ten late AAs to their proper codons. We thus start with different FUCAs (i.e., V6) that already possess different numbers of peptides and RNAs, and the simulation only selects for LUCA as FUCAs that can eventually produce a complete SGC by assigning codons to AAs, one codon to one AA for each wet-and-dry cycle (or two “cycles” in our simulation). Again, vesicles can merge and acquire other vesicles following the same rules as set in Table 3 and Table 4 in the first stage.
For the second stage, we also assume the following rules:
- -
- For simplicity, we skip the evolution of the stop codon. Hence, when all of the 20 AAs have been assigned to their codons, the full SGC has evolved, and we can consider that LUCA now exists. Accordingly, there may be a few LUCAs and they have different genomes, but they all have the same SGC. Very critically, according to our theory, SGC could have only evolved by drawing from “global inventions” via the merger and acquisition of vesicles [5,15,16,17,22] (in this sense, we agree with Herron’s [55] stand that SGC should not be classified as a distinct “major transition” because the evolution of SGC has been a (gradual) process [2]. Notably, while Maynard Smith and Szathmáry [2] identified SGC and protocell (FUCA) as two distinct transitions, Szathmáry later argued that they might have co-evolved in prokaryotic cells. Our thesis holds that they had evolved mostly together, most likely by LUCA [56]).
- -
- Taking cues from the thesis that SGC was a “frozen accident” [50,52,57,58,59,60], we assume that there might have been 4 to 6 nucleotides available for making into the SGC before the SGC was finally fixed. Thus, the total combinations of 20 AAs with the possible codons are anywhere between and , or 1280 to 4320. Thus, for each simulation, the exact number of possible combinations to be assigned is a random number anywhere from 1280 to 4320. For a whole wet-and-dry cycle, FUCAs can only assign one AA to one set of codons, with a fixed probability of 0.999. We are keenly aware that biologically and mathematically, there is a positive feedback mechanism in the evolution of SGC: once one AA has been assigned a set of codons, the remaining AAs will have a smaller pool of codons to be assigned. Hence, an earlier assigning of one AA to a set of codons will accelerate the next cycle of assigning the remaining AAs and codons. As a result, if the probability of assigning the first AA with a set of codons is 10−3, the probability of assigning the next AA with the remaining sets of codons becomes: , with n denoting the number of AAs already assigned. So, for the first AA to be assigned, n is 0, and for the second AA, n is 1, and so on. The ratio is used to magnify the cumulative impact of previous rounds of assigning upon the remaining synchronizations. We initially hoped to implement such dynamics in the simulation. However, due to the fact that many vesicles will die in each cycle (as predicted by our theory [5]), implementing such dynamics requires significant computational resources. We therefore set the probability of successful codon assignment to a fixed probability of 0.999 to speed up the process. Most likely, decreasing the probability will merely prolong the process without fundamentally changing the overall results.
- -
- For simplicity, we assume that when two vesicles with different AAs already assigned to their specific codons merge with each other, the merged vesicle obtains all the codons, and hence the evolution of the universal codon accelerates. For example, vesicle-1 has A1, A2, A3, A4, and A5 assigned, whereas vesicle-2 has A1, A2, A3, A4, and A6, then the merged vesicle of the two vesicles will have A1, A2, A3, A4, A5, and A6 assigned. This is consistent with the dynamics underscored by Vetsigian et al. [61] that SGC had most likely evolved via “collective evolution” by drawing from “global innovations” with “horizontal gene transfer” (HGT). Indeed, according to Tang [5], absorption, acquisition, and merger by vesicles entail extensive “horizontal biomolecule transfer” (HBMT) rather than merely HGT: HBMT thus subsuming HGT because HBMT entails exchange and retention of biological ingredients other than genetic materials. Of course, if two vesicles have the same set of AAs assigned (i.e., when both vesicle-1 and vesicle-2 have A1, A2, A3, A4, and A5 assigned), the newly merged vesicle of the two vesicles does not gain a new AA assigned. But the new merged vesicle can still gain peptides and RNAs, thus also increasing its fitness score according to FS = NA × NP.
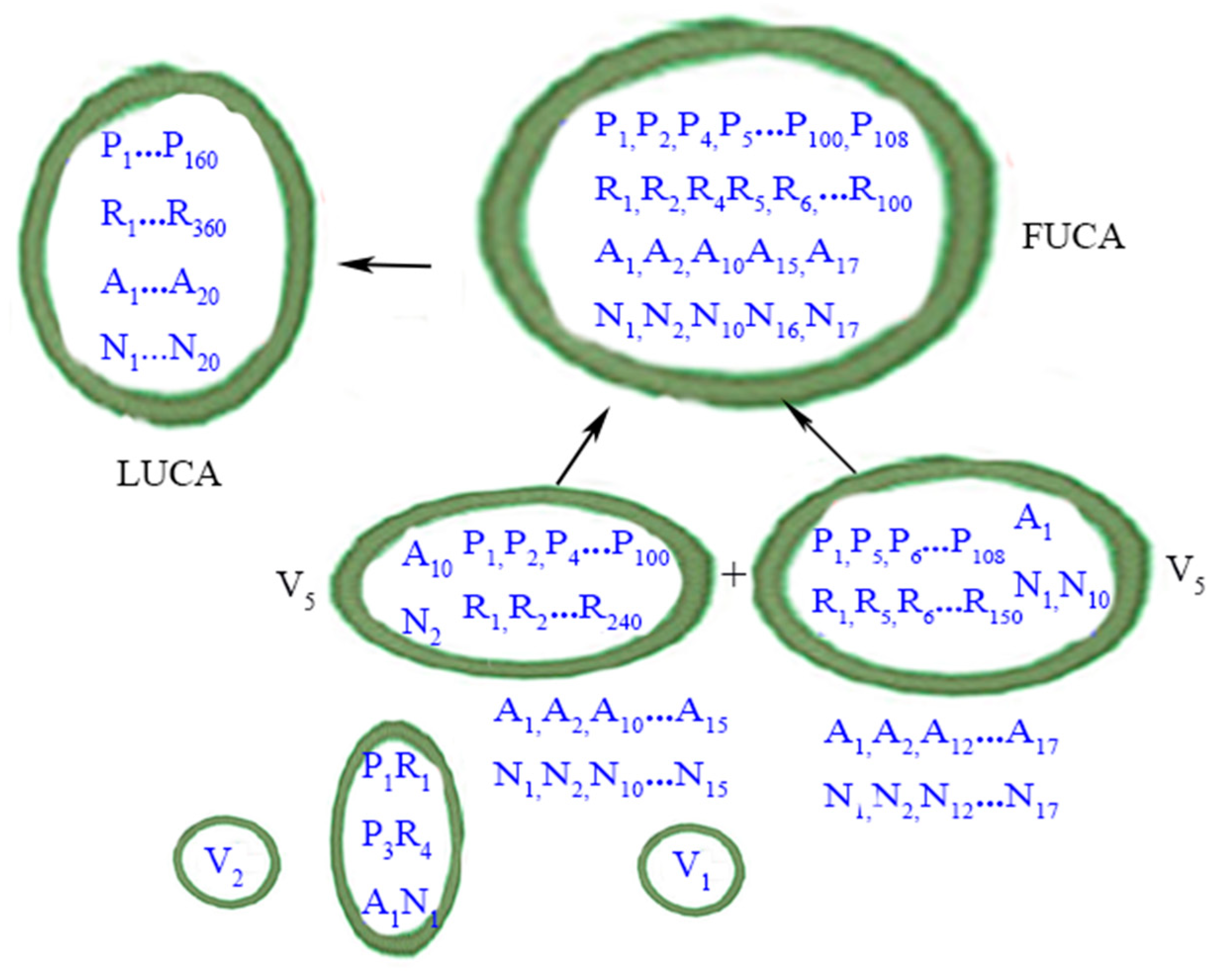
Figure 3 summarizes the evolutionary phase of FUCAs to LUCA.
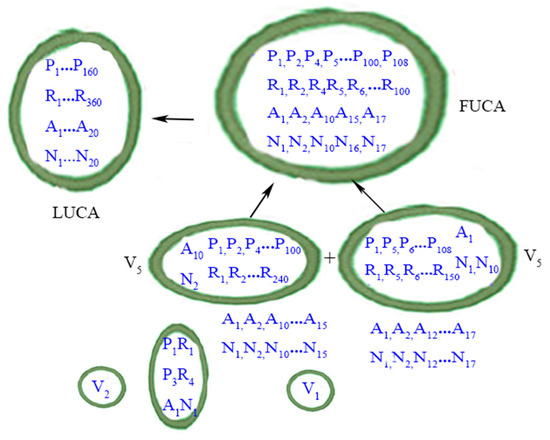
Figure 3.
From FUCAs to LUCA. P: peptide; R: RNAs. A: amino acid; N: nucleotide (as codons). Protocells or vesicles are in closed circles. LUCA is depicted according to the definition in the main text. The perfect matching of amino acids (A) and nucleic acids (N) within LUCA, in a metaphorical sense, implies that the standard genetic code (SGC) had evolved most completely by the time of LUCA. The less than exact matching of amino acids and nucleic acids within the one FUCA and the two V5 vesicles denotes the evolutionary path of SGC from a rudimentary form to a mature form in LUCA. Contents within the two smaller vesicles (i.e., V1 and V2) are now shown. The wet-and-dry cycle part is omitted here. Credit: author.
2.3. The Two Stages Together
To test our theory in a complete setting, we also simulate the two stages together. Again, vesicles can merge and absorb each other. Moreover, all vesicles (or protocells) can absorb and integrate amino acids, short peptides, nucleotides, and short RNA molecules into their existing peptides (or proteins) and RNA molecules according to the probabilities dictated in Table 1, Table 2 and Table 3. We also simulate different volumes within different ponds.
The purpose of these simulations is to show that LUCA can indeed emerge from simple vesicles (i.e., V1 and V2) as long as vesicles are allowed to merge and absorb each other in addition to absorbing and integrating amino acids, short peptides, nucleotides, and short RNA molecules into their existing peptides (or proteins) and RNA molecules.
Again, because small vesicles inevitably perish or are merged and absorbed by other larger vesicles, if vesicles can only be generated de novo and in situ with each pond, simulation time will be extremely long within the constraint of computational resources. We therefore also add exogenously generated V1 and V2 vesicles to each pond to speed up the process. More specifically, 2000 to 3000 V1 or V2 are added in the dry phase, while 4000–5000 V1 or V2 are added in the wet phase.
3. Results
We now present the simulation results.
3.1. The First Stage: The Origin(s) of FUCAs
In various settings, including different volumes of the “warm little pond” and different starting numbers of V1 and V2 vesicles, with about 60–70 cycles, the first FUCA (i.e., V6) will be produced. Eventually, with about 100 to 200 cycles, anywhere from 36 to 284 FUCAs will be produced (Table 5). Numbers of vesicles alive (V1–V6) are the number of vesicles alive within the system when simulations are halted. Snapshots of four specific simulations of the first stage are shown in Figure S1A–D in the Supplementary Materials.

Table 5.
The first stage: evolution of FUCAs under different settings.
3.2. The Second Stage: The Origin of LUCA
With different numbers of FUCAs (i.e., V6 vesicles), eventually a LUCA will appear, even though different FUCAs become the LUCA in different simulations in different time frames (Table 6). Hence, as long as each FUCA is allowed to assign codons to amino acids in each cycle, LUCA is an inevitable evolutionary outcome (see also Figure S2 in the Supplementary Materials for visualizations of two actual synchronization simulations.).

Table 6.
From FUCAs to LUCA (Stage 2): starting with 300 FUCAs.
3.3. The Two Stages Together
With different numbers of V1 and V2 initially, LUCA can emerge with different number of cycles and with different numbers of other protocells or vesicles within a pond. Table 7 summarizes four such simulations. Again, snapshots of four specific simulations of this set of simulations are shown in Figure S3A–D in the Supplementary Materials.

Table 7.
Summary of four simulations with the two stages together.
3.4. Control Simulations: The Two Stages Together
We have also run a set of control simulations with the two stages together. In these simulations, vesicles cannot merge with each other and hence, acquire biomolecules from each other while all initial conditions, parameters, and mechanisms are identical to the four sets of simulations reported in Table 7. We also run these simulations with more cycles than are needed to produce LUCA in our (positive) simulations.
As shown in Table 8, in these control simulations, no FUCA and hence LUCA ever emerges even after more than 1320 to 1440 cycles, which are more than 200 cycles more than the highest number of cycles (1120) needed to produce LUCA in Table 7. These results show that merger and acquisition by vesicles may well be a key, if not indispensable, mechanism that drives the evolution of LUCA, as Tang [5,22] has argued. Screenshots of four specific control simulations are shown in Figure S4A–D in the Supplementary Materials. As shown in these figures, when vesicles cannot merge with and acquire each other, no larger vesicles (i.e., V3 to V6) evolve in the system, and only V1 and V2 vesicles exist even with more cycles lapsed.

Table 8.
Summary of four control simulations with the two stages pooled together.
4. Discussion
According to Tang [5], vesicles’ absorption, acquisition, and fusion via breaking and repacking, proto-endocytosis, proto-endosymbiosis, and other similar processes had been a central and powerful force in the pre-Darwinian evolution before LUCA, long before eukaryogenesis [62,63,64,65], because these processes are processes of variation, selection, and retention.
Absorption, acquisition, and merger are processes of variation because they produce different compartmentalization and hence, different crowding, combination, and coevolution of biomolecules within vesicles. Absorption, acquisition, and merger are also processes of selection and retention because via these processes, some molecules will be retained and integrated within vesicles while some will be excluded from vesicles, and some vesicles will no longer exist. Moreover, absorption, acquisition, and merger entail extensive HBMT rather than merely HGT; HBMT thus subsumes HGT. Indeed, only with HBMT could pre-Darwinian evolution have drawn from “global inventions” and overcome the seemingly insurmountable hurdle of bringing “the overwhelming amount of novelty needed to bring modern cells into existence” [66]. This process of producing LUCA via vesicles’ absorption, acquisition, and merger is far more plausible than the scenario that LUCA has to evolve from a single FUCA de novo and in situ [5].
In this paper, we have shown that the central hypothesis advanced by Tang [5] is indeed viable, at least in computer simulation. If so, we can expect that some of the chemical and biological hypotheses advanced by Tang [5] are also plausible. We therefore hope that our study can stimulate laboratory testing of these hypotheses to show that vesicles’ absorption, acquisition, and merger has indeed been a central force in driving the evolution of LUCA.
Finally, our computer simulation lends more support to the thesis that FUCAs and LUCA had most likely evolved from “fluctuating volcanic hot spring pools” (or Darwin’s “warm little ponds”) on land [6,7,44,67,68,69] rather than from alkaline hydrothermal vents in the ocean [70,71,72]. Most critically, terrestrial hot spring pools allow the wet-and-dry cycles and hence can drive the breakup, repackaging, absorption, merger, and acquisition of vesicles whereas hydrothermal vents do not.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life15010075/s1, Figure S1. Snapshots of four simulations for the evolution of FUCAs (Stage 1); Figure S2. Stage 2: Visualization of the Process of Synchronizing the SGC by two FUCAs; Figure S3. Snapshots, four simulations of the two stages together; Figure S4. Snapshots of four specific control simulations.
Author Contributions
S.T. conceptualized and designed the study. M.G. performed the experiment. S.T. and M.G. analyzed the results together. The authors co-wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author. All computer codes and experimental data are available online. https://github.com/xymbgm/2023-05-Tang-and-Gao-Simulation-of-from-FUCA-to-LUCA-codes (accessed on 1 October 2024).
Acknowledgments
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Woese, C.R.; Fox, G.E. The Concept of Cellular Evolution. J. Mol. Evol. 1977, 10, 1–6. [Google Scholar] [CrossRef]
- Maynard Smith, J.; Szathmáry, E. The Major Transitions in Evolution; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Koonin, E.V. Carl Woese’s Vision of Cellular Evolution and the Domains of Life. RNA Biol. 2014, 11, 197–204. [Google Scholar] [CrossRef]
- Koonin, E.V. The Origin of Cellular Life. Antonie Van Leeuwenhoek 2014, 106, 27–41. [Google Scholar] [CrossRef]
- Tang, S. The Origin(s) of Cell(s): Pre-Darwinian Evolution from FUCAs to LUCA. J. Mol. Evol. 2021, 89, 427–447. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of First Cells at Terrestrial, Anoxic Geothermal Fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef] [PubMed]
- Damer, B.; Deamer, D. Coupled Phases and Combinatorial Selection in Fluctuating Hydrothermal Pools: A Scenario to Guide Experimental Approaches to the Origin of Cellular Life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef] [PubMed]
- Higgs, P.G. The Effect of Limited Diffusion and Wet-Dry Cycling on Reversible Polymerization Reactions: Implications for Prebiotic Synthesis of Nucleic Acids. Life 2016, 6, 24. [Google Scholar] [CrossRef]
- Zhu, T.F.; Adamala, K.; Zhang, N.; Szostak, J.W. Photochemically Driven Redox Chemistry Induces Protocell Membrane Pearling and Division. Proc. Natl. Acad. Sci. USA 2012, 109, 9828–9832. [Google Scholar] [CrossRef] [PubMed]
- Prosdocimi, F.; José, M.V.; de Farias, S.T. The First Universal Common Ancestor (FUCA) as the Earliest Ancestor of LUCA’s (Last UCA) Lineage. In Evolution, Origin of Life, Concepts and Methods; Pontarotti, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 43–54. [Google Scholar]
- Prosdocimi, F.; de Farias, S.T. Origin of Life: Drawing the Big Picture. Prog. Biophys. Mol. Biol. 2023, 180–181, 28–36. [Google Scholar] [CrossRef]
- Prosdocimi, F.; de Farias, S.T. Major Evolutionary Transitions Before Cells: A Journey from Molecules to Organisms. Prog. Biophys. Mol. Biol. 2024, 191, 11–24. [Google Scholar] [CrossRef]
- Lopez, A.; Fiore, M. Investigating Prebiotic Protocells for a Comprehensive Understanding of the Origins of Life: A Prebiotic Systems Chemistry Perspective. Life 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.J.; Goldman, A.D. The Very Early Evolution of Protein Translocation Across Membranes. PLoS Comput. Biol. 2021. [CrossRef]
- Woese, C.R. The Universal Ancestor. Proc. Natl. Acad. Sci. USA 1998, 95, 6854–6859. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R. Interpreting the Universal Phylogenetic Tree. Proc. Natl. Acad. Sci. USA 2000, 97, 8392–8396. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R. On the Evolution of Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8742–8747. [Google Scholar] [CrossRef]
- Klein, A.; Bock, M.; Alt, W. Simple Mechanisms of Early Life-Simulation Model on the Origin of Semi-Cells. Biosystems 2017, 151, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.L.; Lancet, D.; Zidovetzki, R. Replication of Simulated Prebiotic Amphiphilic Vesicles in a Finite Environment Exhibits Complex Behavior That Includes High Progeny Variability and Competition. Astrobiology 2018, 18, 419–430. [Google Scholar] [CrossRef]
- Lancet, D.; Zidovetzki, R.; Markovitch, O. Systems Protobiology: Origin of Life in Lipid Catalytic Networks. J. R. Soc. Interface 2018, 15, 20180159. [Google Scholar] [CrossRef]
- Takagi, Y.A.; Nguyen, D.H.; Wexler, T.B.; Goldman, A.D. The Coevolution of Cellularity and Metabolism Following the Origin of Life. J. Mol. Evol. 2020, 88, 598–617. [Google Scholar] [CrossRef]
- Tang, S. Pre-Darwinian Evolution Before LUCA. Biol. Theory 2020, 15, 175–179. [Google Scholar] [CrossRef]
- Ross, D.S.; Deamer, D. Dry/Wet Cycling and the Thermodynamics and Kinetics of Prebiotic Polymer Synthesis. Life 2016, 6, 28. [Google Scholar] [CrossRef]
- Hargrave, M.; Spencer, S.K.; Deamer, D.W. Computational Models of Polymer Synthesis Driven by Dehydration/Rehydration Cycles: Repurination in Simulated Hydrothermal Fields. J. Mol. Evol. 2018, 86, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yu, C.; Zhang, W.; Hu, J. Nucleotide Synthetase Ribozymes May Have Emerged First in the RNA World. RNA 2007, 13, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.T.; Hu, J.M. Computer Simulation on the Cooperation of Functional Molecules During the Early Stages of Evolution. PLoS ONE 2012, 7, e35454. [Google Scholar] [CrossRef]
- Kim, Y.E.; Higgs, P.G. Co-operation Between Polymerases and Nucleotide Synthetases in the RNA World. PLoS Comput. Biol. 2016, 12, e1005161. [Google Scholar] [CrossRef]
- Müller, F.; Escobar, L.; Xu, F.; Węgrzyn, E.; Nainytė, M.; Amatov, T.; Chan, C.; Pichler, A.; Carell, T. A Prebiotically Plausible Scenario of an RNA-Peptide World. Nature 2022, 605, 279–284. [Google Scholar] [CrossRef]
- Singer, J.N.; Müller, F.M.; Węgrzyn, E.; Hölzl, C.; Hurmiz, H.; Liu, C.; Escobar, L.; Carell, T. Loading of Amino Acids onto RNA in a Putative RNA-Peptide World. Angew. Chem. Int. Ed. 2023, 62, e202302360. [Google Scholar] [CrossRef]
- Radakovic, A.; Lewicka, A.; Todisco, M.; Aitken, H.R.M.; Weiss, Z.; Kim, S.; Bannan, A.; Piccirilli, J.A.; Szostak, J.W. A Potential Role for RNA Aminoacylation Prior to Its Role in Peptide Synthesis. Proc. Natl. Acad. Sci. USA 2024, 121, e2410206121. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Chen, Y.; Yu, C.; Ma, W. From Molecular to Cellular Form: Modeling the First Major Transition During the Arising of Life. BMC Evol. Biol. 2019, 19, 84. [Google Scholar] [CrossRef]
- Nunes Palmeira, R.; Colnaghi, M.; Harrison, S.A.; Pomiankowski, A.; Lane, N. The Limits of Metabolic Heredity in Protocells. Proc. R. Soc. B 2022, 289, 20221469. [Google Scholar] [CrossRef]
- Szathmáry, E. Coevolution of Metabolic Networks and Membranes: The Scenario of Progressive Sequestration. Philos. Trans. R. Soc. B 2007, 362, 1781–1787. [Google Scholar] [CrossRef]
- Charlebois, R.L.; Doolittle, W.F. Computing Prokaryotic Gene Ubiquity: Rescuing the Core from Extinction. Genome Res. 2004, 14, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.K.; Kelley, S.T.; Spiegelman, G.B.; Pace, N.R. The Genetic Core of the Universal Ancestor. Genome Res. 2003, 13, 407–412. [Google Scholar] [CrossRef]
- Koonin, E.V. Comparative Genomics, Minimal Gene-Sets and the Last Universal Common Ancestor. Nat. Rev. Microbiol. 2003, 1, 127–136. [Google Scholar] [CrossRef]
- Ranea, J.A.; Sillero, A.; Thornton, J.M.; Orengo, C.A. Protein Superfamily Evolution and the Last Universal Common Ancestor. J. Mol. Evol. 2006, 63, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.I.; Koonin, E.V. On the Origin of the Translation System and the Genetic Code in the RNA World by Means of Natural Selection, Exaptation, and Subfunctionalization. Biol. Direct 2007, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.D.; Bernhard, T.M.; Dolzhenko, E.; Landweber, L.F. LUCApedia: A Database for the Study of Ancient Life. Nucleic Acids Res. 2013, 41, D1079–D1082. [Google Scholar] [CrossRef]
- Maynard Smith, J.; Szathmáry, E. The Evolution of Chromosome I. Selection for Linkage. J. Theor. Biol. 1993, 164, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Moody, E.R.R.; Álvarez-Carretero, S.; Mahendrarajah, T.A.; Clark, J.W.; Betts, H.C.; Dombrowski, N.; Szánthó, L.L.; Boyle, R.A.; Daines, S.; Chen, X.; et al. The Nature of the Last Universal Common Ancestor and Its Impact on the Early Earth System. Nat. Ecol. Evol. 2024, 8, 1654–1666. [Google Scholar] [CrossRef]
- Jheeta, S.; Chatzitheodoridis, E. Origin of Life. In Conflicting Models for the Origin of Life; Smoukov, S.K., Seckbach, J., Gordon, R., Eds.; Wiley: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Kovacs, N.A.; Petrov, A.S.; Lanier, K.A.; Williams, L.D. Frozen in Time: The History of Proteins. Mol. Biol. Evol. 2017, 34, 1252–1260. [Google Scholar] [CrossRef]
- Damer, B.; Deamer, D. The Hot Spring Hypothesis for an Origin of Life. Astrobiology 2020, 20, 429–452. [Google Scholar] [CrossRef]
- Deamer, D. Assembling Life; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Wong, J.T. A Co-Evolution Theory of the Genetic Code. Proc. Natl. Acad. Sci. USA 1975, 72, 1909–1912. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.T. Coevolution Theory of the Genetic Code at Age Thirty. Bioessays 2005, 27, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Francis, B.R. Evolution of the Genetic Code by Incorporation of Amino Acids That Improved or Changed Protein Function. J. Mol. Evol. 2013, 77, 134–158. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Higgs, P.G. Pathways of Genetic Code Evolution in Ancient and Modern Organisms. J. Mol. Evol. 2015, 80, 229–243. [Google Scholar] [CrossRef]
- Koonin, E.V. Frozen Accident Pushing 50: Stereochemistry, Expansion, and Chance in the Evolution of the Genetic Code. Life 2017, 7, 22. [Google Scholar] [CrossRef]
- Yarus, M. The Genetic Code and RNA-Amino Acid Affinities. Life 2017, 7, 13. [Google Scholar] [CrossRef]
- Yarus, M. Evolution of the Standard Genetic Code. J. Mol. Evol. 2021, 89, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, B.G.; Fenner, T.I.; Galperin, M.Y.; Koonin, E.V. Algorithms for Computing Parsimonious Evolutionary Scenarios for Genome Evolution, the Last Universal Common Ancestor, and Dominance of Horizontal Gene Transfer in the Evolution of Prokaryotes. BMC Evol. Biol. 2003, 3, 2. [Google Scholar] [CrossRef]
- Koonin, E.V.; Novozhilov, A.S. Origin and Evolution of the Universal Genetic Code. Annu. Rev. Genet. 2017, 51, 45–62. [Google Scholar] [CrossRef]
- Herron, M.D. What Are the Major Transitions? Biol. Philos. 2021, 36, 2. [Google Scholar] [CrossRef]
- Szathmáry, E. Toward Major Evolutionary Transitions Theory 2.0. Proc. Natl. Acad. Sci. USA 2015, 112, 10104–10111. [Google Scholar] [CrossRef]
- Woese, C.R. On the Origin of the Genetic Code. Proc. Natl. Acad. Sci. USA 1965, 54, 1546–1552. [Google Scholar] [CrossRef]
- Woese, C.R. The Genetic Code; Harper & Row: New York, NY, USA, 1967. [Google Scholar]
- Crick, F.H. The Origin of the Genetic Code. J. Mol. Biol. 1968, 38, 367–379. [Google Scholar] [CrossRef]
- Orgel, L.E. Evolution of the Genetic Apparatus. J. Mol. Biol. 1968, 38, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Vetsigian, K.; Woese, C.; Goldenfeld, N. Collective Evolution and the Genetic Code. Proc. Natl. Acad. Sci. USA 2006, 103, 10696–10701. [Google Scholar] [CrossRef]
- Sagan, L. On the Origin of Mitosing Cells. J. Theoret Biol. 1967, 14, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Margulis, L. Symbiosis in Cell Evolution: Life and Its Environment on the Early Earth; W. H. Freeman: San Francisco, CA, USA, 1981. [Google Scholar]
- Margulis, L. Symbiogenesis and Symbionticism. In Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; Margulis, L., Fester, R., Eds.; MIT Press: Cambridge, UK, 1991; pp. 1–14. [Google Scholar]
- O’Malley, M.A. Endosymbiosis and Its Implications for Evolutionary Theory. Proc. Natl. Acad. Sci. USA 2014, 112, 10270–10277. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R. A New Biology for a New Century. Microbiol. Mol. Biol. Rev. 2004, 68, 173–186. [Google Scholar] [CrossRef]
- Damer, B. A Field Trip to the Archaean in Search of Darwin’s Warm Little Pond. Life 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Milshteyn, D.; Damer, B.; Havig, J.; Deamer, D. Amphiphilic Compounds Assemble into Membranous Vesicles in Hydrothermal Hot Spring Water but Not in Seawater. Life 2018, 8, 11. [Google Scholar] [CrossRef]
- Deamer, D.W.; Damer, B.; Kompanichenko, V. Hydrothermal Chemistry and the Origin of Cellular Life. Astrobiology 2019, 19, 1523–1537. [Google Scholar] [CrossRef]
- Koonin, E.V.; Martin, W. On the Origin of Genomes and Cells Within Inorganic Compartments. Trends Genet. 2005, 21, 647–654. [Google Scholar] [CrossRef]
- Martin, W.; Russell, M.J. On the Origin of Biochemistry at an Alkaline Hydrothermal Vent. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1887–1925. [Google Scholar] [CrossRef]
- Lane, N.; Martin, W.F. The Origin of Membrane Bioenergetics. Cell 2012, 151, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).