A Hypothesis That Glucagon-like Peptide-1 Receptor Agonists Exert Immediate and Multifaceted Effects by Activating Adenosine Monophosphate-Activate Protein Kinase (AMPK)
Abstract
1. Introduction
2. Known Mechanisms
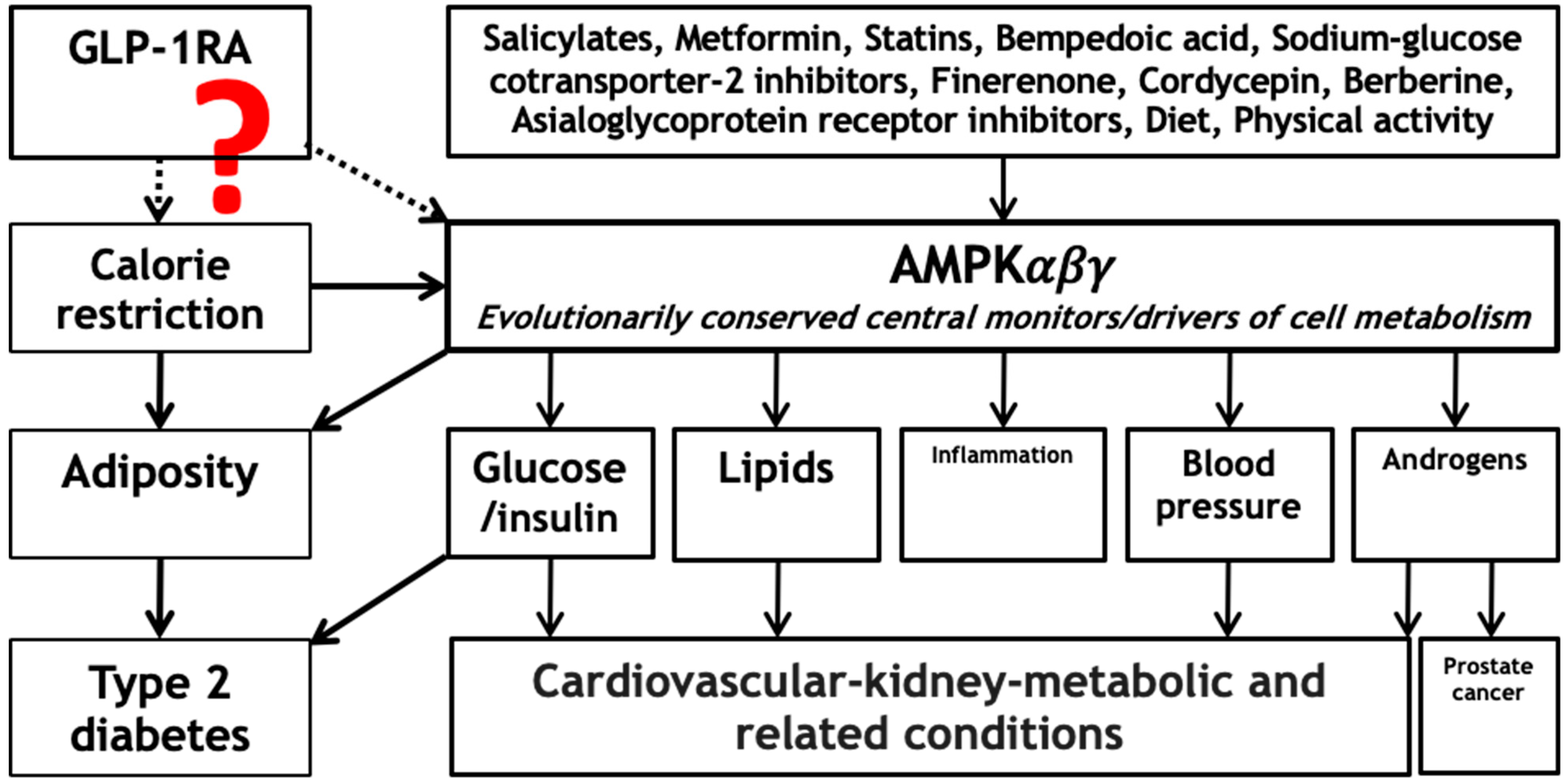
3. Potential Mechanism via Adenosine Monophosphate-Activated Protein Kinase (AMPK)
4. Role of AMPK Activation in Cardiovascular–Kidney–Metabolic Treatments and Other Therapies
5. Next Steps
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPK | adenosine monophosphate-activated protein kinase |
| ASGR1 | asialoglycoprotein receptor 1 |
| CKM | cardiovascular-kidney-metabolic |
| CVD | cardiovascular disease |
| GLP-1RA | glucagon-like peptide-1 receptor agonists |
| MR | Mendelian randomization |
| mTOR | mechanistic target of rapamycin |
| RCT | randomized controlled trial |
| SGLT2 | sodium–glucose cotransporter-2 |
References
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, Y.; Yang, S.; He, G.; Jiang, Z.; Gang, X.; Wang, G. Antidiabetic medications and the risk of prostate cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Pharmacol. Res. 2022, 177, 106094. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Nong, K.; Vandvik, P.O.; Guyatt, G.H.; Schnell, O.; Rydén, L.; Marx, N.; Brosius, F.C., 3rd; Mustafa, R.A.; Agarwal, A.; et al. Benefits and harms of drug treatment for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2023, 381, e074068. [Google Scholar] [CrossRef]
- Fairbank, R. Ozempic keeps wowing: Trial data show benefits for kidney disease. Nature 2024, 630, 16–17. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Neeland, I.J.; Tuttle, K.R.; Chow, S.L.; Mathew, R.O.; Khan, S.S.; Coresh, J.; Baker-Smith, C.M.; Carnethon, M.R.; Després, J.-P.; et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 1636–1664. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.M.; Schooling, C.M. Effect of Glucagon on Ischemic Heart Disease and Its Risk Factors: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2020, 105, e2778–e2788. [Google Scholar] [CrossRef]
- Kalra, S.; Jacob, J.J.; Gupta, Y. Newer antidiabetic drugs and calorie restriction mimicry. Indian J. Endocrinol. Metab. 2016, 20, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Alton, L.A.; Bywater, C.L.; Lombardi, E.J.; Marshall, D.J. Metabolic scaling is the product of life-history optimization. Science 2022, 377, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Zhang, J. Evidence for the role of selection for reproductively advantageous alleles in human aging. Sci. Adv. 2023, 9, eadh4990. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Krupa Das, S.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Strom, A.; Jensen, R.A. Mortality from circulatory diseases in Norway 1940–1945. Lancet 1951, 1, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Hindhede, M. The effect of food restriction during war on mortality in Copenhagen. J. Am. Med. Assoc. 1920, 74, 381–382. [Google Scholar] [CrossRef][Green Version]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef]
- Reis-Barbosa, P.H.; Marcondes-de-Castro, I.A.; Marinho, T.S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. The mTORC1/AMPK pathway plays a role in the beneficial effects of semaglutide (GLP-1 receptor agonist) on the liver of obese mice. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101922. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Hardie, D.G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Tricò, D.; Del Prato, S. Incretins and cardiovascular disease: To the heart of type 2 diabetes? Diabetologia 2023, 66, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Rajani, R.; Pastor-Soler, N.M.; Hallows, K.R. Role of AMP-activated protein kinase in kidney tubular transport, metabolism, and disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 375–383. [Google Scholar] [CrossRef]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Marzolla, V.; Feraco, A.; Gorini, S.; Mammi, C.; Marrese, C.; Mularoni, V.; Boitani, C.; Lombès, M.; Kolkhof, P.; Ciriolo, M.R.; et al. The novel non-steroidal MR antagonist finerenone improves metabolic parameters in high-fat diet-fed mice and activates brown adipose tissue via AMPK-ATGL pathway. Faseb J. 2020, 34, 12450–12465. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, L.; Song, H.; Bao, X.; Niu, S.; Bai, J.; Ma, J.; Yuan, R.; Liu, S.; Guo, J. Cordyceps: Alleviating Ischemic Cardiovascular and Cerebrovascular Injury—A Comprehensive Review. J. Ethnopharmacol. 2024, 332, 118321. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Li, L.L.; Hu, A.; Deng, G.; Wei, J.; Li, Y.F.; Liu, Y.B.; Lu, X.Y.; Qiu, Z.P.; Shi, X.J.; et al. Inhibition of ASGR1 decreases lipid levels by promoting cholesterol excretion. Nature 2022, 608, 413–420. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Schooling, C.M.; Au Yeung, S.L.; Freeman, G.; Cowling, B.J. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ding, B.; Shen, Y.; Yan, R.N.; Li, F.F.; Sun, R.; Jing, T.; Lee, K.O.; Ma, J.H. Rapid Changes in Serum Testosterone in Men With Newly Diagnosed Type 2 Diabetes With Intensive Insulin and Metformin. Diabetes Care 2021, 44, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Schooling, C.M.; Zhao, J.V.; Au Yeung, S.L.; Leung, G.M. Investigating pleiotropic effects of statins on ischemic heart disease in the UK Biobank using Mendelian randomisation. eLife 2020, 9, e58567. [Google Scholar] [CrossRef] [PubMed]
- Elkind-Hirsch, K.E.; Chappell, N.; Shaler, D.; Storment, J.; Bellanger, D. Liraglutide 3 mg on weight, body composition, and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: A randomized placebo-controlled-phase 3 study. Fertil. Steril. 2022, 118, 371–381. [Google Scholar] [CrossRef]
- Lengsfeld, S.; Probst, L.; Emara, Y.; Werlen, L.; Vogt, D.R.; Bathelt, C.; Baur, F.; Caviezel, B.; Vukajlovic, T.; Fischer, M.; et al. Effects of the glucagon-like peptide-1 receptor agonist dulaglutide on sexuality in healthy men: A randomised, double-blind, placebo-controlled crossover study. eBioMedicine 2024, 107, 105284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.V.; Schooling, C.M. The role of testosterone in chronic kidney disease and kidney function in men and women: A bi-directional Mendelian randomization study in the UK Biobank. BMC Med. 2020, 18, 122. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. Macronutrient balance and lifespan. Aging 2009, 1, 875–880. [Google Scholar] [CrossRef]
- Di Francesco, A.; Deighan, A.G.; Litichevskiy, L.; Chen, Z.; Luciano, A.; Robinson, L.; Garland, G.; Donato, H.; Vincent, M.; Schott, W.; et al. Dietary restriction impacts health and lifespan of genetically diverse mice. Nature 2024, 634, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Vara-Ciruelos, D.; Russell, F.M.; Hardie, D.G. The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde? Open Biol. 2019, 9, 190099. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, B.; Lu, Y.; Donahoo, W.T.; Singh Ospina, N.; Kotecha, P.; Lu, Y.; Tong, J.; Smith, S.M.; Rosenberg, E.I.; et al. Assessing the benefit-risk profile of newer glucose-lowering drugs: A systematic review and network meta-analysis of randomized outcome trials. Diabetes Obes. Metab. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Bouras, E.; Constantinescu, A.; Burrows, K.; Bull, C.J.; Vincent, E.E.; Martin, R.M.; Dimopoulou, O.; Lewis, S.J.; Moreno, V.; et al. Genetically proxied glucose-lowering drug target perturbation and risk of cancer: A Mendelian randomisation analysis. Diabetologia 2023, 66, 1481–1500. [Google Scholar] [CrossRef]
- Scirica, B.M.; Lincoff, A.M.; Lingvay, I.; Bogdanski, P.; Buscemi, S.; Colhoun, H.; Craciun, A.E.; Ezhov, M.; Hardt-Lindberg, S.; Kleist Jeppesen, O.; et al. The Effect of Semaglutide on Mortality and COVID-19-Related Deaths: An Analysis From the SELECT Trial. J. Am. Coll. Cardiol. 2024, 84, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J.; et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef] [PubMed]
- Schick, R.R.; Zimmermann, J.P.; vorm Walde, T.; Schusdziarra, V. Peptides that regulate food intake: Glucagon-like peptide 1-(7-36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1427–R1435. [Google Scholar] [CrossRef]
- Burmeister, M.A.; Brown, J.D.; Ayala, J.E.; Stoffers, D.A.; Sandoval, D.A.; Seeley, R.J.; Ayala, J.E. The glucagon-like peptide-1 receptor in the ventromedial hypothalamus reduces short-term food intake in male mice by regulating nutrient sensor activity. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E651–E662. [Google Scholar] [CrossRef]
- Huang, K.P.; Acosta, A.A.; Ghidewon, M.Y.; McKnight, A.D.; Almeida, M.S.; Nyema, N.T.; Hanchak, N.D.; Patel, N.; Gbenou, Y.S.K.; Adriaenssens, A.E.; et al. Dissociable hindbrain GLP1R circuits for satiety and aversion. Nature 2024, 632, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Briski, K.P. Hindbrain dorsal vagal complex AMPK controls hypothalamic gluco-regulatory transmitter and counter-regulatory hormone responses to hypoglycemia. Brain Res. Bull. 2019, 144, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.P.; Arnold, M.; Pettersen, K.G.; Lossel, P.; Langhans, W.; Lee, S.J. Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia. Diabetes 2016, 65, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Journel, M.; Chaumontet, C.; Darcel, N.; Fromentin, G.; Tomé, D. Brain responses to high-protein diets. Adv. Nutr. 2012, 3, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Song, J.; Yang, L.; Wang, D.; Yuan, G.; Zhao, L. Mechanistic insights into GLP-1 receptor agonist-induced weight loss through ceRNA network analysis. Genomics 2025, 117, 110988. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.R.; Harris, D.D.; Broadwin, M.; Kanuparthy, M.; Nho, J.-W.; Yalamanchili, K.; Hamze, J.; Abid, M.R.; Sellke, F.W. Semaglutide Improves Myocardial Perfusion and Performance in a Large Animal Model of Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2024, 45, 285–297. [Google Scholar] [CrossRef]
- Hurtado-Carneiro, V.; Sanz, C.; Roncero, I.; Vazquez, P.; Blazquez, E.; Alvarez, E. Glucagon-like peptide 1 (GLP-1) can reverse AMP-activated protein kinase (AMPK) and S6 kinase (P70S6K) activities induced by fluctuations in glucose levels in hypothalamic areas involved in feeding behaviour. Mol. Neurobiol. 2012, 45, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Velasco, C.; Comesaña, S.; Conde-Sieira, M.; Míguez, J.M.; Soengas, J.L. Effects of CCK-8 and GLP-1 on fatty acid sensing and food intake regulation in trout. J. Mol. Endocrinol. 2019, 62, 101–116. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef]
- Mosenzon, O.; Capehorn, M.S.; De Remigis, A.; Rasmussen, S.; Weimers, P.; Rosenstock, J. Impact of semaglutide on high-sensitivity C-reactive protein: Exploratory patient-level analyses of SUSTAIN and PIONEER randomized clinical trials. Cardiovasc. Diabetol. 2022, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Rached, F.H.; Miname, M.H. Insights into the Role of Inflammation in the Management of Atherosclerosis. J. Inflamm. Res. 2023, 16, 2223–2239. [Google Scholar] [CrossRef]
- Shaw, R.J. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol. 2009, 196, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.; Dib, M.J.; Cronjé, H.T.; Karhunen, V.; Woolf, B.; Gagnon, E.; Daghlas, I.; Nyberg, M.; Drakeman, D.; Burgess, S. Common pitfalls in drug target Mendelian randomization and how to avoid them. BMC Med. 2024, 22, 473. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schooling, C.M.; Yang, G.; Soliman, G.A.; Leung, G.M. A Hypothesis That Glucagon-like Peptide-1 Receptor Agonists Exert Immediate and Multifaceted Effects by Activating Adenosine Monophosphate-Activate Protein Kinase (AMPK). Life 2025, 15, 253. https://doi.org/10.3390/life15020253
Schooling CM, Yang G, Soliman GA, Leung GM. A Hypothesis That Glucagon-like Peptide-1 Receptor Agonists Exert Immediate and Multifaceted Effects by Activating Adenosine Monophosphate-Activate Protein Kinase (AMPK). Life. 2025; 15(2):253. https://doi.org/10.3390/life15020253
Chicago/Turabian StyleSchooling, C. Mary, Guoyi Yang, Ghada A. Soliman, and Gabriel M. Leung. 2025. "A Hypothesis That Glucagon-like Peptide-1 Receptor Agonists Exert Immediate and Multifaceted Effects by Activating Adenosine Monophosphate-Activate Protein Kinase (AMPK)" Life 15, no. 2: 253. https://doi.org/10.3390/life15020253
APA StyleSchooling, C. M., Yang, G., Soliman, G. A., & Leung, G. M. (2025). A Hypothesis That Glucagon-like Peptide-1 Receptor Agonists Exert Immediate and Multifaceted Effects by Activating Adenosine Monophosphate-Activate Protein Kinase (AMPK). Life, 15(2), 253. https://doi.org/10.3390/life15020253





