Vitamin C: From Self-Sufficiency to Dietary Dependence in the Framework of Its Biological Functions and Medical Implications
Abstract
:1. Introduction
2. Normal Vitamin C Levels in the Body and Its Daily Requirements
3. Debates on Vitamin C Regarding Health and Disease
3.1. Vitamin C Is Crucial for Supporting the Immune System
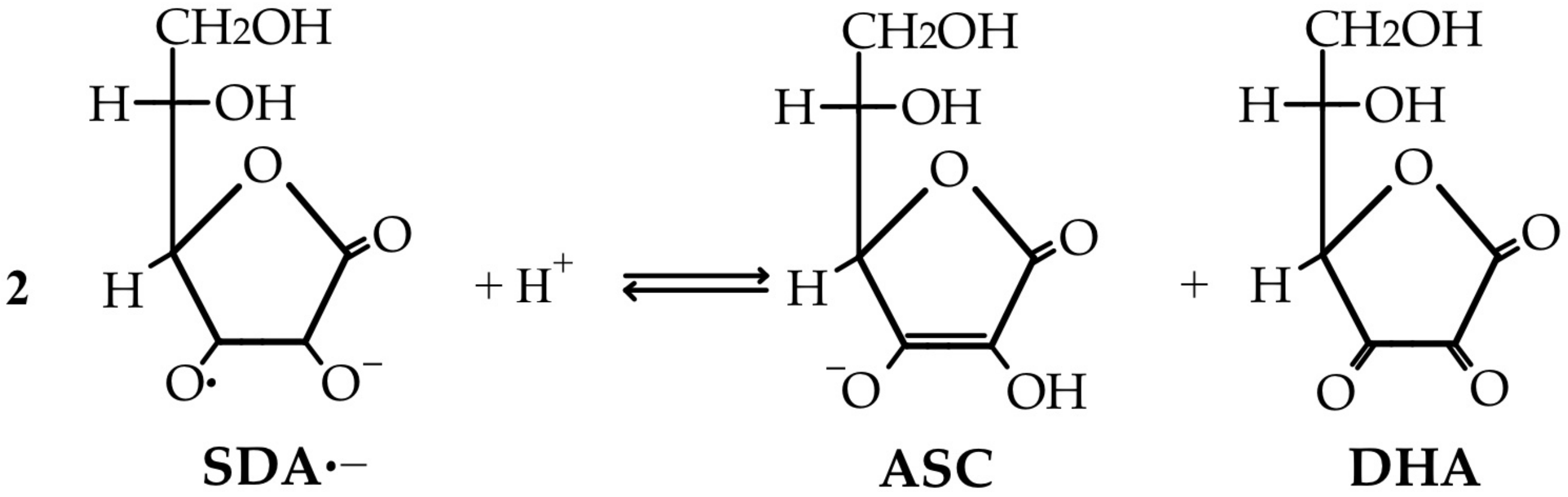
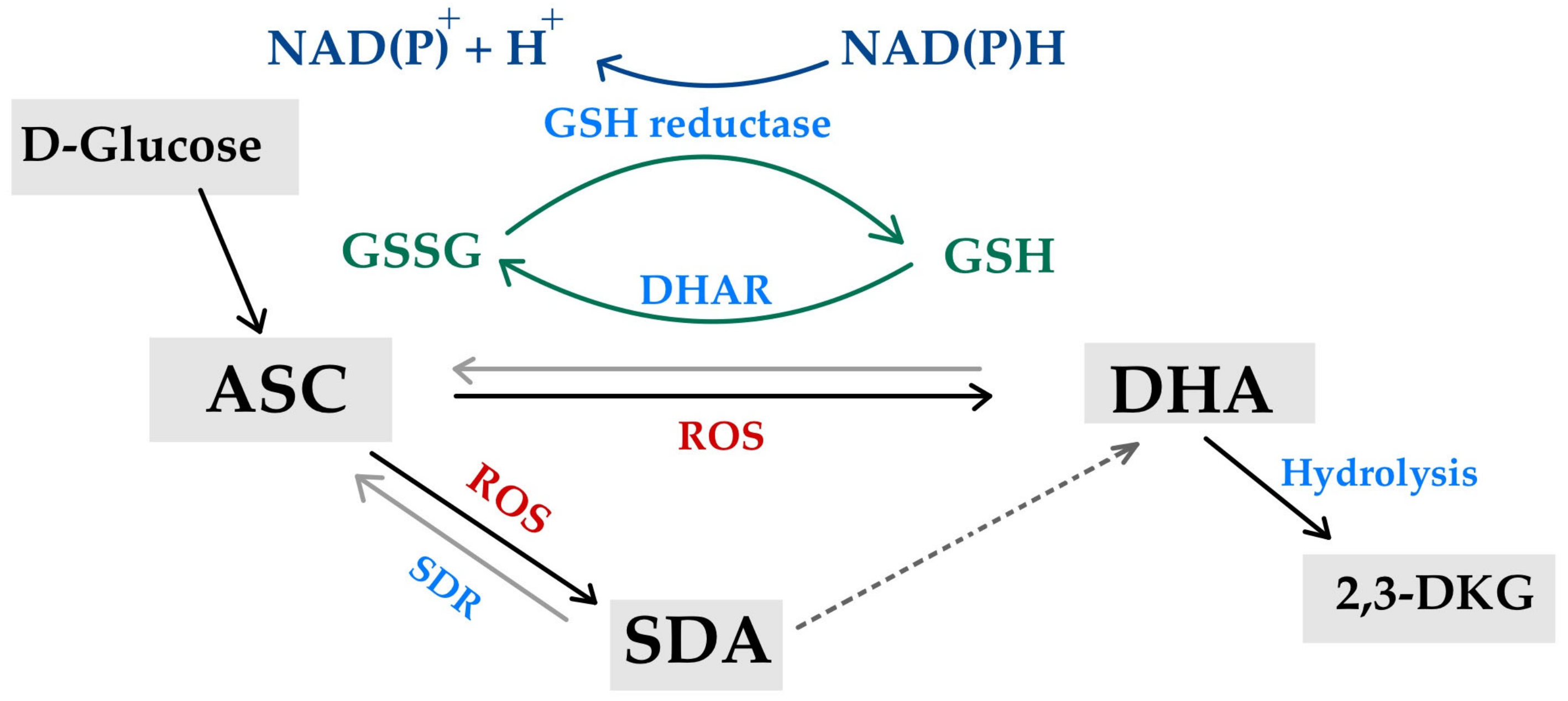
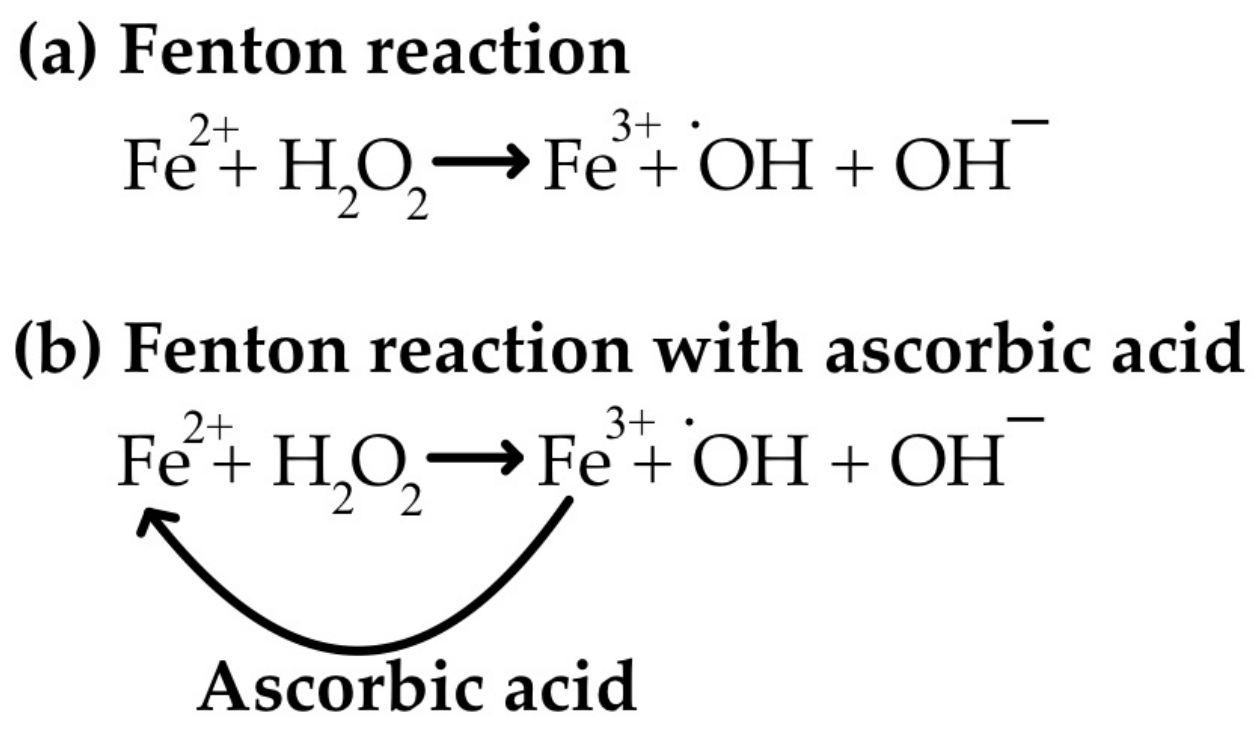
3.2. Anti-Oxidant Properties of Vitamin C and the Reverse of Pro-Oxidant Action
3.3. Archaic Association of Vitamin C with Scurvy Prevention, Related Molecular Mechanisms of Co-Factoring Activities for Collagen Synthesis, and Subsequent Positive Effects on Wound Healing and Tissue Remodeling
3.4. Vitamin C and Its Enzymatic Cofactor Activities in Various Metabolisms
- (i)
- Vitamin C is an enzymatic cofactor for carnitine synthesis: Carnitine is an essential cofactor in the transport of long-chain fatty acids into mitochondria in order to produce ATP via beta-oxidation. Although current results are contradictory regarding the essentiality of vitamin C in the biosynthesis of carnitine [113], it remains an important cofactor for the activities of two enzymes (6-N-trimethyllysine dioxygenase and gamma-butyrobetaine dioxygenase) involved in the carnitine biosynthetic pathway, which has long been considered essential in this process [114].
- (ii)
- Vitamin C is an enzymatic cofactor for catecholamine norepinephrine synthesis: The highest concentrations of vitamin C in the body are found in brain and neuroendocrine tissues, such as adrenal [38,69,115]. Vitamin C is a cofactor in the biosynthesis of norepinephrine, firstly in a step mediated by tyrosine hydroxylase, and of tyrosine hydroxylation into 3,4 dihydroxy–l-phenylalanine (L-DOPA) metabolite, followed by the conversion of dopamine (formed after decarboxylation of L-DOPA by aromatic amino acid decarboxylase) to norepinephrine by dopamine beta-hydroxylase [21,116,117,118].
- (iii)
- Vitamin C is an enzymatic cofactor for peptide amidation: Vitamin C acts as a cofactor for peptidylglycine alpha-amidating mono-oxygenase (a copper- and ascorbate-dependent type I membrane protein), the only known enzyme able to catalyze the reaction of amidation of the terminal carboxyl (C-terminal α-amidation) as the final and essential step in the biosynthesis of neuropeptides and peptide hormones [21,119,120,121].
- (iv)
- Vitamin C is an enzymatic cofactor for tyrosine metabolism: Vitamin C is a cofactor of 4-hydroxyphenylpyruvate dioxygenase, a vitamin-C-dependent dioxygenase with a ferrous ion in the active site. This enzyme is involved in tyrosine catabolism, catalyzing the conversion of 4-hydroxyphenylpyruvate to homogentisate (2,5-dihydroxyphenylacetate) [4] through decarboxylation, substituent migration, and aromatic oxygenation in a single catalytic cycle [122]. The final products of tyrosine degradation are fumarate and acetyl coenzyme A (acetyl-CoA), with both playing important roles in energy production [123].
3.5. Vitamin C Is an Enzymatic Cofactor That Plays a Role in Gene Transcription
3.6. Vitamin C Is an Enzymatic Cofactor That Plays a Role in Epigenetic Regulation
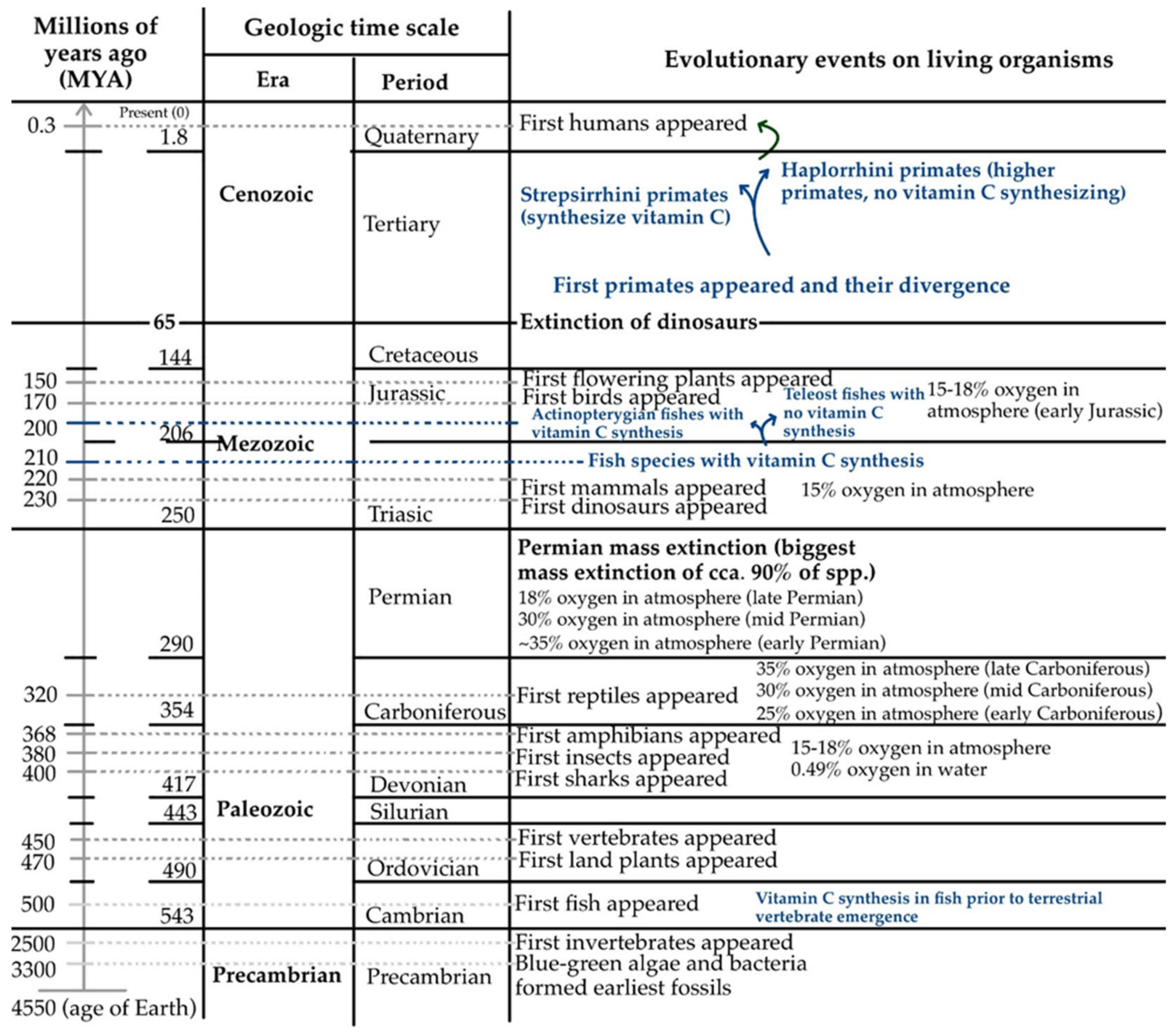
4. Endogenous Vitamin C: A General Frame of Interrupted Biosynthesis Pathway
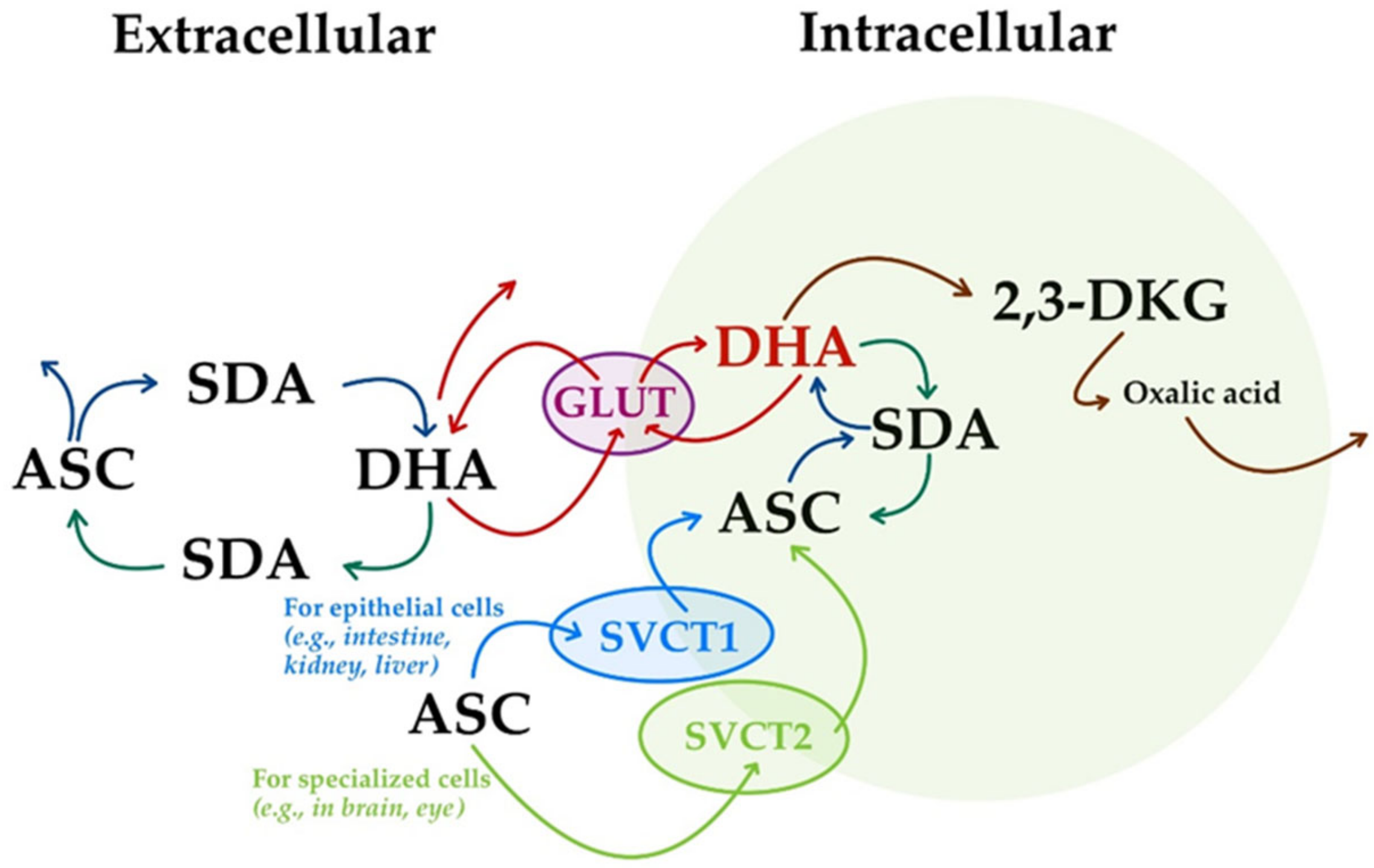
5. Molecular Mechanisms in Vitamin C Transport, Homeostatic Distribution, and Recycling
- (i)
- The transport of ASC through the body involves sodium-dependent vitamin C transporters (SVCTs). Two sodium-dependent vitamin C transporters were reported, namely, SVCT1 and SVCT2 [18,37,46,115,138]. They are now considered as the primary means of ASC homeostasis systemic control [22], being crucial players in the process of ASC being taken up by cells in unidirectional mediated transport [4]. The majority of ASC is transported by SVCT1 in epithelial cells (e.g., intestine, kidney, and liver), while the remainder is transported by SVCT2 in specialized cells (e.g., brain and eye) (Figure 7) [38].
- (ii)
- The oxidized form of ASC, DHA, is transported in the body using glucose transporters (GLUTs) (Figure 7) [37,115]. Thus, ASC is oxidized at the extracellular level to form DHA, and is subsequently transported intracellularly using GLUT1 (SLC2A1 in humans) and GLUT3 (SLC2A3 in humans) glucose transporter isoforms [18]. The isoform GLUT4 (SLC2A4 in humans) seems to be used only for insulin-sensitive tissues [20,36,141]. However, unlike unidirectional transport facilitated by SVCTs, the GLUTs mediate bidirectional DHA-facilitated transport (Figure 7) [4].
6. Fluctuating Gene Activity and the Evolutionary Puzzle of GULO Loss of Function
- (i)
- A relatively high metabolic cost: While the exact metabolic cost can vary depending on factors such as species, metabolic rate, and dietary conditions, it is generally considered to be relatively high considering the required energy and many enzymes involved. First of all, the vitamin C biosynthesis pathway starts with glucose [128], a simple sugar derived from food. Converting glucose into intermediates necessary for vitamin C synthesis consumes energy, primarily in the form of ATP (adenosine triphosphate). Furthermore, there are several enzyme-mediated reactions with different enzymes involved, each catalyzing a specific reaction. Some catalytic enzymes require cofactors (such as NADPH—nicotinamide adenine dinucleotide phosphate) to function and whose regeneration is also energy-consuming [154]. All of these have several evolutionary implications regarding adaptation to reduce the metabolic burden associated with vitamin C synthesis, freeing up energy for other essential functions in many vertebrates, including humans [155].
- (ii)
- Dietary availability: As vertebrates evolved and diversified, many species adapted to diets rich in vitamin-C-containing fruits and vegetables, which made the metabolic cost of internal synthesis less necessary and reduced the selective pressure to maintain the ability to synthesize vitamin C internally. This led to an evolutionary adaptation that allowed these species to reduce their metabolic burden and focus on other essential functions [156].
- (iii)
- Genetic drift: Over time, genetic drift (random changes in gene frequencies) may have led to the loss of the gene responsible for vitamin C synthesis in certain vertebrate lineages. This could have occurred especially in species with abundant dietary sources of vitamin C. Genetic drift is a random process that can cause changes in gene frequencies within a population, especially in small populations. It is a key mechanism of evolution, and it likely played a role in the loss of the vitamin C synthesis gene in vertebrates [157].
- (iv)
- Additional hypotheses can also be taken into account, such as selection for other factors or the intervention of environmental factors. For example, it is possible that the gene involved in vitamin C synthesis was repurposed for other functions, making its loss advantageous; while there is no definitive evidence that this has happened in vertebrates, it is a possibility that scientists are exploring. Repurposing of genes often occurs when a gene’s original function becomes less important or when a new function offers a selective advantage [150]. Hypothetical examples of how the vitamin C synthesis gene might have been repurposed take into account the possibility that the enzymes involved in vitamin C synthesis were readapted to participate in other metabolic pathways, such as those involved in energy production or detoxification. Alternatively, the gene products may have evolved new functions in cellular signaling, allowing them to regulate various cellular processes, or the synthesizing gene could have been co-opted to play a role in gene expression, such as controlling the activity of other genes [162]. However, it is important to note that these are speculative examples. More research is needed to determine if the vitamin C synthesis gene has indeed been repurposed in vertebrates and, if so, what its new functions might be.
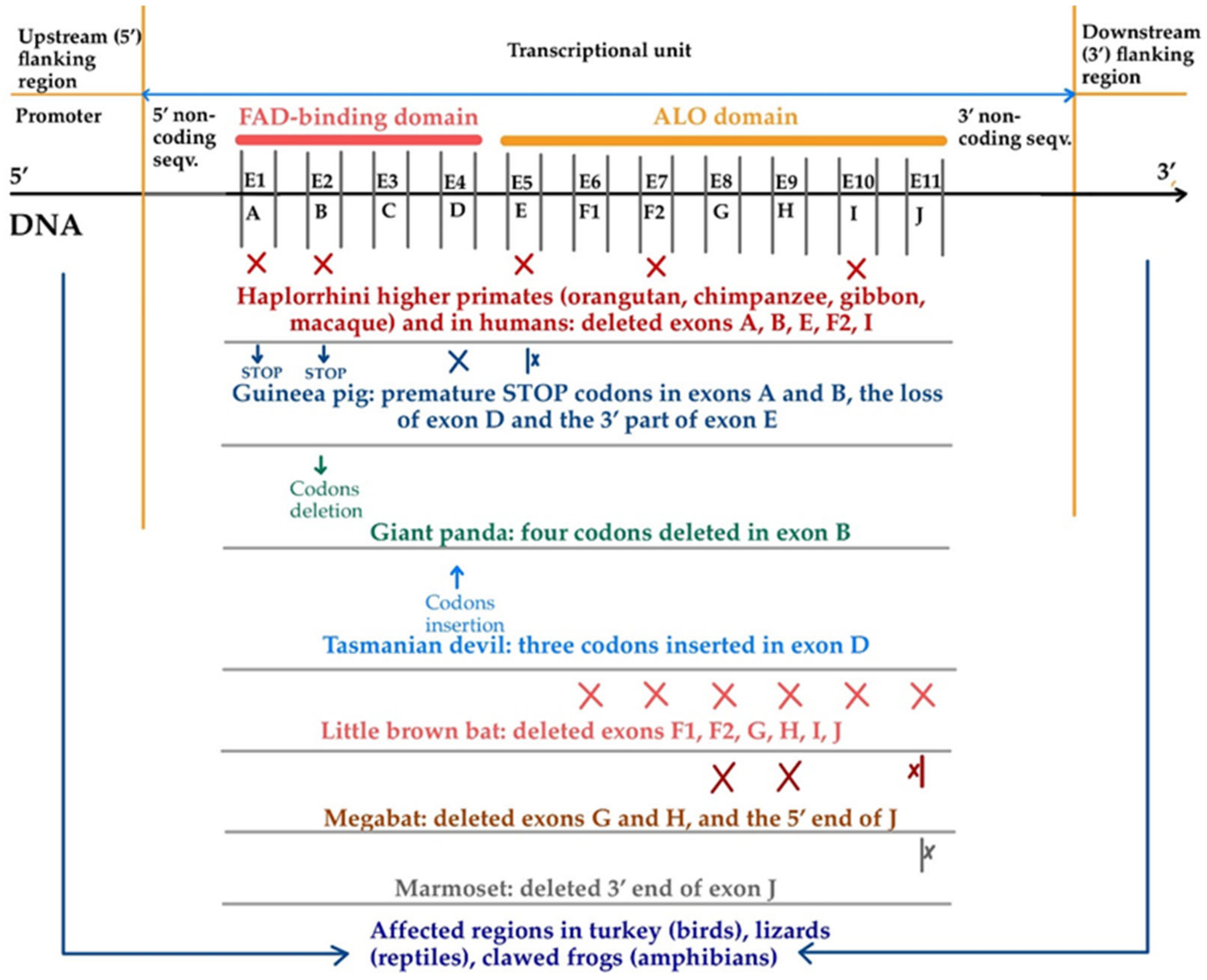
7. Gene to Pseudogene Transformation: A Journey into Genetic Silence
8. The Role of Vitamin C Gene Production and Pseudogene Transformation in Human Health
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ginter, E. Optimum intake of vitamin C for the human organism. Nutr. Health 1982, 1, 66–77. [Google Scholar] [CrossRef]
- Carpenter, K.J. The discovery of vitamin C. Ann. Nutr. Metab. 2012, 61, 259–264. [Google Scholar] [CrossRef]
- De Tullio, M.C. Beyond the antioxidant: The double life of vitamin C, Subcell. Biochem. 2012, 56, 49–65. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Kujovská Krčmová, L.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C—Sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Linster, C.L.; Van Schaftingen, E. Vitamin C biosynthesis, recycling and degradation in mammals. FEBS J. 2006, 274, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Loewus, F.A. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry 1999, 52, 193–210. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in plants: Novel concepts, new perspectives, and outstanding issues. Antioxid. Redox Signal 2020, 32, 463–485. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.-U.; Chung, S.-M.; Kumar, M. Regulation of reactive oxygen species during salt stress in plants and their crosstalk with other signaling molecules—Current perspectives and future directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Pannala, V.R.; Bazil, J.N.; Camara, A.K.S.; Dash, R.K. A biophysically based mathematical model for the catalytic mechanism of glutathione reductase. Free Radic. Biol. Med. 2013, 65, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ruiz, M.; Houmani, H.; Muñoz-Vargas, M.A.; Palma, J.M.; Corpas, F.J. Detection of ascorbate peroxidase (APX) activity in plant tissues: Using non-denaturing PAGE and spectrophotometric sssay. Methods Mol. Biol. 2024, 2798, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Bruno, E.J., Jr.; Ziegenfuss, T.N.; Landis, J. Vitamin C: Research update. Curr. Sports Med. Rep. 2006, 5, 177–181. [Google Scholar] [CrossRef]
- Çolak, N.G.; Eken, N.T.; Ülger, M.; Frary, A.; Doğanlar, S. Mapping of quantitative trait loci for antioxidant molecules in tomato fruit: Carotenoids, vitamins C and E, glutathione and phenolic acids. Plant Sci. 2020, 292, 110393. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.A. Vitamin C in human and disease is still a mystery? An overview, Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 2007, 137, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Hussain, F.H.S.; Samad, A. Cure and prevention of diseases with vitamin C into perspective: An overview. J. Crit. Rev. 2020, 7, 289–293. [Google Scholar] [CrossRef]
- Daruwala, R.; Song, J.; Koh, W.S.; Rumsey, S.C.; Levine, M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett. 1999, 460, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Yang, H. Conserved or lost: Molecular evolution of the key gene GULO in vertebrate vitamin C biosynthesis. Biochem. Genet. 2013, 51, 413–425. [Google Scholar] [CrossRef]
- Lane, D.J.R.; Richardson, D.R. The active role of vitamin C in mammalian iron metabolism: Much more than just enhanced iron absorption. Free Radic. Biol. Med. 2014, 75, 69–83. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. On the effect of vitamin C intake on human health: How to (mis)interprete the clinical evidence. Redox Biol. 2020, 34, 101532. [Google Scholar] [CrossRef]
- Sharma, Y.; Popescu, A.; Horwood, C.; Hakendorf, P.; Thompson, C. Relationship between vitamin C deficiency and cognitive impairment in older hospitalised patients: A cross—Sectional study. Antioxidants 2022, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Mandl, J.; Szarka, A.; Bánhegyi, G. Vitamin C: Update on physiology and pharmacology. Br. J. Pharmacol. 2009, 157, 1097–1110. [Google Scholar] [CrossRef]
- Deruelle, F.; Baron, B. Vitamin C: Is the supplementation necessary for optimal health? J. Altern. Complement. Med. 2008, 14, 1291–1298. [Google Scholar] [CrossRef]
- Susa, F.; Pisano, R. Advances in ascorbic acid (vitamin C) manufacturing: Green extraction techniques from natural sources. Processes 2023, 11, 3167. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Smith, V.H. Vitamin C deficiency is an under-diagnosed contributor to degenerative disc disease in the elderly. Med. Hypotheses 2010, 74, 695–697. [Google Scholar] [CrossRef]
- Mitmesser, S.H.; Ye, Q.; Evans, M.; Combs, M. Determination of plasma and leukocyte vitamin C concentrations in a randomized, double-blind, placebo-controlled trial with Ester-C(®). Springerplus 2016, 5, 1161. [Google Scholar] [CrossRef] [PubMed]
- McCall, S.J.; Clark, A.B.; Luben, R.N.; Wareham, N.J.; Khaw, K.T.; Myint, P.K. Plasma vitamin C levels: Risk factors for deficiency and association with self-reported functional health in the European prospective investigation into cancer-Norfolk. Nutrients 2019, 11, 1552. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, M.; Tveden-Nyborg, P.; Lykkesfeldt, J. Regulation of vitamin C homeostasis during deficiency. Nutrients 2013, 5, 2860–2879. [Google Scholar] [CrossRef]
- Rowe, S.; Carr, A.C. Global Vitamin C status and prevalence of deficiency: A cause for concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral. Diseases. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; De Pinto, M.C. Vitamin C in plants: From functions to biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1170. [Google Scholar] [CrossRef]
- Nusgens, B.V.; Humbert, P.; Rougier, A.; Colige, A.C.; Haftek, M.; Lambert, C.A.; Richard, A.; Creidi, P.; Lapière, C.M. Topically applied vitamin C enhances the m RNA levels of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J. Investig. Dermatol. 2001, 116, 853–859. [Google Scholar] [CrossRef]
- Carr, A.C.; Vissers, M.C.M. Synthetic or food-derived vitamin C—Are they equally bioavailable? Nutrients 2013, 5, 4284–4304. [Google Scholar] [CrossRef]
- Pacier, C.; Martirosyan, D.M. Vitamin C: Optimal dosages, supplementation and use in disease prevention. Funct. Foods Health Dis. 2015, 5, 89–107. [Google Scholar] [CrossRef]
- Clemens, Z.; Tóth, C. Vitamin C and disease: Insights from the evolutionary perspective. J. Evol. Health 2016, 1, 13. [Google Scholar] [CrossRef]
- Lim, D.J.; Sharma, Y.; Thompson, C.H. Vitamin C and alcohol: A call to action. Bmjnph 2018, 1, 17–22. [Google Scholar] [CrossRef]
- Carr, A.C.; Vlasiuk, E.; Zawari, M.; Lunt, H. Understanding the additional impact of prediabetes and type 2 diabetes mellitus on vitamin C requirements in people living with obesity. Nutr. Res. 2024, 130, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, Z.; Ni, Y. Interaction between aspirin and vitamin C with human serum albumin as binary and ternary systems. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 236, 118356. [Google Scholar] [CrossRef] [PubMed]
- Gerster, H. Human vitamin C requirements. Z. Für Ernährungswissenschaft 1987, 26, 125–137. [Google Scholar] [CrossRef]
- Xu, K.; Peng, R.; Zou, Y.; Jiang, X.; Sun, Q.; Song, C. Vitamin C intake and multiple health outcomes: An umbrella review of systematic reviews and meta-analyses. Int. J. Food Sci. Nutr. 2022, 73, 588–599. [Google Scholar] [CrossRef]
- Suriyaprom, K.; Kaewprasert, S.; Putpadungwipon, P.; Namjuntra, P.; Klongthalay, S. Association of antioxidant status and inflammatory markers with metabolic syndrome in Thais. J. Health Popul. Nutr. 2019, 38, 1. [Google Scholar] [CrossRef] [PubMed]
- Ballaz, S.J.; Rebec, G.V. Neurobiology of vitamin C: Expanding the focus from antioxidant to endogenous neuromodulator. Pharmacol. Res. 2019, 146, 104321. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broeke, L.T.; Gräslund, A.; Larsson, P.H.; Nilsson, J.L.; Wahlberg, J.E.; Scheynius, A.; Karlberg, A.T. Free radicals as potential mediators of metal allergy: Effect of ascorbic acid on lymphocyte proliferation and IFN-gamma production in contact allergy to Ni2+ and CO2+. Acta Derm. Venereol. 1998, 78, 95–98. [Google Scholar] [CrossRef]
- Atanassova, B.D.; Tzatchev, K.N. Ascorbic acid--important for iron metabolism. Folia Med. 2008, 50, 11–16. [Google Scholar]
- Lynch, S.R.; Cook, J.D. Interaction of vitamin C and iron. Ann. N.Y. Acad. Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, G.; Wu, W.; Zhang, M.; Liu, W.; Chen, Q.; Wang, X. The efficacy and safety of vitamin C for iron supplementation in adult patients with iron deficiency anemia: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2023644. [Google Scholar] [CrossRef]
- Finkelstein, F.O.; Juergensen, P.; Wang, S.; Santacroce, S.; Levine, M.; Kotanko, P.; Levin, N.W.; Handelman, G.J. Hemoglobin and plasma vitamin C levels in patients on peritoneal dialysis. Perit. Dial. Int. 2011, 31, 74–79. [Google Scholar] [CrossRef]
- Luo, X.; Ng, C.; He, J.; Yang, M.; Luo, X.; Herbert, T.P.; Whitehead, J.P. Vitamin C protects against hypoxia, inflammation, and ER stress in primary human preadipocytes and adipocytes. Mol. Cell Endocrinol. 2022, 556, 111740. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S. Obesity, cardiovascular disease, and role of vitamin C on inflammation: A review of facts and underlying mechanisms. Inflammopharmacology 2017, 25, 313–328. [Google Scholar] [CrossRef]
- Michael, A.J.; Alexopoulos, C.; Pontiki, E.A.; Hadjipavlou-Litina, D.J.; Saratsis, P.; Ververidis, H.N.; Boscos, C.M. Quality and reactive species of extended canine semen after vitamin C supplementation. Theriogenology 2008, 70, 827–835. [Google Scholar] [CrossRef]
- Selman, C.; McLaren, J.S.; Meyer, C.; Duncan, J.S.; Redman, P.; Collins, A.R.; Duthie, G.G.; Speakman, J.R. Life-long vitamin C supplementation in combination with cold exposure does not affect oxidative damage or lifespan in mice, but decreases expression of antioxidant protecting genes. Mech. Ageing Dev. 2006, 127, 897–904. [Google Scholar] [CrossRef]
- Selman, C.; McLaren, J.S.; Collins, A.R.; Duthie, G.G.; Speakman, J.R. Deleterious consequences of antioxidant supplementation on lifespan in a wild-derived mammal. Biol. Lett. 2013, 9, 20130432. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.N.; Niu, Z.Y.; Sun, T.T.; Wang, Z.P.; Jiao, P.X.; Zi, B.B.; Chen, P.P.; Tian, D.L.; Liu, F.Z. Vitamin E and vitamin C supplementation improves antioxidant status and immune function in oxidative-stressed breeder roosters by up-regulating expression of GSH-Px gene. Poult. Sci. 2018, 97, 1238–1244. [Google Scholar] [CrossRef]
- Aumailley, L.; Bousrassa, S.; Gotti, C.; Droit, A.; Lebel, M. Vitamin C modulates the levels of several proteins of the mitochondrial complex III and its activity in the mouse liver. Redox Biol. 2022, 57, 10249. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A.; Machlin, L.J.; Scandurra, O. The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Meulmeester, F.L.; Luo, J.; Martens, L.G.; Mills, K.; van Heemst, D.; Noordam, R. Antioxidant supplementation in oxidative stress-related diseases: What have we learned from studies on alpha-tocopherol? Antioxidants 2022, 11, 2322. [Google Scholar] [CrossRef]
- Hanschmann, E.M.; Godoy, J.R.; Berndt, C.; Hudemann, C.; Lillig, C.H. Thioredoxins, glutaredoxins, and peroxiredoxins-molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid. Redox Signal 2013, 19, 1539–1605. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Karlenius, T.C.; Tonissen, K.F. Thioredoxin and cancer: A role for thioredoxin in all states of tumor oxygenation. Cancers 2010, 2, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system. Antioxid. Redox Signal 2017, 27, 989–1010. [Google Scholar] [CrossRef]
- Shcholok, T.; Eftekharpour, E. Insights into the multifaceted roles of thioredoxin-1 system: Exploring knockout murine models. Biology 2024, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Codini, M. Why vitamin C could be an excellent complementary remedy to conventional therapies for breast cancer. Int. J. Mol. Sci. 2020, 21, 8397. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Rus, E.; Amaya, I.; Valpuesta, V. The challenge of increasing vitamin C content in plant foods. Biotechnol. J. 2012, 7, 1–12. [Google Scholar] [CrossRef]
- Gramlich, G.; Zhang, J.; Nau, W.M. Increased antioxidant reactivity of vitamin C at low pH in model membranes. J. Am. Chem. Soc. 2002, 124, 11252–11253. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Jelodar, G.; Nazifi, S.; Sajedianfard, J. An overview of the characteristics and function of vitamin C in various tissues: Relying on its antioxidant function. Zahedan J. Res. Med. Sci. 2016, 18, e4037. [Google Scholar] [CrossRef]
- Wells, W.W.; Xu, D.P. Dehydroascorbate reduction. J. Bioenerg. Biomembr. 1994, 26, 369–377. [Google Scholar] [CrossRef]
- Rege, S.; Momin, S.; Bhowmick, D. Effect of ascorbic acid on the oxidative stability of water-in-oil emulsion in the presence of lipophilic antioxidants. Int. J. Food Prop. 2014, 18, 259–265. [Google Scholar] [CrossRef]
- Szarka, A.; Kapuy, O.; Lőrincz, T.; Bánhegyi, G. Vitamin C and cell death. Antioxid. Redox Signal 2021, 34, 831–844. [Google Scholar] [CrossRef]
- Diliberto, E.J., Jr.; Dean, G.; Carter, C.; Allen, P.L. Tissue, subcellular, and submitochondrial distributions of semidehydroascorbate reductase: Possible role of semidehydroascorbate reductase in cofactor regeneration. J. Neurochem. 1982, 39, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; He, M.; Zhang, Y.; Wang, C.; Qin, Z.; Li, Q.; Yang, T.; Meng, F.; Zhou, Y.; Ge, H.; et al. Unraveling the 2,3-diketo-l-gulonic acid-dependent and -independent impacts of l-ascorbic acid on somatic cell reprogramming. Cell Biosci. 2023, 13, 218. [Google Scholar] [CrossRef]
- Do, H.; Kim, I.S.; Jeon, B.W.; Lee, C.W.; Park, A.K.; Wi, A.R.; Shin, S.C.; Park, H.; Kim, Y.S.; Yoon, H.S.; et al. Structural understanding of the recycling of oxidized ascorbate by dehydroascorbate reductase (OsDHAR) from Oryza sativa L. japonica. Sci. Rep. 2016, 6, 19498. [Google Scholar] [CrossRef]
- Pehlivan, F.E. Vitamin C: An antioxidant agent. In Vitamin C; Hamza, A.H., Ed.; InTech: Houston, TX, USA, 2017; pp. 23–35. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and anti-inflammatory activity of ascorbic acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Kim, I.S.; Kim, Y.S.; Kim, Y.H.; Park, A.K.; Kim, H.W.; Lee, J.H.; Yoon, H.S. Potential application of the Oryza sativa Monodehydroascorbate Reductase Gene (OsMDHAR) to improve the dtress tolerance and fermentative capacity of Saccharomyces Cerevisiae. PLoS ONE 2016, 11, e0158841. [Google Scholar] [CrossRef]
- Terai, Y.; Ueno, H.; Ogawa, T.; Sawa, Y.; Miyagi, A.; Kawai-Yamada, M.; Ishikawa, T.; Maruta, T. Dehydroascorbate reductases and glutathione set a threshold for high-light–induced ascorbate accumulation. Plant Physiol. 2020, 183, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Cidlowski, J.A. Glutathione efflux and cell death. Antioxid. Redox Signal 2012, 17, 1694–1713. [Google Scholar] [CrossRef]
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. Pro- and antioxidant effects of vitamin C in cancer in correspondence to its dietary and pharmacological concentrations. Oxid. Med. Cell Longev. 2019, 2020, 7286737. [Google Scholar] [CrossRef] [PubMed]
- Alsulami, F.J.; Shaheed, S.U. Role of natural antioxidants in cancer. Cancer Treat. Res. 2024, 191, 95–117. [Google Scholar] [CrossRef]
- Hässig, A.; Liang, W.X.; Schwabl, H.; Stampfli, K. Flavonoids and tannins: Plant-based antioxidants with vitamin character. Med. Hypotheses 1999, 52, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; McGill, M.; Raia, N.R.; Hasturk, O.; Kaplan, D.L. Silk hydrogels crosslinked by the Fenton reaction. Adv. Heal. Mater. 2019, 8, e1900644. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two faces of vitamin C—Antioxidative and pro-oxidative agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Abe, C.; Miyazawa, T.; Miyazawa, T. Current use of Fenton reaction in drugs and food. Molecules 2022, 27, 5451. [Google Scholar] [CrossRef] [PubMed]
- Farris, P.K.; Valacchi, G. Ultraviolet light protection: Is it really enough? Antioxidants 2022, 11, 1484. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox interactions of vitamin C and iron: Inhibition of the pro-oxidant activity by deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Chourasia, P.; Sangani, V.; Errabelli, P.K.; Patel, S.S.; Adapa, S. Megadose vitamin C prescription through alternative medicine leading to end-stage renal disease: Case study and literature review. J. Investig. Med. High. Impact Case Rep. 2023, 11, 23247096231158954. [Google Scholar] [CrossRef]
- Halliwell, B. Vitamin C and genomic stability. Mutat. Res. 2001, 475, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Pizzorno, J.E.; Murray, M. Textbook of Natural Medicine, 5th ed.; Elsevier: North York, NY, USA, 2021. [Google Scholar]
- May, C.N.; Bellomo, R.; Lankadeva, Y.R. Therapeutic potential of megadose vitamin C to reverse organ dysfunction in sepsis and COVID-19. Br. J. Pharmacol. 2021, 178, 3864–3868. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, B.; Patterson, G.T.; Kumar, S.; Vyavahare, S.; Mishra, S.; Isales, C.; Fulzele, S. Vitamin C supplementation for the treatment of osteoarthritis: Perspectives on the past, present, and future. Ther. Adv. Chronic Dis. 2021, 12, 20406223211047026. [Google Scholar] [CrossRef] [PubMed]
- Moritz, B.; Schmitz, A.E.; Rodrigues, A.L.S.; Dafre, A.L.; Cunha, M.P. The role of vitamin C in stress-related disorders. J. Nutr. Biochem. 2020, 85, 108459. [Google Scholar] [CrossRef]
- Shen, X.; Wang, J.; Deng, B.; Chen, S.; John, C.; Zhao, Z.; Sinha, N.; Haag, J.; Sun, W.; Kong, W.; et al. High-dose ascorbate exerts anti-tumor activities and improves inhibitory effect of carboplatin through the pro-oxidant function pathway in uterine serous carcinoma cell lines. Gynecol. Oncol. 2024, 183, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Böttger, F.; Vallés-Martí, A.; Cahn, L.; Jimenez, C.R. High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J. Exp. Clin. Cancer Res. 2021, 40, 343. [Google Scholar] [CrossRef]
- Mikkelsen, S.U.; Gillberg, L.; Lykkesfeldt, J.; Grønbæk, K. The role of vitamin C in epigenetic cancer therapy. Free Radic. Biol. Med. 2021, 170, 179–193. [Google Scholar] [CrossRef] [PubMed]
- González-Montero, J.; Chichiarelli, S.; Eufemi, M.; Altieri, F.; Saso, L.; Rodrigo, R. Ascorbate as a bioactive compound in cancer therapy: The old classic strikes back. Molecules 2022, 27, 3818. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, G.; Ishikawa, T.; Pornsaksit, V.; Smirnoff, N. Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes. eLife 2015, 4, e06369. [Google Scholar] [CrossRef] [PubMed]
- Granger, M.; Eck, P. Dietary vitamin C in human health. Adv. Food Nutr. Res. 2018, 83, 281–310. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S. Vitamin C. In Present Knowledge in Nutrition, 11th ed.; Basic nutrition and metabolism; Marriott, M.P., Birt, D.F., Stallings, V.A., Yates, A.A., Eds.; Academic Press (Elsevier): London, UK, 2020; Volume 1, pp. 155–170. [Google Scholar]
- Hacişevki, A. An overview of ascorbic acid biochemistry. J. Fac. Pharm. 2009, 38, 233–255. [Google Scholar]
- Duarte, T.L.; Cooke, M.S.; Jones, G.D.D. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free Radic. Biol. Med. 2009, 46, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Magana, A.A.; Reed, R.L.; Koluda, R.; Miranda, C.L.; Maier, C.S.; Stevens, J.F. Vitamin C activates the folate-mediated one-carbon cycle in C2C12 myoblasts. Antioxidants 2020, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Urban, K.; Höhling, H.J.; Lüttenberg, B.; Szuwart, T.; Plate, U.; Biomineralisation Research Unit. An in vitro study of osteoblast vitality influenced by the vitamins C and E. Head. Face Med. 2012, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, P.; Hall, S.; Wongworawat, M.D.; Mohan, S. The roles and mechanisms of actions of vitamin C in bone: New developments. J. Bone Min. Res. 2015, 30, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Cantatore, F.P.; Loperfido, M.C.; Magli, D.M.; Mancini, L.; Carrozzo, M. The importance of vitamin C for hydroxylation of vitamin D3 to 1,25(OH)2D3 in man. Clin. Rheumatol. 1991, 10, 162–167. [Google Scholar] [CrossRef]
- Furusawa, H.; Sato, Y.; Tanaka, Y.; Inai, Y.; Amano, A.; Iwama, M.; Kondo, Y.; Handa, S.; Murata, A.; Nishikimi, M.; et al. Vitamin C is not essential for carnitine biosynthesis in vivo: Verification in vitamin C-depleted senescence marker protein-30/gluconolactonase knockout mice. Biol. Pharm. Bull. 2008, 31, 1673–1679. [Google Scholar] [CrossRef]
- Rebouche, C.J. Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1147S–1152S. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Yoshida-Hiroi, M.; Sotiriou, S.; Levine, M.; Hartwig, H.G.; Nussbaum, R.L.; Eisenhofer, G. Impaired adrenal catecholamine system function in mice with deficiency of the ascorbic acid transporter (SVCT2). FASEB J. 2003, 17, 1928–1930. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, B.J. Is there an acute exercise-induced physiological/biochemical threshold which triggers increased speed of cognitive functioning? A meta-analytic investigation. J. Sport. Health Sci. 2015, 4, 4–13. [Google Scholar] [CrossRef]
- Benarroch, E.E. What is the role of ascorbic acid in norepinephrine synthesis and orthostatic hypotension? Neurology 2020, 95, 913–916. [Google Scholar] [CrossRef]
- Carlson, K.; Pomerantz, S.C.; Vafa, O.; Naso, M.; Stroh, W.; Mains, R.E.; Eipper, B.A. Optimizing production of Fc-amidated peptides by Chinese hamster ovary cells. BMC Biotechnol. 2015, 15, 95. [Google Scholar] [CrossRef]
- Carr, A.C.; McCall, C. The role of vitamin C in the treatment of pain: New insights. J. Transl. Med. 2017, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Bergmann, A.; Melander, O. Novel insights into peptide amidation and amidating activity in the human circulation. Sci. Rep. 2021, 11, 15791. [Google Scholar] [CrossRef]
- Moran, G.R. 4-Hydroxyphenylpyruvate dioxygenase. Arch. Biochem. Biophys. 2005, 433, 117–128. [Google Scholar] [CrossRef]
- Shan, C.; Lu, Z.; Li, Z.; Sheng, H.; Fan, J.; Qi, Q.; Liu, S.; Zhang, S. 4-hydroxyphenylpyruvate dioxygenase promotes lung cancer growth via pentose phosphate pathway (PPP) flux mediated by LKB1-AMPK/HDAC10/G6PD axis. Cell Death Dis. 2019, 10, 525. [Google Scholar] [CrossRef]
- Huff, T.C.; Sant, D.W.; Camarena, V.; Van Booven, D.; Andrade, N.S.; Mustafi, S.; Monje, P.V.; Wang, G. Vitamin C regulates Schwann cell myelination by promoting DNA demethylation of pro-myelinating genes. J. Neurochem. 2021, 157, 1759–1773. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martínez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef]
- Ramezankhani, B.; Taha, M.F.; Javeri, A. Vitamin C counteracts miR-302/367-induced reprogramming of human breast cancer cells and restores their invasive and proliferative capacity. J. Cell Physiol. 2018, 234, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, L.; Chen, L.; Wang, X.; Wu, H.; Ai, Z.; Du, J.; Liu, Y.; Shi, X.; Wu, Y.; et al. Vitamin C facilitates pluripotent stem cell maintenance by promoting pluripotency gene transcription. Biochimie 2013, 95, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Boverio, A.; Jamil, N.; Mannucci, B.; Mascotti, M.L.; Fraaije, M.W.; Mattevi, A. Structure, mechanism, and evolution of the last step in vitamin C biosynthesis. Nat. Commun. 2024, 15, 4158. [Google Scholar] [CrossRef]
- Linster, C.L.; Gomez, T.A.; Christensen, K.C.; Adler, L.N.; Young, B.D.; Brenner, C.; Clarke, S.G. Arabidopsis VTC2 encodes a GDP-L-galactose phosphorylase, the last unknown enzyme in the Smirnoff-Wheeler pathway to ascorbic acid in plants. J. Biol. Chem. 2007, 282, 18879–18885. [Google Scholar] [CrossRef]
- Figueroa-Méndez, R.; Rivas-Arancibia, S. Vitamin C in health and disease: Its role in the metabolism of cells and redox state in the brain. Front. Physiol. 2015, 6, 397. [Google Scholar] [CrossRef] [PubMed]
- Mellidou, I.; Kanellis, A.K. Genetic control of ascorbic acid biosynthesis and recycling in horticultural crops. Front. Chem. 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Duque, P.; Vieira, C.P.; Bastos, B.; Vieira, J. The evolution of vitamin C biosynthesis and transport in animals. BMC Ecol. Evol. 2022, 22, 84. [Google Scholar] [CrossRef]
- Bulley, S.; Laing, W. The regulation of ascorbate biosynthesis. Curr. Opin. Plant Biol. 2016, 33, 15–22. [Google Scholar] [CrossRef]
- Nandi, A.; Mukhopadhyay, C.K.; Ghosh, M.K.; Chattopadhyay, D.J.; Chatterjee, I.B. Evolutionary significance of vitamin C biosynthesis in terrestrial vertebrates. Free Radic. Biol. Med. 1997, 22, 1047–1054. [Google Scholar] [CrossRef]
- Corpe, C.P.; Tu, H.; Eck, P.; Wang, J.; Faulhaber-Walter, R.; Schnermann, J.; Margolis, S.; Padayatty, S.; Sun, H.; Wang, Y.; et al. Vitamin C transporter SLC23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survive in mice. J. Clin. Investig. 2010, 120, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Ebenuwa, I.; Violet, P.C. Vitamin C. In Essential and Toxic Trace Elements and Vitamins in Human Health; Prasad, A.S., Brewer, G.J., Eds.; Academic Press (Elsevier): London, UK, 2020; pp. 241–262. [Google Scholar] [CrossRef]
- May, J.M. The SLC23 family of ascorbate transporters: Ensuring that you get and keep your daily dose of vitamin C. Br. J. Pharmacol. 2011, 164, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.S.; Srinivasan, P.; Wildman, A.J.; Marchant, J.S.; Said, H.M. Molecular mechanism(s) involved in differential expression of vitamin C transporters along the intestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G340–G347. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, B.; Illing, A.C.; Hediger, M.A. Transport model of the human Na+-coupled L-ascorbic acid (vitamin C) transporter SVCT1, Am. J. Physiol. Cell Physiol. 2008, 294, C451–C459. [Google Scholar] [CrossRef]
- Harrison, F.E.; Dawes, S.M.; Meredith, M.E.; Babaev, V.R.; Li, L.; May, J.M. Low vitamin C and increased oxidative stress and cell death in mice that lack the sodium-dependent vitamin C transporter SVCT2. Free Radic. Biol. Med. 2010, 49, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Shaghaghi, M.A.; Kloss, O.; Eck, P. Genetic variation in human vitamin C transporter gene in common complex diseases. Adv. Nutr. 2016, 7, 287–298. [Google Scholar] [CrossRef]
- Stratakis, C.A.; Taymans, S.E.; Daruwala, R.; Song, J.; Levine, M. Mapping of the human genes (SLC23A2 and SLC23A1) coding for vitamin C transporters 1 and 2 (SVCT1 and SVCT2) to 5q23 and 20p12, respectively. J. Med. Genet. 2000, 37, e20. [Google Scholar] [CrossRef]
- Senthilkumari, S.; Talwar, B.; Dharmalingam, K.; Ravindran, R.D.; Jayanthi, R.; Sundaresan, P.; Saravanan, C.; Young, I.S.; Dangour, A.D.; Fletcher, A.E. Polymorphisms in sodium-dependent vitamin C transporter genes and plasma, aqueous humor and lens nucleus ascorbate concentrations in an ascorbate depleted setting. Exp. Eye Res. 2014, 124, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.D.; Sundaresan, P.; Krishnan, T.; Vashist, P.; Maraini, G.; Saravanan, V.; Chakravarthy, U.; Smeeth, L.; Nitsch, D.; Young, I.S.; et al. Genetic variants in a sodium-dependent vitamin C transporter gene and age-related cataract. Br. J. Ophthalmol. 2019, 103, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Michels, A.J.; Hagen, T.M.; Frei, B. Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu. Rev. Nutr. 2013, 33, 45–70. [Google Scholar] [CrossRef]
- Zheng, J.S.; Luan, J.; Sofianopoulou, E.; Imamura, F.; Stewart, I.D.; Day, F.R.; Pietzner, M.; Wheeler, E.; Lotta, L.A.; Gundersen, T.E.; et al. Plasma vitamin C and type 2 diabetes: Genome-wide association study and Mendelian randomization analysis in European populations. Diabetes Care 2021, 44, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Keske, M.A.; Wadley, G.D. Effects of vitamin C supplementation on glycemic control and cardiovascular risk factors in people with type 2 diabetes: A grade-assessed systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2021, 44, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.A.M.; Sirko, A. L-gulono-γ-lactone Oxidase, the Key Enzyme for L-Ascorbic Acid Biosynthesis. Curr. Issues Mol. Biol. 2024, 46, 11057–11074. [Google Scholar] [CrossRef] [PubMed]
- El Basuini, M.F.; Shahin, S.A.; Teiba, I.I.; Zaki, M.A.A.; El Hais, A.M.; Sewilam, H.; Almeer, R.; Abdelkhalek, N.; Dawood, M.A.O. The influence of dietary coenzyme Q10 and vitamin C on the growth rate, immunity, oxidative-related genes, and the resistance against Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 531, 735862. [Google Scholar] [CrossRef]
- Mansueto, A.; Good, D.J. Conservation of a chromosome 8 inversion and exon mutations confirm common Gulonolactone oxidase gene evolution among primates, including H. Neanderthalensis. J. Mol. Evol. 2024, 92, 266–277. [Google Scholar] [CrossRef]
- Cui, J.; Pan, Y.H.; Zhang, Y.; Jones, G.; Zhang, S. Progressive pseudogenization: Vitamin C synthesis and its loss in bats. Mol. Biol. Evol. 2011, 28, 1025–1031. [Google Scholar] [CrossRef]
- Lachapelle, M.Y.; Dronin, G. Inactivation dates of the human and guinea pig vitamin C genes. Genetica 2011, 139, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, B.S.; Kaesler, R.L. Prehistoric Life: Evolution and the Fossil Record. Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Kietzmann, T. Vitamin C: From nutrition to oxygen sensing and epigenetics. Redox Biol. 2023, 63, 102753. [Google Scholar] [CrossRef]
- Duque, P.; Vieira, C.P.; Vieira, J. Advances in novel animal vitamin C biosynthesis pathways and the role of prokaryote-based inferences to understand their origin. Genes 2022, 13, 1917. [Google Scholar] [CrossRef]
- Duan, W.; Song, X.; Liu, T.; Huang, Z.; Ren, J.; Hou, X.; Du, J.; Li, Y. Patterns of evolutionary conservation of ascorbic acid-related genes following whole-genome triplication in Brassica rapa. Genome Biol. Evol. 2014, 7, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Glez-Peña, D.; López-Fernández, H.; Duque, P.; Vieira, C.P.; Vieira, J. Inferences on the evolution of the ascorbic acid synthesis pathway in insects using Phylogenetic Tree Collapser (PTC), a tool for the automated collapsing of phylogenetic trees using taxonomic information. J. Integr. Bioinform. 2024, 21, 20230051. [Google Scholar] [CrossRef]
- Star, B.; Spencer, H.G. Effects of genetic drift and gene flow on the selective maintenance of genetic variation. Genetics 2013, 194, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Tenaillon, O.; Matic, I. The impact of neutral mutations on genome evolvability. Curr. Biol. 2020, 30, R527–R534. [Google Scholar] [CrossRef]
- Lucena-Perez, M.; Kleinman-Ruiz, D.; Marmesat, E.; Saveljev, A.P.; Schmidt, K.; Godoy, J.A. Bottleneck-associated changes in the genomic landscape of genetic diversity in wild lynx populations. Evol. Appl. 2021, 14, 2664–2679. [Google Scholar] [CrossRef]
- Blakeslee, A.M.H.; Haram, L.E.; Altman, I.; Kennedy, K.; Ruiz, G.M.; Miller, A.W. Founder effects and species introductions: A host versus parasite perspective. Evol. Appl. 2019, 13, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Drouin, G.; Godin, J.R.; Pagé, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef]
- Jewell, D.E.; Motsinger, L.A.; Paetau-Robinson, I. Effect of dietary antioxidants on free radical damage in dogs and cats. J. Anim. Sci. 2024, 102, skae153. [Google Scholar] [CrossRef] [PubMed]
- Gable, T.D.; Windels, S.K.; Bruggink, J.G. Estimating biomass of berries consumed by gray wolves. Wildl. Soc. Bull. 2017, 41, 129–131. [Google Scholar] [CrossRef]
- Bosch, G.; Hagen-Plantinga, E.A.; Hendriks, W.H. Dietary nutrient profiles of wild wolves: Insights for optimal dog nutrition? Br. J. Nutr. 2015, 113, S40–S54. [Google Scholar] [CrossRef]
- Pallauf, K.; Bendall, J.K.; Scheiermann, C.; Watschinger, K.; Hoffmann, J.; Roeder, T.; Rimbach, G. Vitamin C and lifespan in model organisms. Food Chem. Toxicol. 2013, 58, 255–263. [Google Scholar] [CrossRef]
- Qian, S.H.; Chen, L.; Xiong, Y.L.; Chen, Z.X. Evolution and function of developmentally dynamic pseudogenes in mammals. Genome Biol. 2022, 23, 235. [Google Scholar] [CrossRef] [PubMed]
- Pink, R.C.; Wicks, K.; Caley, D.P.; Punch, E.K.; Jacobs, L.; Carter, D.R. Pseudogenes: Pseudo-functional or key regulators in health and disease? Rna 2011, 17, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.D.; Frankish, A.; Hunt, T.; Harrow, J.; Gerstein, M. Identification and analysis of unitary pseudogenes: Historic and contemporary gene losses in humans and other primates. Genome Biol. 2010, 11, R26. [Google Scholar] [CrossRef]
- Mighell, A.J.; Smith, N.R.; Robinson, P.A.; Markham, A.F. Vertebrate pseudogenes. FEBS Lett. 2000, 468, 109–114. [Google Scholar] [CrossRef]
- Zhu, J.; Sanborn, J.Z.; Diekhans, M.; Lowe, C.B.; Pringle, T.H.; Haussler, D. Comparative genomics search for losses of long-established genes on the human lineage. PLoS Comput. Biol. 2007, 3, e247. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, S.W.; Faulkner, G.J.; Dinger, M.E. Overcoming challenges and dogmas to understand the functions of pseudogenes. Nat. Rev. Genet. 2020, 21, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Magrinelli, F.; Lohmann, K. PRKRAP1 and other pseudogenes in movement disorders: The troublemakers in genetic analyses are more than genomic fossils. Mov. Disord. Clin. Pr. 2022, 9, 698–702. [Google Scholar] [CrossRef]
- Takuno, S.; Nishio, T.; Satta, Y.; Innan, H. Preservation of a pseudogene by gene conversion and diversifying selection. Genetics 2008, 180, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Henriques, S.F.; Duque, P.; López-Fernández, H.; Vázquez, N.; Fdez-Riverola, F.; Reboiro-Jato, M.; Vieira, C.P.; Vieira, J. Multiple independent L-gulonolactone oxidase (GULO) gene losses and vitamin C synthesis reacquisition events in non-Deuterostomian animal species. BMC Evol. Biol. 2019, 19, 126. [Google Scholar] [CrossRef]
- Inai, Y.; Ohta, Y.; Nishikimi, M. The whole structure of the human nonfunctional L-gulono-γ-lactone oxidase gene—The gene responsible for scurvy—And the evolution of repetitive sequences thereon. J. Nutr. Sci. Vitaminol. 2003, 49, 315–319. [Google Scholar] [CrossRef]
- Brand, A.; Brand, H.; Schulte in den Bäumen, T. The impact of genetics and genomics on public health. Eur. J. Hum. Genet. 2008, 16, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Rowe, S. Factors affecting vitamin C status and prevalence of deficiency: A global health perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef]
- Rivera, S.; Clements, A.; Hess, J.; Gerhartz, N.; Downs, A.; Kruit, L. Scurvy presenting as blood loss anemia in the United States. Off. Publ. State Med. Soc. Wis. 2023, 122, 63–66. [Google Scholar]
- Delanghe, J.R.; Langlois, M.R.; De Buyzere, M.L.; Na, N.; Ouyang, J.; Speeckaert, M.M.; Torck, M.A. Vitamin C deficiency: More than just a nutritional disorder. Genes. Nutr. 2011, 6, 341–346. [Google Scholar] [CrossRef]
- Wang, Q.; Zennadi, R. The role of RBC oxidative stress in sickle cell disease: From the molecular basis to pathologic implications. Antioxidants 2021, 10, 1608. [Google Scholar] [CrossRef]
- Shi, L.; Niedzwiecki, A.; Rath, M. Age and dietary vitamin C intake affect brain physiology in genetically modified mice expressing human lipoprotein(A) and unable to synthesize vitamin C. Curr. Aging Sci. 2021, 14, 223–234. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizeșan, I.; Vlase, L.; Popa, D.S. Antioxidants in age-related diseases and anti-aging strategies. Antioxidants 2022, 11, 1868. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Karwowski, B.T. Nutrition can help DNA repair in the case of aging. Nutrients 2020, 12, 3364. [Google Scholar] [CrossRef]
- Harrison, F.E. A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Kondo, Y.; Ishigami, A. The evidence to date: Implications of l-ascorbic acid in the pathophysiology of aging. J. Physiol. Sci. 2024, 74, 29. [Google Scholar] [CrossRef]
- Carr, A.C.; Zawari, M. Does aging have an impact on vitamin C status and requirements? A scoping review of Comparative Studies of Aging and Institutionalisation. Nutrients 2023, 15, 915. [Google Scholar] [CrossRef] [PubMed]


| Tissue | Mean Value (µmol/L) |
|---|---|
| Brain | 800–900 |
| Lungs | 400 |
| Skeletal muscle | 200–300 |
| Spleen, liver, pancreas | 600–900 |
| Adrenals | 1700–2300 |
| Kidneys | 300–900 |
| Saliva | 0.6 |
| Gastric juice | 136 |
| Urine | 200 |
| Blood plasma | 50 |
| Neutrophil | 1350 |
| Monocyte | 3100 |
| Lymphocyte | 3800 |
| Platelet | 2790 |
| Red blood cells | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grădinaru, A.C.; Popa, S. Vitamin C: From Self-Sufficiency to Dietary Dependence in the Framework of Its Biological Functions and Medical Implications. Life 2025, 15, 238. https://doi.org/10.3390/life15020238
Grădinaru AC, Popa S. Vitamin C: From Self-Sufficiency to Dietary Dependence in the Framework of Its Biological Functions and Medical Implications. Life. 2025; 15(2):238. https://doi.org/10.3390/life15020238
Chicago/Turabian StyleGrădinaru, Andrei Cristian, and Setalia Popa. 2025. "Vitamin C: From Self-Sufficiency to Dietary Dependence in the Framework of Its Biological Functions and Medical Implications" Life 15, no. 2: 238. https://doi.org/10.3390/life15020238
APA StyleGrădinaru, A. C., & Popa, S. (2025). Vitamin C: From Self-Sufficiency to Dietary Dependence in the Framework of Its Biological Functions and Medical Implications. Life, 15(2), 238. https://doi.org/10.3390/life15020238







