An Evaluation of Morphometric Characteristics of Honey Bee (Apis cerana) Populations in the Qinghai–Tibet Plateau in China
Abstract
1. Introduction
2. Materials and Methods
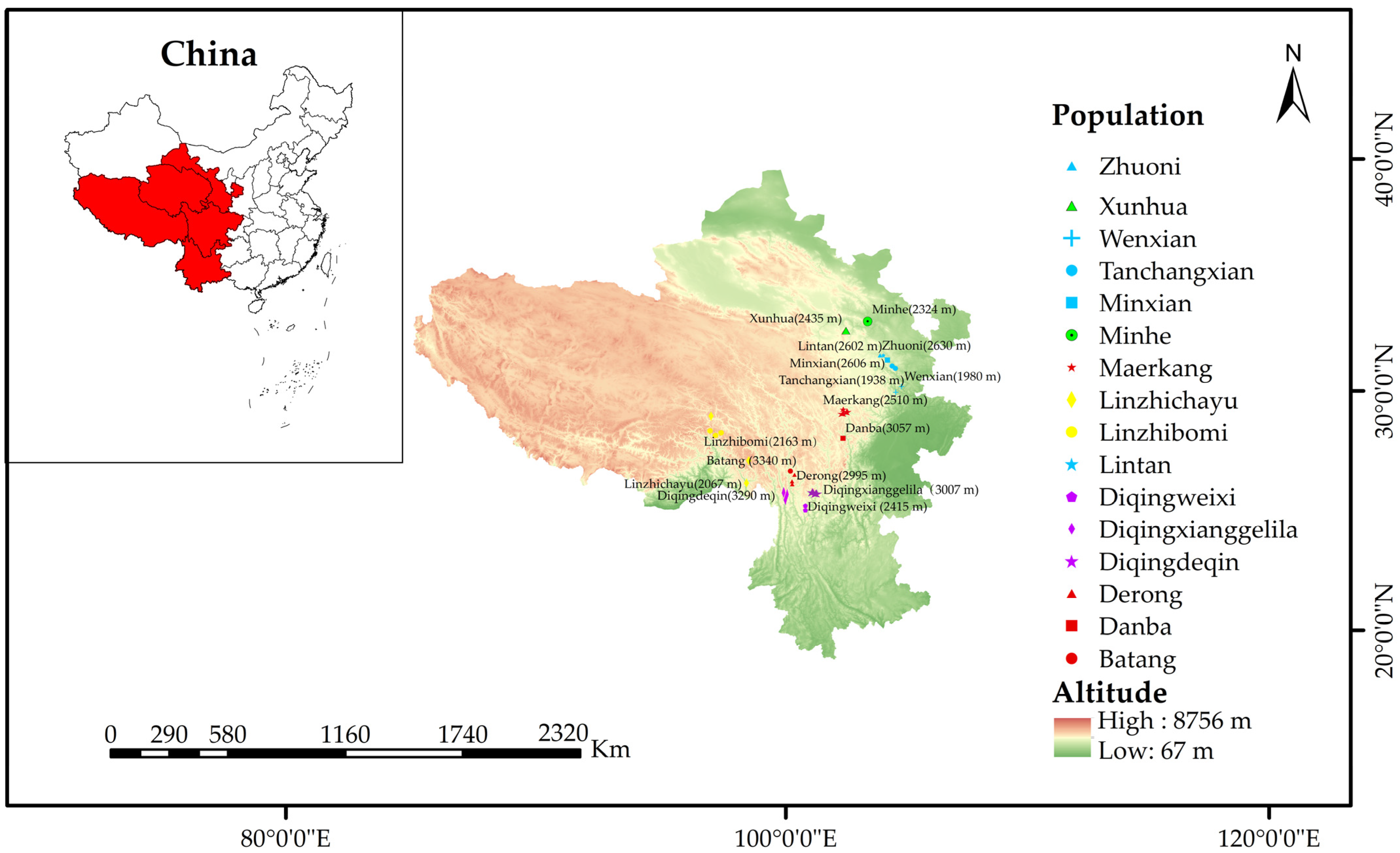
2.1. Description of the Area of Study
2.2. Collection of Honey Bees
2.3. Sample Preparation and Morphometric Measurements
2.4. Statistical Analyses
3. Results
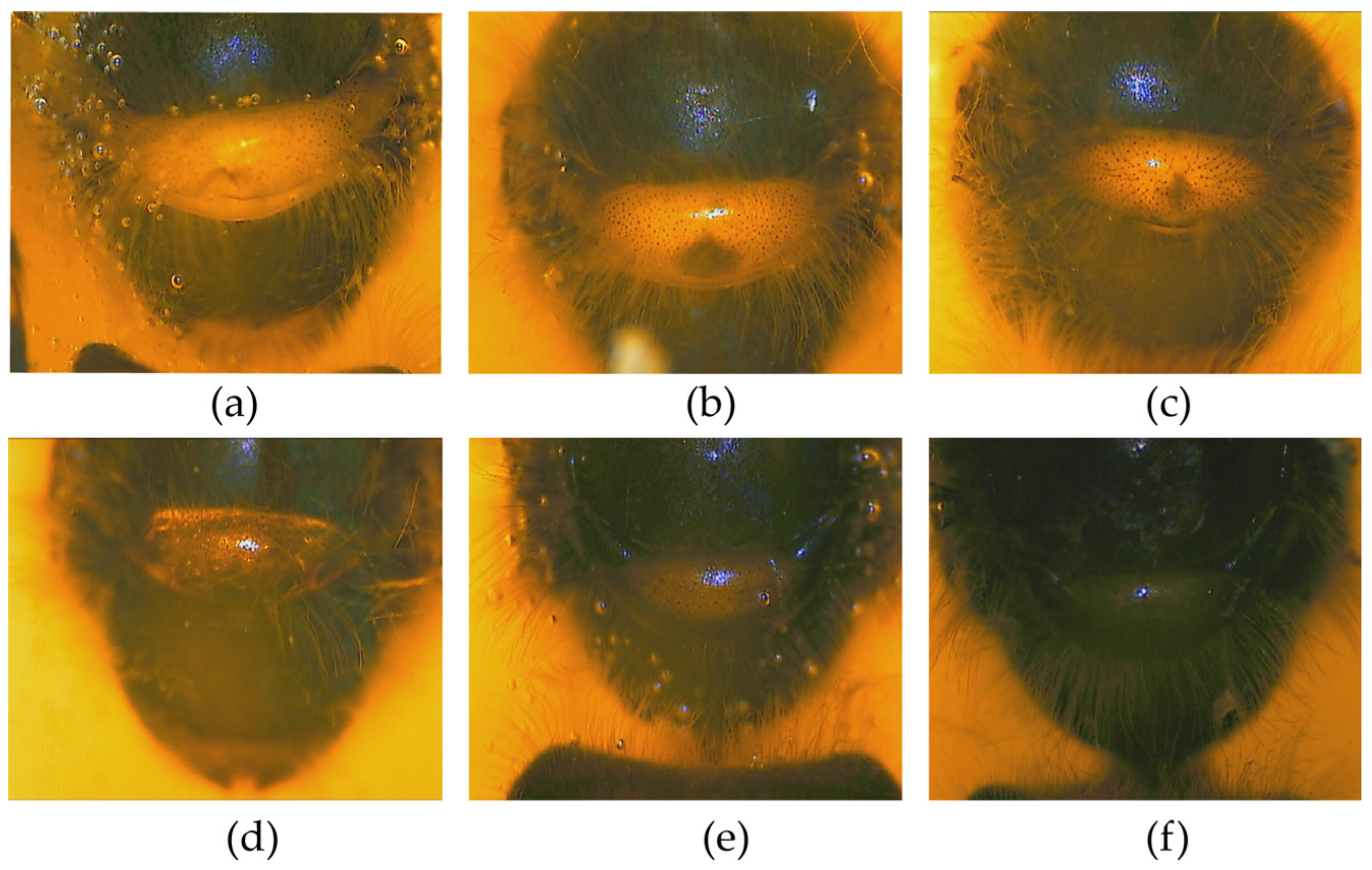
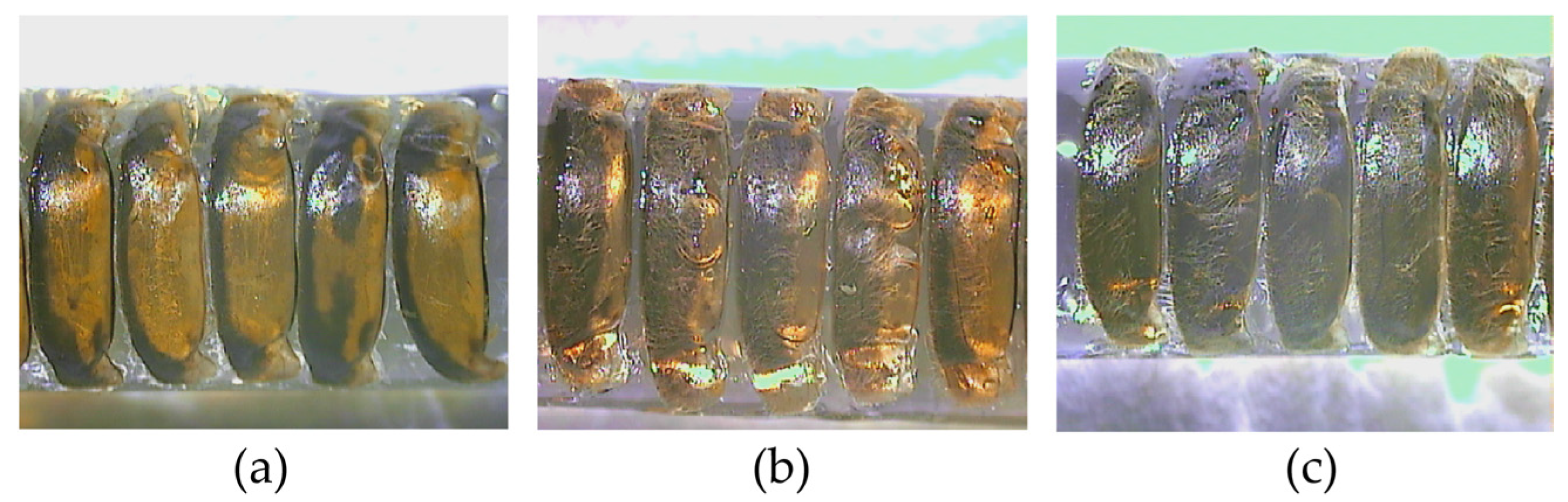
3.1. Pigmentation of Labrum, Scutellum, and Tergite
3.2. Body Size Characteristics
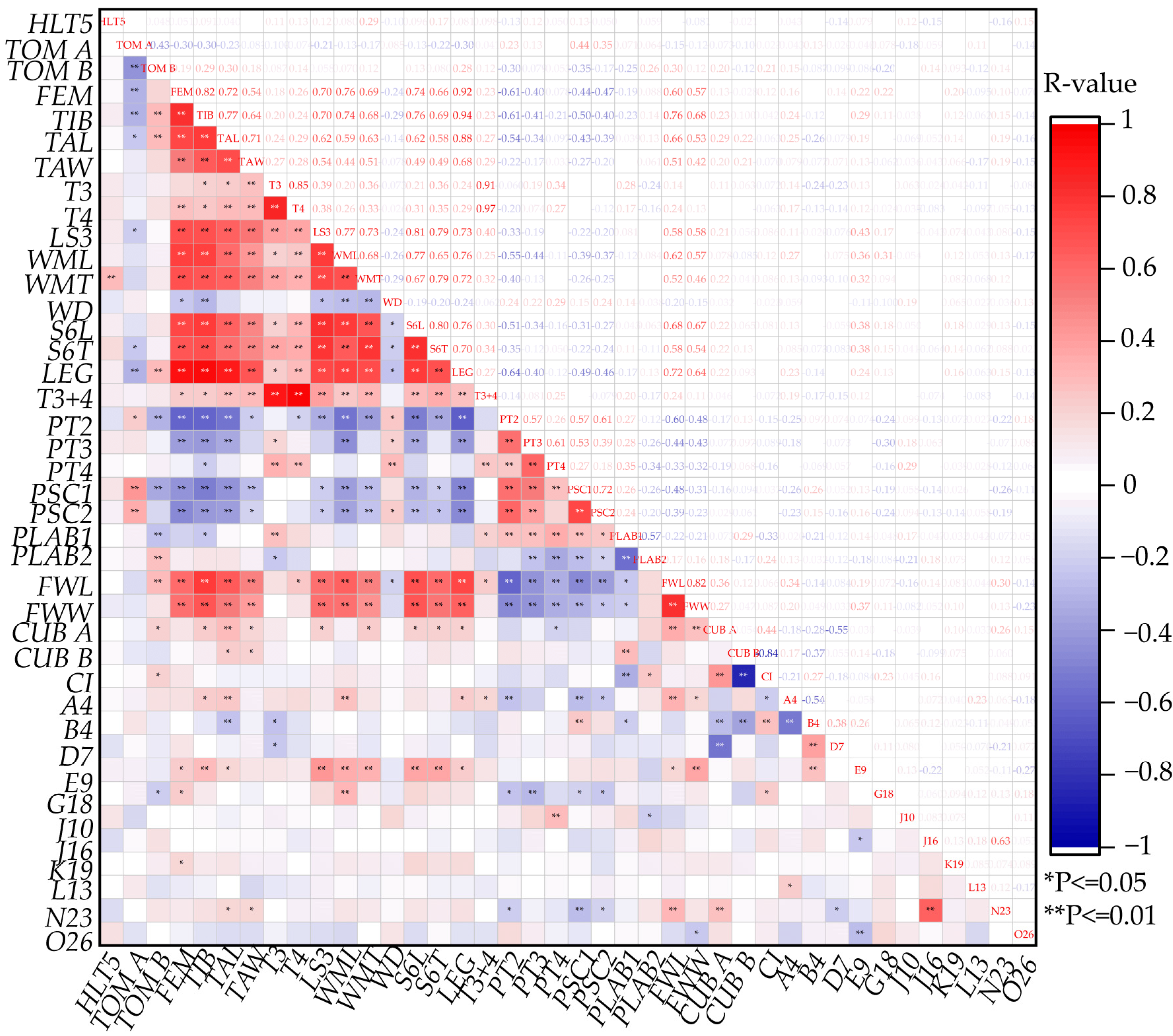
3.3. Correlation Coefficient of Morphology Features
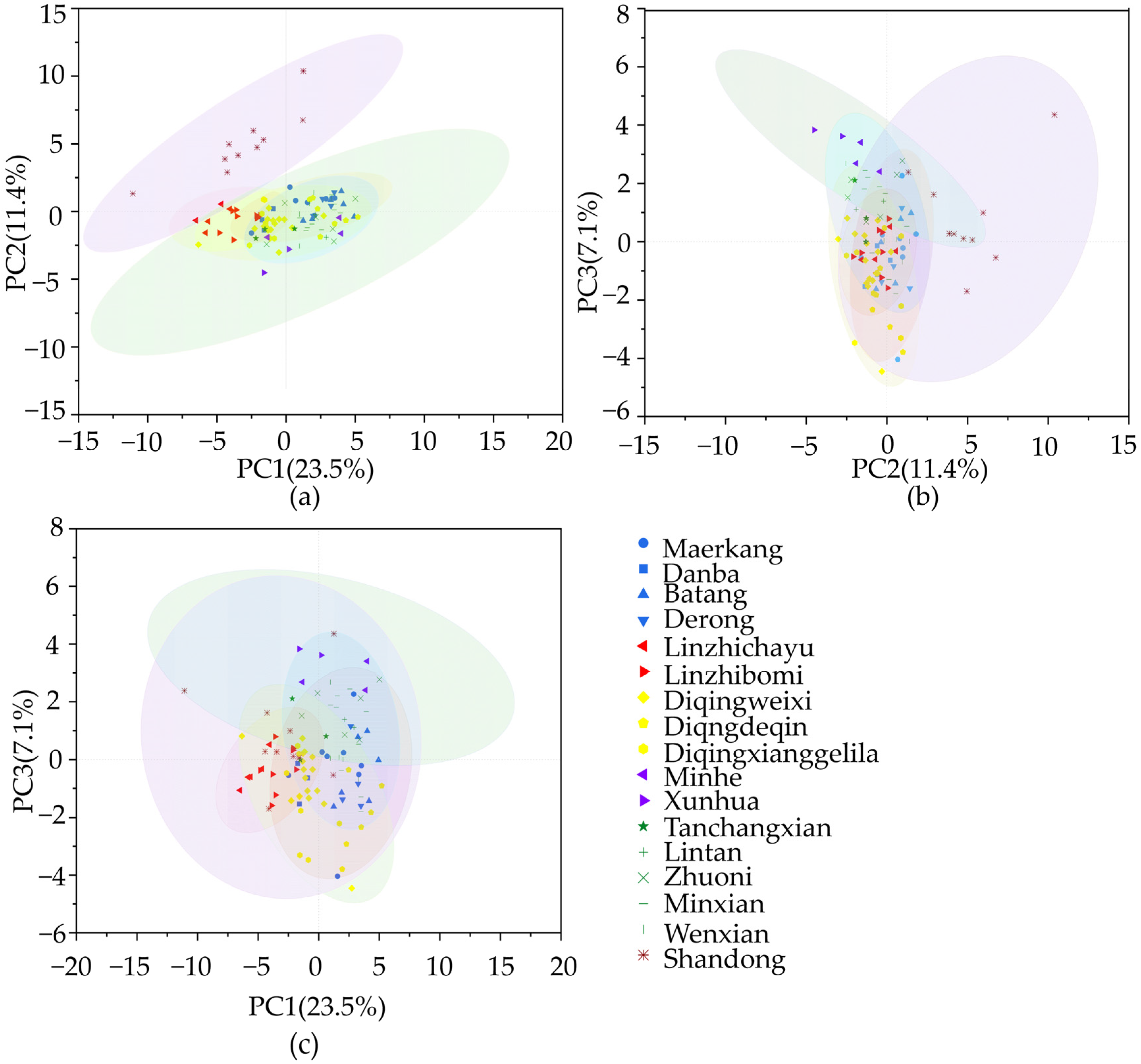
3.4. Principal Components Analysis of Morphology Features
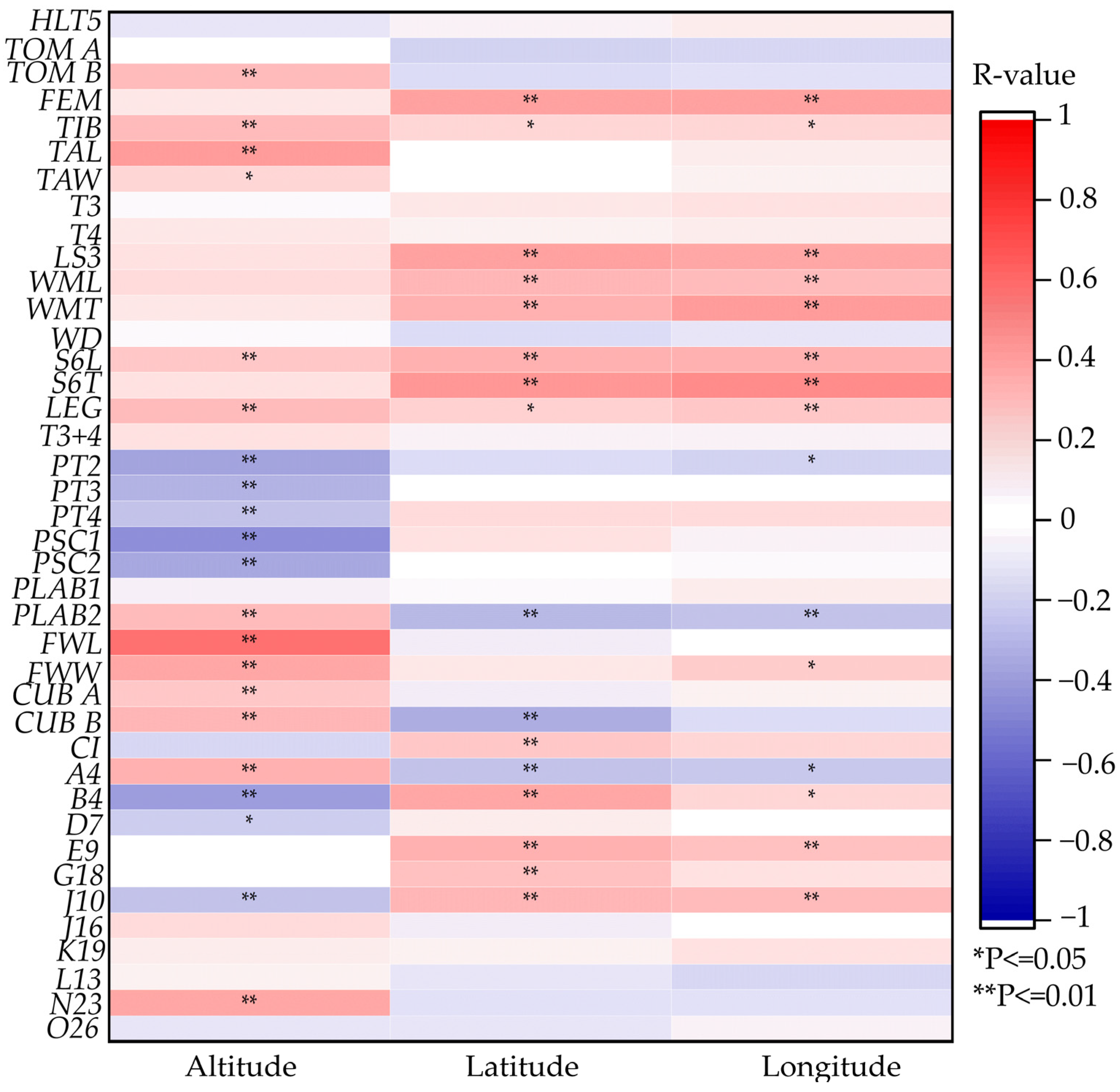
3.5. Correlation Analysis of Morphology Features and Geographical Features
3.6. Cluster Analysis of Morphologic Features
3.7. Stepwise Discriminant Analysis of Morphometric Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, P.; Zhou, J.; Song, H.; Wu, Y.; Lan, L.; Tang, X.; Ma, Z.; Vossbrinck, C.R.; Vossbrinck, B.; Zhou, Z.; et al. Genomic analysis of Asian honeybee populations in China reveals evolutionary relationships and adaptation to abiotic stress. Ecol. Evol. 2020, 10, 13427–13438. [Google Scholar] [CrossRef] [PubMed]
- Abrol, D.P. Asiatic Honeybee Apis Cerana: Biodiversity Conservation and Agricultural Production; Springer Nature: Berlin/Heidelberg, Germany, 2013; p. 1019. [Google Scholar] [CrossRef]
- China National Commission of Animal Genetic Resources. Animal genetic resources in China: Bees. In The Formation of Chinese Honey Bees; China Agriculture Press: Beijing, China, 2011; p. 7. (In Chinese) [Google Scholar]
- Luo, Y.X.; Chen, L.H. The Current Situation and Suggestions for the Rearing of Chinese Bees in China. J. Anim. Sci. 2014, 24, 22–23. [Google Scholar]
- Luo, Y.X.; Chen, L.D. The Current Situation of Chinese Bee Breeding in China. Apic. China 2018, 69, 22–24. [Google Scholar]
- Wang, Y.C.; Zeng, B.; Deng, M.Q.; Zhao, T.; Liao, Y.; Ren, R.Q.; Wang, H.; Yuan, Y. Whole-genome resequencing reveals genetic diversity and adaptive evolution in Chinese honeybee (Apis cerana cerana) in Guizhou, China. Front. Genet. 2024, 15, 1352455. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.R.; Araújo, E.D.; Gramacho, K.P.; Nunes, L.A. Bee’s morphometrics and behavior in response to seasonal effects from ecoregions. Genet. Mol. Res. 2016, 15, gmr7597. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Wang, Z.; Jie, H.L.; Gao, F.C.; Cai, M.Q.; Wang, K.; Chen, D.F.; Guo, R.; Lin, Z.G.; et al. A key gene for the climatic adaptation of Apis cerana populations in China according to selective sweep analysis. BMC Genom. 2023, 24, 100. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Luo, J.Y. Research progress of the population genetic differentication and environmental adaptation mechanisms in Apis cerana cerana (Hymenoptera:Apidae). Acta Ecol. Sin. 2023, 66, 1258–1270. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, B.Y.; Zheng, D. A discussion on the boundary and area of the Tibetan Plateau in China. Geogr. Res. 2022, 21, 1–8. [Google Scholar] [CrossRef]
- The State Council Information Office of the People’s Republic of China. Ecological Progress on the Qinghai-Tibet Plateau. Available online: http://www.scio.gov.cn/zfbps/ndhf/2018n/202207/t20220704_130607.html (accessed on 18 July 2018).
- Liu, J.; Milne, R.; Cadotte, M.W.; Wu, Z.-Y.; Provan, J.L.; Zhu, G.-F.; Gao, L.; Li, D.Z. Protect Third Pole’s fragile ecosystem. Science 2018, 362, 1368. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Y.; Song, H.L.; Shi, P.; Zhang, X.Y.; Tang, Z.H.; Wang, W.F.; Zha, L.; Chen, X.L.; Zhou, Z.Y.; Xu, J.S. Whole-genome resequencing reveals the genetic diversity and adaptive evolution of Apis cerana (Hymenoptera: Apidae) on the eastern and southeastern edges of the Qinghai-Tibet Plateau. Acta Entomol. Sin. 2022, 65, 638–647. [Google Scholar] [CrossRef]
- Yang, G.H. Chinese Honeybee; Agricultural Science and Technology Press: Beijing, China, 2001. (In Chinese) [Google Scholar]
- Yu, Y.L.; Zhou, S.J.; Zhu, X.J.; Xu, X.J.; Wang, W.F.; Zha, L.; Wang, P.; Wang, J.W.; Lai, K.; Wang, S.H.; et al. Genetic Differentiation of Eastern Honey Bee (Apis cerana) Populations Across Qinghai-Tibet Plateau-Valley Landforms. Front. Genet. 2019, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.C.; Shi, W.; Tang, J.; Ji, T.; Gao, J.L.; Liu, F.; Shan, J.Q.; Chen, X.; Chen, C. Morphometrical analyses revealed high diversity of the eastern honey bee (Apis cerana) in mountains and islands in China. J. Apic. Res. 2023, 62, 647–655. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.d.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Theisen-Jones, H.; Bienefeld, K. The Asian Honey Bee (Apis cerana) is Significantly in Decline. Bee World 2017, 93, 90–97. [Google Scholar] [CrossRef]
- Sommer, R.J. Phenotypic Plasticity: From Theory and Genetics to Current and Future Challenges. Genetics 2020, 215, 1–13. [Google Scholar] [CrossRef]
- Stearns, S.C. The Evolutionary Significance of Phenotypic Plasticity. BioScience 1989, 39, 436–445. [Google Scholar] [CrossRef]
- Holloway, G.J. Phenotypic Plasticity: Beyond Nature and Nurture. Heredity 2002, 89, 410. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: Oxford, UK, 2003. [Google Scholar] [CrossRef]
- Forsman, A. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 2014, 115, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Radloff, S.E.; Hepburn, C.; Hepburn, R.; Fuchs, S.; Hadisoesilo, S.; Tan, K.; Engel, M.S.; Kuznetsov, V. Population structure and classification of Apis cerana. Apidologie 2010, 41, 589–601. [Google Scholar] [CrossRef]
- Zhu, X.J.; Zhou, B.F.; Wang, Y.; Wu, X.D.; Han, X.Q. Study on morphological genetics analytical method of Apis cerana cerana population. Chin. J. Appl. Entomol. 2011, 48, 202–206. [Google Scholar]
- Ruttner, F.; Tassencourt, L.; Louveaux, J. Biometrical-Statistical Analysis of the Geographic Variability of Apis mellifera L.* I. Material and Methods. Apidologie 1978, 9, 363–381. [Google Scholar] [CrossRef]
- Ruttner, F. Morphometric Analysis and Classification. In Biogeography and Taxonomy of Honeybees, 1st ed.; Springer: New York, NY, USA, 1988; pp. 66–78. [Google Scholar] [CrossRef]
- Bouga, M.; Alaux, C.; Bienkowska, M.; Büchler, R.; Carreck, N.L.; Cauia, E.; Chlebo, R.; Dahle, B.; Dall’Olio, R.; De la Rúa, P.; et al. A review of methods for discrimination of honey bee populations as applied to European beekeeping. J. Apic. Res. 2015, 50, 51–84. [Google Scholar] [CrossRef]
- Meixner, M.D.; Pinto, M.A.; Bouga, M.; Kryger, P.; Ivanova, E.; Fuchs, S. Standard methods for characterising subspecies and ecotypes of Apis mellifera. J. Apic. Res. 2015, 52, 1–28. [Google Scholar] [CrossRef]
- Tan, K.; Stefan, F.; Nikolaus, K.; Zan, R.g. Morphological characterization of Apis cerana in the Yunnan Province of China. Apidologie 2003, 34, 553–561. [Google Scholar] [CrossRef]
- Tan, K.; Qu, Y.F.; Wang, Z.W.; Liu, Z.W.; Engel, M.S. Haplotype diversity and genetic similarity among populations of the Eastern honey bee from Himalaya-Southwest China and Nepal (Hymenoptera: Apidae). Apidologie 2015, 47, 197–205. [Google Scholar] [CrossRef][Green Version]
- Liu, N.N.; Liu, H.M.; Ju, Y.; Li, X.a.; Li, Y.; Wang, T.J.; He, J.M.; Niu, Q.S.; Mei, X.X. Geometric morphology and population genomics provide insights into the adaptive evolution of Apis cerana in Changbai Mountain. BMC Genom. 2022, 23, 64. [Google Scholar] [CrossRef]
- Zhu, X.J.; Zhou, S.J.; Xu, X.J.; Wang, J.W.; Yu, Y.L.; Yang, K.-c.; Luo, Q.; Xu, Y.Y.; Wang, S.H.; Zhou, B.F. Morphological differentiation in Asian honey bee (Apis cerana) populations in the basin and highlands of southwestern China. J. Apic. Res. 2017, 56, 203–209. [Google Scholar] [CrossRef]
- Zhu, X.J.; Zhou, S.J.; Xu, X.J.; Yu, Y.L.; Hu, J.J.; Zhang, Z.Y.; Qi, W.Z.; Wang, B.; Yuan, C.Y.; Xi, F.G.; et al. Morphological differentiation in the Asian honey bees (Apis cerana) in China. Acta Entomol. Sin. 2022, 65, 912–926. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates, Inc.: Mahwah, NJ, USA, 1988; p. 567. [Google Scholar]
- Gong, Y.L.; Lei, Y.; Yan, Z.Q.; Liu, X.W.; Zhang, D.S.; Wu, J.Q.; Zhu, S.S. Comprehensive Evaluation of Phenotype Genetic Diversity in Japonica Rice Germplasm Resources in Different Ecological Zones. Crops 2020, 35, 71–79. [Google Scholar] [CrossRef]
- Lukens, L.; Li, Z.; Coffey, L.; Garfin, J.; Miller, N.D.; White, M.R.; Spalding, E.P.; de Leon, N.; Kaeppler, S.M.; Schnable, P.S.; et al. Genotype-by-environment interactions affecting heterosis in maize. PLoS ONE 2018, 13, e0191321. [Google Scholar] [CrossRef]
- Chen, C.; Wang, H.H.; Liu, Z.G.; Chen, X.; Tang, J.; Meng, F.M.; Shi, W.; Innan, H. Population Genomics Provide Insights into the Evolution and Adaptation of the Eastern Honey Bee (Apis cerana). Mol. Biol. Evol. 2018, 35, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Shi, P.; Song, H.L.; Tang, X.Y.; Zhou, J.Y.; Yang, J.D.; Yang, M.X.; Xu, J.S. De Novo Genome Assembly of Chinese Plateau Honeybee Unravels Intraspecies Genetic Diversity in the Eastern Honeybee, Apis cerana. Insects 2021, 12, 891. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Chao, T.L.; Fan, Y.H.; Lou, D.L.; Wang, G.Z. Population genomics and morphological features underlying the adaptive evolution of the eastern honey bee (Apis cerana). BMC Genom. 2019, 20, 869. [Google Scholar] [CrossRef]
- Liu, F.; Shi, T.F.; Huang, S.S.; Yu, L.S.; Bi, S.D. Genetic structure of Mount Huang honey bee (Apis cerana) populations: Evidence from microsatellite polymorphism. Hereditas 2016, 153, 8. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.H.; Zhu, X.; Li, M.S.; Chen, M.; Zhang, S.B.; Yang, X.Y.; Zheng, Z.Y.; Qin, Y.K.; Zhang, Y.; Lv, S.H. Creation and Verification of a High-Resolution Multi-Parameter Surface Meteorological Assimilation Dataset for the Tibetan Plateau for 2010–2020 Available Online. Remote Sens. 2023, 15, 2906. [Google Scholar] [CrossRef]
- Mao, K.S.; Wang, Y.; Liu, J.Q. Evolutionary origin of species diversity on the Qinghai–Tibet Plateau. J. Syst. Evol. 2021, 59, 1142–1158. [Google Scholar] [CrossRef]
- Thorax of the Honey Bee. Available online: https://bee-health.extension.org/thorax-of-the-honey-bee/ (accessed on 20 August 2019).
- Matherne, M.E.; Anyanwu, G.; Leavey, J.K.; Hu, D.L. How honey bees carry pollen. In Proceedings of the 70th Annual Meeting of the American Physical Society Division of Fluid Dynamics, Denver, CO, USA, 19–21 November 2017. [Google Scholar]
- Zeng, Z.J. Analysis of correlative degree between morphological characters of worker bees and quantity of collect honey. Apic. China 1992, 1, 12–23. (In Chinese) [Google Scholar]
- Barry, R.G. Mountain Weather and Climate, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Chen, S.A.; Zhang, Q.; Wan, R.; Zhao, S.W.; Liu, Z.T.; Luo, J.C.; Ye, Y.L.; Zhang, X.W. Response of food resources and trophic niche of Apis cerana cerana to an altitudinal gradient in the north valley of the Lancang River. Acta Ecol. Sin. 2017, 37, 3201–3211. [Google Scholar] [CrossRef]
- Montero-Mendieta, S.; Tan, K.; Christmas, M.J.; Olsson, A.; Vilà, C.; Wallberg, A.; Webster, M.T. The genomic basis of adaptation to high-altitude habitats in the eastern honey bee (Apis cerana). Mol. Ecol. 2019, 28, 746–760. [Google Scholar] [CrossRef]
- Harrison, J.F.; Haddad, G.G. Effects of oxygen on growth and size: Synthesis of molecular, organismal, and evolutionary studies with Drosophila melanogaster. Annu. Rev. Physiol. 2011, 73, 95–113. [Google Scholar] [CrossRef]
- Callier, V.; Nijhout, H.F. Control of body size by oxygen supply reveals size-dependent and size-independent mechanisms of molting and metamorphosis. Proc. Natl. Acad. Sci. USA 2011, 108, 14664–14669. [Google Scholar] [CrossRef]
- Nijhout, H.F.; Callier, V. Developmental Mechanisms of Body Size and Wing-Body Scaling in Insects. Annu. Rev. Entomol. 2015, 60, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Klok, C.J.; Harrison, J.F. Atmospheric Hypoxia Limits Selection for Large Body Size in Insects. PLoS ONE 2009, 4, e3876. [Google Scholar] [CrossRef][Green Version]
- Azevedo, S.V.; Caranton, O.A.M.; de Oliveira, T.L.; Hartfelder, K. Differential expression of hypoxia pathway genes in honey bee (Apis mellifera L.) caste development. J. Insect Physiol. 2011, 57, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Gorr, T.A.; Gassmann, M.; Wappner, P. Sensing and responding to hypoxia via HIF in model invertebrates. J. Insect Physiol. 2006, 52, 349–364. [Google Scholar] [CrossRef]
- Harrison, J.F.; Kaiser, A.; VandenBrooks, J.M. Atmospheric oxygen level and the evolution of insect body size. Proc. R. Soc. B 2010, 277, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Mittelbach, G.G.; Schemske, D.W.; Cornell, H.V.; Allen, A.P.; Brown, J.M.; Bush, M.B.; Harrison, S.P.; Hurlbert, A.H.; Knowlton, N.; Lessios, H.A.; et al. Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecol. Lett. 2007, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.M.V.; Anderson, M.J.; Eme, D.; Liggins, L.; Roberts, C.D.; Griffen, B. Changes in key traits versus depth and latitude suggest energy-efficient locomotion, opportunistic feeding and light lead to adaptive morphologies of marine fishes. J. Anim. Ecol. 2019, 89, 309–322. [Google Scholar] [CrossRef]
- Morales Vargas, R.E.; Ya-umphan, P.; Phumala-Morales, N.; Komalamisra, N.; Dujardin, J.P. Climate associated size and shape changes in Aedes aegypti (Diptera: Culicidae) populations from Thailand. Infect. Genet. Evol. 2010, 10, 580–585. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Lu, J.; Qu, X.; Chen, X. An Evaluation of Morphometric Characteristics of Honey Bee (Apis cerana) Populations in the Qinghai–Tibet Plateau in China. Life 2025, 15, 255. https://doi.org/10.3390/life15020255
Zhang X, Lu J, Qu X, Chen X. An Evaluation of Morphometric Characteristics of Honey Bee (Apis cerana) Populations in the Qinghai–Tibet Plateau in China. Life. 2025; 15(2):255. https://doi.org/10.3390/life15020255
Chicago/Turabian StyleZhang, Xinru, Jian Lu, Xinying Qu, and Xiao Chen. 2025. "An Evaluation of Morphometric Characteristics of Honey Bee (Apis cerana) Populations in the Qinghai–Tibet Plateau in China" Life 15, no. 2: 255. https://doi.org/10.3390/life15020255
APA StyleZhang, X., Lu, J., Qu, X., & Chen, X. (2025). An Evaluation of Morphometric Characteristics of Honey Bee (Apis cerana) Populations in the Qinghai–Tibet Plateau in China. Life, 15(2), 255. https://doi.org/10.3390/life15020255






