Thrombocytopenia in Sepsis
Abstract
:1. Introduction
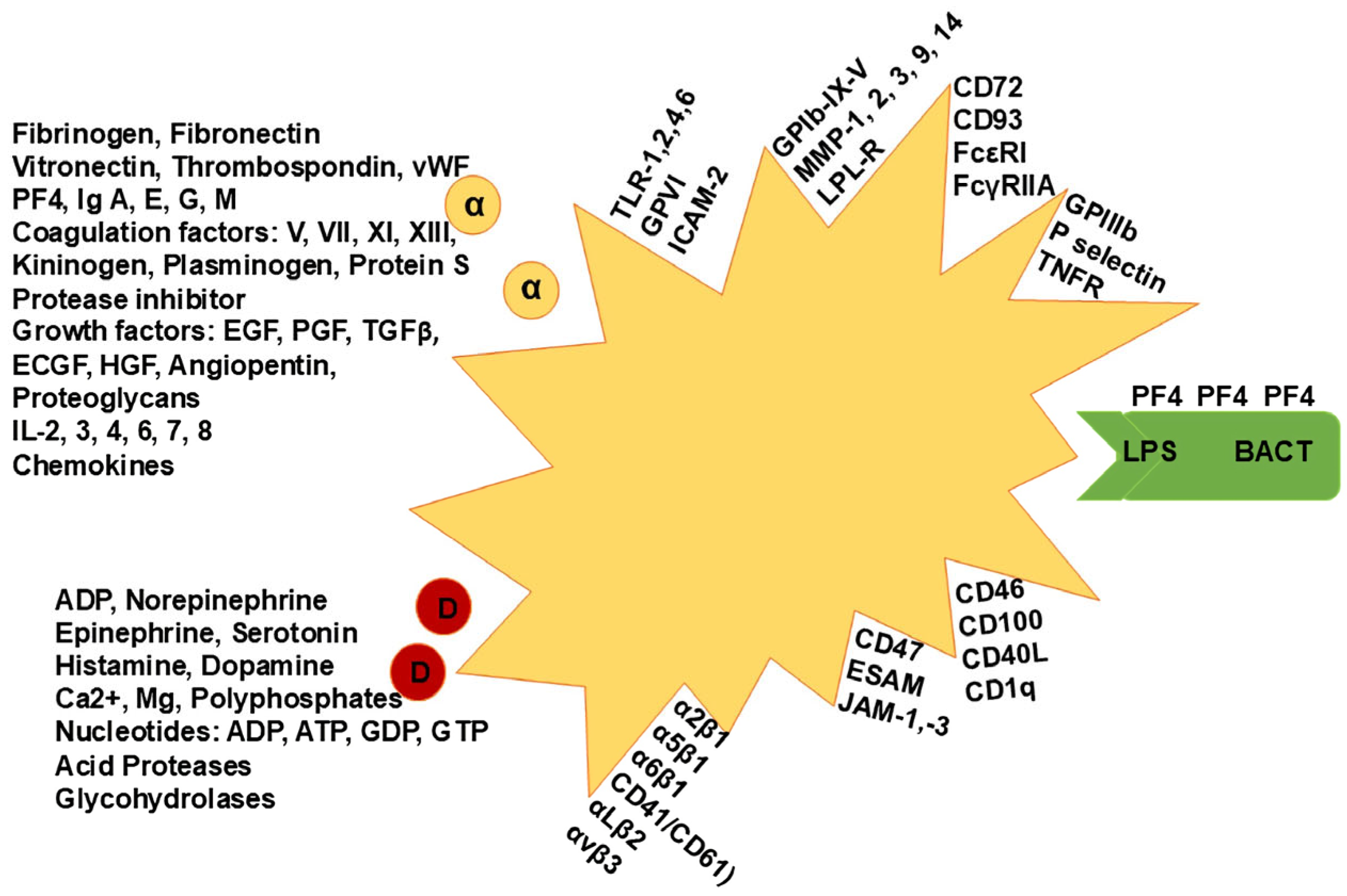
2. Additional Platelet Physiological Roles
3. Immune-Associated Platelet Functions in Sepsis
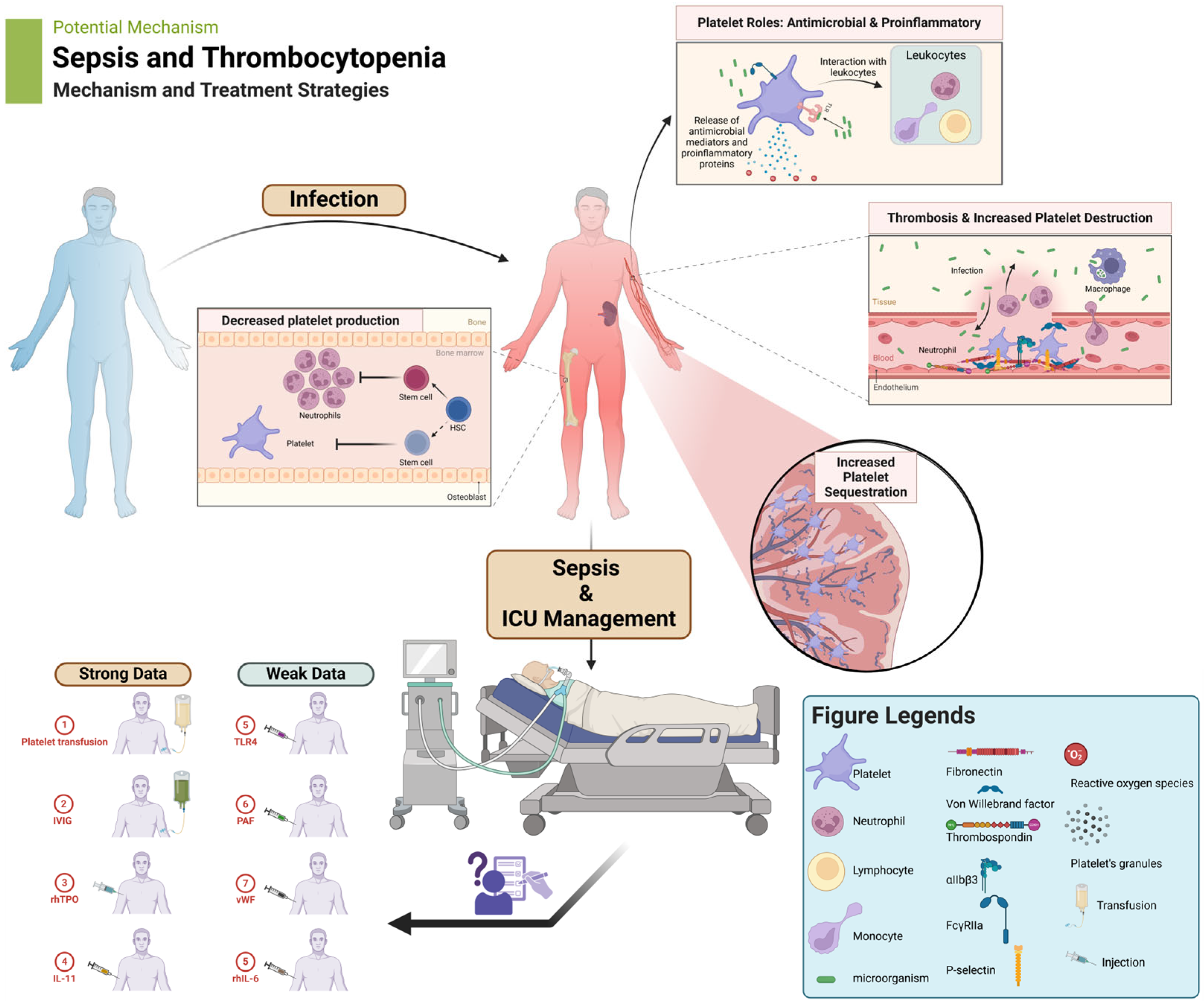
4. Clinical Implications
5. Management of Thrombocytopenia
5.1. Platelet Transfusions
5.2. Intravenous Immunoglobulins (IVIG)
5.3. Recombinant Human Thrombopoietin (rhTPO)
5.4. Recombinant Human IL-11
5.5. Recombinant Human IL-6
5.6. TLR4 Inhibition
5.7. Platelet-Activating Factor (PAF) Inhibition
5.8. Von Willebrand Factor (vWF)-Binding Function
6. Biomarkers
7. Future Research Directions
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holinstat, M. Normal platelet function. Cancer Metastasis Rev. 2017, 36, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Xiao, W.; Maitta, R.W. Steady increment of immature platelet fraction is suppressed by irradiation in single-donor platelet components during storage. PLoS ONE 2014, 9, e85465. [Google Scholar] [CrossRef] [PubMed]
- Repsold, L.; Joubert, A.M. Platelet Function, Role in Thrombosis, Inflammation, and Consequences in Chronic Myeloproliferative Disorders. Cells 2021, 10, 3034. [Google Scholar] [CrossRef] [PubMed]
- Lefrancais, E.; Ortiz-Munoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.; Nurden, P. Advances in our understanding of the molecular basis of disorders of platelet function. J. Thromb. Haemost. 2011, 9 (Suppl. 1), 76–91. [Google Scholar] [CrossRef]
- Broos, K.; Feys, H.B.; De Meyer, S.F.; Vanhoorelbeke, K.; Deckmyn, H. Platelets at work in primary hemostasis. Blood Rev. 2011, 25, 155–167. [Google Scholar] [CrossRef]
- Arman, M.; Krauel, K.; Tilley, D.O.; Weber, C.; Cox, D.; Greinacher, A.; Kerrigan, S.W.; Watson, S.P. Amplification of bacteria-induced platelet activation is triggered by FcgammaRIIA, integrin alphaIIbbeta3, and platelet factor 4. Blood 2014, 123, 3166–3174. [Google Scholar] [CrossRef]
- Wen, H. Sepsis induced by cecal ligation and puncture. Methods Mol. Biol. 2013, 1031, 117–124. [Google Scholar] [CrossRef]
- Bojalil, R.; Ruíz-Hernández, A.; Villanueva-Arias, A.; Amezcua-Guerra, L.M.; Cásarez-Alvarado, S.; Hernández-Dueñas, A.M.; Rodríguez-Galicia, V.; Pavón, L.; Marquina, B.; Becerril-Villanueva, E.; et al. Two murine models of sepsis: Immunopathological differences between the sexes—Possible role of TGFβ1 in female resistance to endotoxemia. Biol. Res. 2023, 56, 54. [Google Scholar] [CrossRef]
- Corral, J.; Yélamos, J.; Hernández-Espinosa, D.; Monreal, Y.; Mota, R.; Arcas, I.; Miñano, A.; Parrilla, P.; Vicente, V. Role of Lipopolysaccharide and Cecal Ligation and Puncture on Blood Coagulation and Inflammation in Sensitive and Resistant Mice Models. Am. J. Pathol. 2005, 166, 1089–1098. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, W.; Liu, Z.; Pei, Y.; Zhu, Y.; Chen, F.; Zou, L.; Jiang, Y.; Liu, X.; Huang, J.; et al. Early predictive value of platelet function for clinical outcome in sepsis. J. Infect. 2022, 84, 628–636. [Google Scholar] [CrossRef]
- Li, M.-F.; Li, X.-L.; Fan, K.-L.; Yu, Y.-Y.; Gong, J.; Geng, S.-Y.; Liang, Y.-F.; Huang, L.; Qiu, J.-H.; Tian, X.-H.; et al. Platelet desialylation is a novel mechanism and a therapeutic target in thrombocytopenia during sepsis: An open-label, multicenter, randomized controlled trial. J. Hematol. Oncol. 2017, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, W.; Li, Y.; Qin, Y.; Zou, T. Association between minimal decrease in platelet counts and outcomes in septic patients: A retrospective observational study. BMJ Open 2023, 13, e069027. [Google Scholar] [CrossRef] [PubMed]
- Garma, L.D.; Deng, H.; Goldschmidt, E. Integrated analysis of transcriptomic data reveals the platelet response in COVID-19 disease. Sci. Rep. 2022, 12, 6851. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Joo, J.-Y.; Kang, J.; Yu, Y.; Kim, Y.H.; Park, H.R. Single-cell analysis of platelets from patients with periodontitis and diabetes. Res. Pract. Thromb. Haemost. 2023, 7, 100099. [Google Scholar] [CrossRef] [PubMed]
- Supernat, A.; Popęda, M.; Pastuszak, K.; Best, M.G.; Grešner, P.; Veld, S.I.T.; Siek, B.; Bednarz-Knoll, N.; Rondina, M.T.; Stokowy, T.; et al. Transcriptomic landscape of blood platelets in healthy donors. Sci. Rep. 2021, 11, 15679. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Litvinov, R.I.; Weisel, J.W.; Stalker, T.J. Use of electron microscopy to study platelets and thrombi. Platelets 2020, 31, 580–588. [Google Scholar] [CrossRef]
- Leslie, M. Cell biology. Beyond clotting: The powers of platelets. Science 2010, 328, 562–564. [Google Scholar] [CrossRef]
- Elzey, B.D.; Tian, J.; Jensen, R.J.; Swanson, A.K.; Lees, J.R.; Lentz, S.R.; Stein, C.S.; Nieswandt, B.; Wang, Y.; Davidson, B.L.; et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity 2003, 19, 9–19. [Google Scholar] [CrossRef]
- Diacovo, T.G.; Puri, K.D.; Warnock, R.A.; Springer, T.A.; von Andrian, U.H. Platelet-mediated lymphocyte delivery to high endothelial venules. Science 1996, 273, 252–255. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Bayer, A.S. Antimicrobial peptides from platelets. Drug Resist. Updates 1999, 2, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Q.; Yeaman, M.R.; Selsted, M.E. Antimicrobial peptides from human platelets. Infect. Immun. 2002, 70, 6524–6533. [Google Scholar] [CrossRef] [PubMed]
- Smyth, S.S.; McEver, R.P.; Weyrich, A.S.; Morrell, C.N.; Hoffman, M.R.; Arepally, G.M.; French, P.A.; Dauerman, H.L.; Becker, R.C.; Platelet Colloquium, P. Platelet functions beyond hemostasis. J. Thromb. Haemost. 2009, 7, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.T.; Corken, A.; Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015, 126, 582–588. [Google Scholar] [CrossRef]
- Krauel, K.; Potschke, C.; Weber, C.; Kessler, W.; Furll, B.; Ittermann, T.; Maier, S.; Hammerschmidt, S.; Broker, B.M.; Greinacher, A. Platelet factor 4 binds to bacteria, [corrected] inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood 2011, 117, 1370–1378. [Google Scholar] [CrossRef]
- Greinacher, A.; Kohlmann, T.; Strobel, U.; Sheppard, J.A.; Warkentin, T.E. The temporal profile of the anti-PF4/heparin immune response. Blood 2009, 113, 4970–4976. [Google Scholar] [CrossRef]
- Neuhaus, F.C.; Baddiley, J. A continuum of anionic charge: Structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 686–723. [Google Scholar] [CrossRef]
- Krauel, K.; Weber, C.; Brandt, S.; Zahringer, U.; Mamat, U.; Greinacher, A.; Hammerschmidt, S. Platelet factor 4 binding to lipid A of Gram-negative bacteria exposes PF4/heparin-like epitopes. Blood 2012, 120, 3345–3352. [Google Scholar] [CrossRef]
- Senchenkova, E.Y.; Ansari, J.; Becker, F.; Vital, S.A.; Al-Yafeai, Z.; Sparkenbaugh, E.M.; Pawlinski, R.; Stokes, K.Y.; Carroll, J.L.; Dragoi, A.M.; et al. Novel Role for the AnxA1-Fpr2/ALX Signaling Axis as a Key Regulator of Platelet Function to Promote Resolution of Inflammation. Circulation 2019, 140, 319–335. [Google Scholar] [CrossRef]
- Kapur, R.; Semple, J.W. Platelets as immune-sensing cells. Blood Adv. 2016, 1, 10–14. [Google Scholar] [CrossRef]
- Vallance, T.M.; Zeuner, M.T.; Williams, H.F.; Widera, D.; Vaiyapuri, S. Toll-Like Receptor 4 Signalling and Its Impact on Platelet Function, Thrombosis, and Haemostasis. Mediat. Inflamm. 2017, 2017, 9605894. [Google Scholar] [CrossRef] [PubMed]
- Andonegui, G.; Kerfoot, S.M.; McNagny, K.; Ebbert, K.V.; Patel, K.D.; Kubes, P. Platelets express functional Toll-like receptor-4. Blood 2005, 106, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Zhang, G.; Guo, L.; Li, X.A.; Morris, A.J.; Daugherty, A.; Whiteheart, S.W.; Smyth, S.S.; Li, Z. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat. Commun. 2013, 4, 2657. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xu, R.; Xie, P.; Liu, X.; Ling, C.; Liu, Y.; Zhang, X.; Xia, Z.; Chen, Z.; Tang, J. EGFR of platelet regulates macrophage activation and bacterial phagocytosis function. J. Inflamm. 2024, 21, 10. [Google Scholar] [CrossRef]
- Clemetson, K.J. Platelets and pathogens. Cell Mol. Life Sci. 2010, 67, 495–498. [Google Scholar] [CrossRef]
- Ojha, A.; Nandi, D.; Batra, H.; Singhal, R.; Annarapu, G.K.; Bhattacharyya, S.; Seth, T.; Dar, L.; Medigeshi, G.R.; Vrati, S.; et al. Platelet activation determines the severity of thrombocytopenia in dengue infection. Sci. Rep. 2017, 7, 41697. [Google Scholar] [CrossRef]
- Claushuis, T.A.M.; de Vos, A.F.; Nieswandt, B.; Boon, L.; Roelofs, J.; de Boer, O.J.; van ’t Veer, C.; van der Poll, T. Platelet glycoprotein VI aids in local immunity during pneumonia-derived sepsis caused by gram-negative bacteria. Blood 2018, 131, 864–876. [Google Scholar] [CrossRef]
- Dutting, S.; Bender, M.; Nieswandt, B. Platelet GPVI: A target for antithrombotic therapy?! Trends Pharmacol. Sci. 2012, 33, 583–590. [Google Scholar] [CrossRef]
- Wolff, M.; Handtke, S.; Palankar, R.; Wesche, J.; Kohler, T.P.; Kohler, C.; Gruel, Y.; Hammerschmidt, S.; Greinacher, A. Activated platelets kill Staphylococcus aureus, but not Streptococcus pneumoniae-The role of FcgammaRIIa and platelet factor 4/heparinantibodies. J. Thromb. Haemost. 2020, 18, 1459–1468. [Google Scholar] [CrossRef]
- Guo, Y.L.; Liu, D.Q.; Bian, Z.; Zhang, C.Y.; Zen, K. Down-regulation of platelet surface CD47 expression in Escherichia coli O157:H7 infection-induced thrombocytopenia. PLoS ONE 2009, 4, e7131. [Google Scholar] [CrossRef]
- King, M.; McDermott, P.; Schreiber, A.D. Characterization of the Fc gamma receptor on human platelets. Cell Immunol. 1990, 128, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Xie, F.; Gibson, A.W.; Edberg, J.C.; Kimberly, R.P.; Wu, J. Functional expression of IgA receptor FcalphaRI on human platelets. J. Leukoc. Biol. 2008, 84, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, S.; Tolley, N.D.; Dixon, D.A.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A.; Weyrich, A.S. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J. Cell Biol. 2001, 154, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Henn, V.; Slupsky, J.R.; Grafe, M.; Anagnostopoulos, I.; Forster, R.; Muller-Berghaus, G.; Kroczek, R.A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef]
- Danese, S.; Katz, J.A.; Saibeni, S.; Papa, A.; Gasbarrini, A.; Vecchi, M.; Fiocchi, C. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut 2003, 52, 1435–1441. [Google Scholar] [CrossRef]
- Yacoub, D.; Hachem, A.; Theoret, J.F.; Gillis, M.A.; Mourad, W.; Merhi, Y. Enhanced levels of soluble CD40 ligand exacerbate platelet aggregation and thrombus formation through a CD40-dependent tumor necrosis factor receptor-associated factor-2/Rac1/p38 mitogen-activated protein kinase signaling pathway. Arter. Thromb. Vasc. Biol. 2010, 30, 2424–2433. [Google Scholar] [CrossRef]
- Del Conde, I.; Cruz, M.A.; Zhang, H.; Lopez, J.A.; Afshar-Kharghan, V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 2005, 201, 871–879. [Google Scholar] [CrossRef]
- Wong, C.H.; Jenne, C.N.; Petri, B.; Chrobok, N.L.; Kubes, P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat. Immunol. 2013, 14, 785–792. [Google Scholar] [CrossRef]
- Nagasawa, T.; Nakayasu, C.; Rieger, A.M.; Barreda, D.R.; Somamoto, T.; Nakao, M. Phagocytosis by Thrombocytes is a Conserved Innate Immune Mechanism in Lower Vertebrates. Front. Immunol. 2014, 5, 445. [Google Scholar] [CrossRef]
- Meseguer, J.; Esteban, M.A.; Rodriguez, A. Are thrombocytes and platelets true phagocytes? Microsc. Res. Tech. 2002, 57, 491–497. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoon, B.R.; Kim, H.Y.; Yoo, S.J.; Kang, S.W.; Lee, W.W. Activated Platelets Convert CD14(+)CD16(-) Into CD14(+)CD16(+) Monocytes with Enhanced FcgammaR-Mediated Phagocytosis and Skewed M2 Polarization. Front. Immunol. 2020, 11, 611133. [Google Scholar] [CrossRef]
- Carestia, A.; Kaufman, T.; Schattner, M. Platelets: New Bricks in the Building of Neutrophil Extracellular Traps. Front. Immunol. 2016, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Carestia, A.; Kaufman, T.; Rivadeneyra, L.; Landoni, V.I.; Pozner, R.G.; Negrotto, S.; D’Atri, L.P.; Gomez, R.M.; Schattner, M. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J. Leukoc. Biol. 2016, 99, 153–162. [Google Scholar] [CrossRef]
- Schrottmaier, W.C.; Kral-Pointner, J.B.; Salzmann, M.; Mussbacher, M.; Schmuckenschlager, A.; Pirabe, A.; Brunnthaler, L.; Kuttke, M.; Maier, B.; Heber, S.; et al. Platelet p110beta mediates platelet-leukocyte interaction and curtails bacterial dissemination in pneumococcal pneumonia. Cell Rep. 2022, 41, 111614. [Google Scholar] [CrossRef]
- Mateo, J.; Ganji, G.; Lemech, C.; Burris, H.A.; Han, S.W.; Swales, K.; Decordova, S.; DeYoung, M.P.; Smith, D.A.; Kalyana-Sundaram, S.; et al. A First-Time-in-Human Study of GSK2636771, a Phosphoinositide 3 Kinase Beta-Selective Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 5981–5992. [Google Scholar] [CrossRef]
- Weyrich, A.S.; Zimmerman, G.A. Platelets: Signaling cells in the immune continuum. Trends Immunol. 2004, 25, 489–495. [Google Scholar] [CrossRef]
- Cox, D. Bacteria-platelet interactions. J. Thromb. Haemost. 2009, 7, 1865–1866. [Google Scholar] [CrossRef]
- Fitzgerald, J.R.; Foster, T.J.; Cox, D. The interaction of bacterial pathogens with platelets. Nat. Rev. Microbiol. 2006, 4, 445–457. [Google Scholar] [CrossRef]
- Kerrigan, S.W.; Jakubovics, N.S.; Keane, C.; Maguire, P.; Wynne, K.; Jenkinson, H.F.; Cox, D. Role of Streptococcus gordonii surface proteins SspA/SspB and Hsa in platelet function. Infect. Immun. 2007, 75, 5740–5747. [Google Scholar] [CrossRef]
- O’Seaghdha, M.; van Schooten, C.J.; Kerrigan, S.W.; Emsley, J.; Silverman, G.J.; Cox, D.; Lenting, P.J.; Foster, T.J. Staphylococcus aureus protein A binding to von Willebrand factor A1 domain is mediated by conserved IgG binding regions. FEBS J. 2006, 273, 4831–4841. [Google Scholar] [CrossRef] [PubMed]
- Lourbakos, A.; Yuan, Y.P.; Jenkins, A.L.; Travis, J.; Andrade-Gordon, P.; Santulli, R.; Potempa, J.; Pike, R.N. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: A new trait in microbial pathogenicity. Blood 2001, 97, 3790–3797. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sharma, M.; Majumder, M.; Steier, W.; Sangal, A.; Kalawar, M. Thrombocytopenia in septic shock patients—A prospective observational study of incidence, risk factors and correlation with clinical outcome. Anaesth. Intensive Care 2007, 35, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, S.; De Weerdt, A.; Malbrain, M.; Vankersschaever, D.; Frans, E.; Wilmer, A.; Bobbaers, H. Thrombocytopenia and prognosis in intensive care. Crit. Care Med. 2000, 28, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Ravi, S.; Budhathoki, R.; Arjyal, L.; Hamal, S.; Bista, A.; Khadka, S.; Uprety, D. Current understanding and future implications of sepsis-induced thrombocytopenia. Eur. J. Haematol. 2021, 106, 301–305. [Google Scholar] [CrossRef]
- Jiménez-Zarazúa, O.; González-Carrillo, P.L.; Vélez-Ramírez, L.N.; Alcocer-León, M.; Salceda-Muñoz, P.A.T.; Palomares-Anda, P.; Nava-Quirino, O.A.; Escalante-Martínez, N.; Sánchez-Guzmán, S.; Mondragón, J.D. Survival in septic shock associated with thrombocytopenia. Heart Lung 2021, 50, 268–276. [Google Scholar] [CrossRef]
- Prasad Sahu, D.; Wasnik, M.; Kannauje, P.K. Factors Influencing Corrected Count Increment After Platelet Transfusion in Thrombocytopenic Patients. Cureus 2023, 15, e46161. [Google Scholar] [CrossRef]
- Reizine, F.; Le Marec, S.; Le Meur, A.; Consigny, M.; Berteau, F.; Bodenes, L.; Geslain, M.; McQuilten, Z.; Le Niger, C.; Huntzinger, J.; et al. Prophylactic platelet transfusion response in critically ill patients: A prospective multicentre observational study. Crit. Care 2023, 27, 373. [Google Scholar] [CrossRef]
- Zhou, W.; Fan, C.; He, S.; Chen, Y.; Xie, C. Impact of Platelet Transfusion Thresholds on Outcomes of Patients with Sepsis: Analysis of the MIMIC-IV Database. Shock 2022, 57, 486–493. [Google Scholar] [CrossRef]
- He, S.; Fan, C.; Ma, J.; Tang, C.; Chen, Y. Platelet Transfusion in Patients With Sepsis and Thrombocytopenia: A Propensity Score-Matched Analysis Using a Large ICU Database. Front. Med. 2022, 9, 830177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Z.; Xiao, W.; Hua, T.; Zheng, Y.; Yang, M. Efficacy and Safety of Recombinant Human Thrombopoietin on Sepsis Patients With Thrombocytopenia: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Feng, T.; Xie, Y.; Huang, P.; Xie, H.; Tian, R.; Qian, B.; Wang, R. The effect of recombinant human thrombopoietin (rhTPO) on sepsis patients with acute severe thrombocytopenia: A study protocol for a multicentre randomised controlled trial (RESCUE trial). BMC Infect. Dis. 2019, 19, 780. [Google Scholar] [CrossRef] [PubMed]
- Guclu, E.; Durmaz, Y.; Karabay, O. Effect of severe sepsis on platelet count and their indices. Afr. Health Sci. 2013, 13, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, L.; Bercovitz, R.S.; Sholapur, N.S.; Heddle, N.M.; Stanworth, S.J.; Arnold, D.M. Platelet transfusions for critically ill patients with thrombocytopenia. Blood 2014, 123, 1146–1151. [Google Scholar] [CrossRef]
- Kaufman, R.M.; Djulbegovic, B.; Gernsheimer, T.; Kleinman, S.; Tinmouth, A.T.; Capocelli, K.E.; Cipolle, M.D.; Cohn, C.S.; Fung, M.K.; Grossman, B.J.; et al. Platelet Transfusion: A Clinical Practice Guideline From the AABB. Ann. Intern. Med. 2015, 162, 205–213. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Chang, Y.H.; Yang, S.H.; Wang, T.F.; Lin, T.Y.; Yang, K.L.; Chen, S.H. Complete blood count reference values of cord blood in Taiwan and the influence of gender and delivery route on them. Pediatr. Neonatol. 2011, 52, 155–160. [Google Scholar] [CrossRef]
- Walka, M.M.; Sonntag, J.; Kage, A.; Dudenhausen, J.W.; Obladen, M. Complete blood counts from umbilical cords of healthy term newborns by two automated cytometers. Acta Haematol. 1998, 100, 167–173. [Google Scholar] [CrossRef]
- Kreuger, A.L.; Makelburg, A.B.U.; Somers, J.A.E.; Tomson, B.; van de Watering, L.M.G.; van der Bom, J.G.; van Kraaij, M.G.J.; Weller, C.M. HLA-matched platelet transfusions are effective only in refractory patients with positive HLA antibody screening. Transfusion 2019, 59, 3303–3307. [Google Scholar] [CrossRef]
- van Baarle, F.L.F.; van de Weerdt, E.K.; van der Velden, W.; Ruiterkamp, R.A.; Tuinman, P.R.; Ypma, P.F.; van den Bergh, W.M.; Demandt, A.M.P.; Kerver, E.D.; Jansen, A.J.G.; et al. Platelet Transfusion before CVC Placement in Patients with Thrombocytopenia. N. Engl. J. Med. 2023, 388, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, Q.; Pan, J.; Zhou, A. Platelet transfusion and mortality in patients with sepsis-induced thrombocytopenia: A propensity score matching analysis. Vox. Sang. 2022, 117, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Belsher, J.; Yilmaz, M.; Afessa, B.; Winters, J.L.; Moore, S.B.; Hubmayr, R.D.; Gajic, O. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest 2007, 131, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Ness, P.M.; Takemoto, C.M.; Krishnamurti, L.; King, K.E.; Tobian, A.A.R. Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Blood 2015, 125, 1470–1476. [Google Scholar] [CrossRef]
- Levy, J.H.; Neal, M.D.; Herman, J.H. Bacterial contamination of platelets for transfusion: Strategies for prevention. Crit. Care 2018, 22, 271. [Google Scholar] [CrossRef]
- Chen, B.-Z.; Xia, R. Pro-inflammatory effects after platelet transfusion: A review. Vox Sang. 2020, 115, 349–357. [Google Scholar] [CrossRef]
- Barry, M.; Pati, S. Targeting repair of the vascular endothelium and glycocalyx after traumatic injury with plasma and platelet resuscitation. Matrix Biol. Plus 2022, 14, 100107. [Google Scholar] [CrossRef]
- Ho-Tin-Noé, B.; Demers, M.; Wagner, D.D. How platelets safeguard vascular integrity. J. Thromb. Haemost. 2011, 9 (Suppl. 1), 56–65. [Google Scholar] [CrossRef]
- Mohammadi, A.; Youssef, D.; Mohammadi, A. Unusual Presentation of Corynebacterium Endocarditis in a Patient Without Conventional Risk Factors: A Case Report. Cureus 2024, 16, e54970. [Google Scholar] [CrossRef]
- Heitink-Pollé, K.M.J.; Uiterwaal, C.; Porcelijn, L.; Tamminga, R.Y.J.; Smiers, F.J.; van Woerden, N.L.; Wesseling, J.; Vidarsson, G.; Laarhoven, A.G.; de Haas, M.; et al. Intravenous immunoglobulin vs observation in childhood immune thrombocytopenia: A randomized controlled trial. Blood 2018, 132, 883–891. [Google Scholar] [CrossRef]
- Hansen, R.J.; Balthasar, J.P. Intravenous immunoglobulin mediates an increase in anti-platelet antibody clearance via the FcRn receptor. Thromb. Haemost. 2002, 88, 898–899. [Google Scholar] [CrossRef] [PubMed]
- Barsam, S.J.; Psaila, B.; Forestier, M.; Page, L.K.; Sloane, P.A.; Geyer, J.T.; Villarica, G.O.; Ruisi, M.M.; Gernsheimer, T.B.; Beer, J.H.; et al. Platelet production and platelet destruction: Assessing mechanisms of treatment effect in immune thrombocytopenia. Blood 2011, 117, 5723–5732. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fu, H.; Zhu, X.; Huang, Q.; Chen, Q.; Wu, J.; Chen, Y.; Zhao, P.; Liu, K.-Y.; Zhang, X. The Combination of Intravenous Immunoglobulin (IVIG) and Low Does Recombinant Human Thrombopoietin (rhTPO) for the Management of Corticosteroid/IVIG Monotherapy-Resistant Immune Thrombocytopenia in Pregnancy. Blood 2023, 142, 1213. [Google Scholar] [CrossRef]
- Burns, E.R.; Lee, V.; Rubinstein, A. Treatment of septic thrombocytopenia with immune globulin. J. Clin. Immunol. 1991, 11, 363–368. [Google Scholar] [CrossRef]
- Matzdorff, A.; Meyer, O.; Ostermann, H.; Kiefel, V.; Eberl, W.; Kühne, T.; Pabinger, I.; Rummel, M. Immune Thrombocytopenia-Current Diagnostics and Therapy: Recommendations of a Joint Working Group of DGHO, ÖGHO, SGH, GPOH, and DGTI. Oncol. Res. Treat. 2018, 41, 1–30. [Google Scholar] [CrossRef]
- Martincic, Z.; Skopec, B.; Rener, K.; Mavric, M.; Vovko, T.; Jereb, M.; Lukic, M. Severe immune thrombocytopenia in a critically ill COVID-19 patient. Int. J. Infect. Dis. 2020, 99, 269–271. [Google Scholar] [CrossRef]
- Zhang, S.S.; Du, J.; Cui, N.; Yang, X.; Zhang, L.; Zhang, W.X.; Yue, M.; Wu, Y.X.; Yang, T.; Zhang, X.A.; et al. Clinical efficacy of immunoglobulin on the treatment of severe fever with thrombocytopenia syndrome: A retrospective cohort study. eBioMedicine 2023, 96, 104807. [Google Scholar] [CrossRef]
- Pan, B.; Sun, P.; Pei, R.; Lin, F.; Cao, H. Efficacy of IVIG therapy for patients with sepsis: A systematic review and meta-analysis. J. Transl. Med. 2023, 21, 765. [Google Scholar] [CrossRef]
- Warkentin, T.E. High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: A review. Expert Rev. Hematol. 2019, 12, 685–698. [Google Scholar] [CrossRef]
- Padmanabhan, A.; Jones, C.G.; Pechauer, S.M.; Curtis, B.R.; Bougie, D.W.; Irani, M.S.; Bryant, B.J.; Alperin, J.B.; Deloughery, T.G.; Mulvey, K.P.; et al. IVIg for Treatment of Severe Refractory Heparin-Induced Thrombocytopenia. Chest 2017, 152, 478–485. [Google Scholar] [CrossRef]
- Kuter, D.J. Managing thrombocytopenia associated with cancer chemotherapy. Oncology 2015, 29, 282–294. [Google Scholar] [PubMed]
- Wu, Q.; Ren, J.; Wu, X.; Wang, G.; Gu, G.; Liu, S.; Wu, Y.; Hu, D.; Zhao, Y.; Li, J. Recombinant human thrombopoietin improves platelet counts and reduces platelet transfusion possibility among patients with severe sepsis and thrombocytopenia: A prospective study. J. Crit. Care 2014, 29, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, G.; Sun, J.; Wang, X.; Guo, L. Recombinant human thrombopoietin in critically ill patients with sepsis-associated thrombocytopenia: A clinical study. Int. J. Infect. Dis. 2020, 98, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Vadhan-Raj, S.; Verschraegen, C.F.; Bueso-Ramos, C.; Broxmeyer, H.E.; Kudelkà, A.P.; Freedman, R.S.; Edwards, C.L.; Gershenson, D.; Jones, D.; Ashby, M.; et al. Recombinant human thrombopoietin attenuates carboplatin-induced severe thrombocytopenia and the need for platelet transfusions in patients with gynecologic cancer. Ann. Intern. Med. 2000, 132, 364–368. [Google Scholar] [CrossRef]
- Wang, S.; Yang, R.; Zou, P.; Hou, M.; Wu, D.; Shen, Z.; Lu, X.; Li, Y.; Chen, X.; Niu, T.; et al. A multicenter randomized controlled trial of recombinant human thrombopoietin treatment in patients with primary immune thrombocytopenia. Int. J. Hematol. 2012, 96, 222–228. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, B.; Wang, S.; Zhang, J.; Liu, Y.; Wang, J.; Fan, Z.; Lv, Y.; Zhang, X.; He, L.; et al. Recombinant human thrombopoietin promotes hematopoietic reconstruction after severe whole body irradiation. Sci. Rep. 2015, 5, 12993. [Google Scholar] [CrossRef]
- Dorner, A.J.; Goldman, S.J.; Keith, J.C., Jr. Interleukin-11. BioDrugs 1997, 8, 418–429. [Google Scholar] [CrossRef]
- Du, X.; Williams, D.A. Interleukin-11: Review of molecular, cell biology, and clinical use. Blood 1997, 89, 3897–3908. [Google Scholar] [CrossRef]
- Sands, B.E.; Bank, S.; Sninsky, C.A.; Robinson, M.; Katz, S.; Singleton, J.W.; Miner, P.B.; Safdi, M.A.; Galandiuk, S.; Hanauer, S.B.; et al. Preliminary evaluation of safety and activity of recombinant human interleukin 11 in patients with active Crohn’s disease. Gastroenterology 1999, 117, 58–64. [Google Scholar] [CrossRef]
- Wan, B.; Zhang, H.; Fu, H.; Chen, Y.; Yang, L.; Yin, J.; Wan, Y.; Shi, Y. Recombinant human interleukin-11 (IL-11) is a protective factor in severe sepsis with thrombocytopenia: A case-control study. Cytokine 2015, 76, 138–143. [Google Scholar] [CrossRef]
- Song, J.; Park, D.W.; Moon, S.; Cho, H.J.; Park, J.H.; Seok, H.; Choi, W.S. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: A prospective controlled study according to the Sepsis-3 definitions. BMC Infect. Dis. 2019, 19, 968. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ture, S.K.; Nieves-Lopez, B.; Blick-Nitko, S.K.; Maurya, P.; Livada, A.C.; Stahl, T.J.; Kim, M.; Pietropaoli, A.P.; Morrell, C.N. Thrombocytopenia Independently Leads to Changes in Monocyte Immune Function. Circ. Res. 2024, 134, 970–986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, L.; Shen, A.; Chen, Y.; Qi, Z. Rational Use of Tocilizumab in the Treatment of Novel Coronavirus Pneumonia. Clin. Drug Investig. 2020, 40, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [CrossRef]
- Cheng, J.; Zeng, H.; Chen, H.; Fan, L.; Xu, C.; Huang, H.; Tang, T.; Li, M. Current knowledge of thrombocytopenia in sepsis and COVID-19. Front. Immunol. 2023, 14, 1213510. [Google Scholar] [CrossRef]
- Ridker, P.M.; Devalaraja, M.; Baeres, F.M.M.; Engelmann, M.D.M.; Hovingh, G.K.; Ivkovic, M.; Lo, L.; Kling, D.; Pergola, P.; Raj, D.; et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021, 397, 2060–2069. [Google Scholar] [CrossRef]
- Soares, J.B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol. Int. 2010, 4, 659–672. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Li, Y.; Feng, G. TLR4 inhibitor alleviates sepsis-induced organ failure by inhibiting platelet mtROS production, autophagy, and GPIIb/IIIa expression. J. Bioenerg. Biomembr. 2022, 54, 155–162. [Google Scholar] [CrossRef]
- Aslam, R.; Speck, E.R.; Kim, M.; Crow, A.R.; Bang, K.W.A.; Nestel, F.P.; Ni, H.; Lazarus, A.H.; Freedman, J.; Semple, J.W. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-α production in vivo. Blood 2006, 107, 637–641. [Google Scholar] [CrossRef]
- Carnevale, R.; Cammisotto, V.; Bartimoccia, S.; Nocella, C.; Castellani, V.; Bufano, M.; Loffredo, L.; Sciarretta, S.; Frati, G.; Coluccia, A.; et al. Toll-Like Receptor 4-Dependent Platelet-Related Thrombosis in SARS-CoV-2 Infection. Circ. Res. 2023, 132, 290–305. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Yang, X.; Li, Y.; Wang, X.; Tan, S.; Chen, F. LPS enhances platelets aggregation via TLR4, which is related to mitochondria damage caused by intracellular ROS, but not extracellular ROS. Cell Immunol. 2018, 328, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Galgano, L.; Guidetti, G.F.; Torti, M.; Canobbio, I. The Controversial Role of LPS in Platelet Activation In Vitro. Int. J. Mol. Sci. 2022, 23, 10900. [Google Scholar] [CrossRef] [PubMed]
- Keane, C.; Tilley, D.; Cunningham, A.; Smolenski, A.; Kadioglu, A.; Cox, D.; Jenkinson, H.F.; Kerrigan, S.W. Invasive Streptococcus pneumoniae trigger platelet activation via Toll-like receptor 2. J. Thromb. Haemost. 2010, 8, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Luo, X.; Zhang, P.; Gao, Y.; Xie, S.; Xu, K.; Chang, J.; Ma, L. Strains of Group B streptococci from septic patients induce platelet activation via Toll-like Receptor 2. Clin. Exp. Pharmacol. Physiol. 2017, 44, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Lopes Pires, M.E.; Clarke, S.R.; Marcondes, S.; Gibbins, J.M. Lipopolysaccharide potentiates platelet responses via toll-like receptor 4-stimulated Akt-Erk-PLA2 signalling. PLoS ONE 2017, 12, e0186981. [Google Scholar] [CrossRef]
- Xia, Y.M.; Guan, Y.Q.; Liang, J.F.; Wu, W.D. TAK-242 improves sepsis-associated acute kidney injury in rats by inhibiting the TLR4/NF-kappaB signaling pathway. Ren. Fail. 2024, 46, 2313176. [Google Scholar] [CrossRef]
- Ono, Y.; Maejima, Y.; Saito, M.; Sakamoto, K.; Horita, S.; Shimomura, K.; Inoue, S.; Kotani, J. TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Sci. Rep. 2020, 10, 694. [Google Scholar] [CrossRef]
- Schattner, M. Platelet TLR4 at the crossroads of thrombosis and the innate immune response. J. Leukoc. Biol. 2019, 105, 873–880. [Google Scholar] [CrossRef]
- Tsuchiya, R.; Kyotani, K.; Scott, M.A.; Nishizono, K.; Ashida, Y.; Mochizuki, T.; Kitao, S.; Yamada, T.; Kobayashi, K. Role of platelet activating factor in development of thrombocytopenia and neutropenia in dogs with endotoxemia. Am. J. Vet. Res. 1999, 60, 216–221. [Google Scholar] [CrossRef]
- Schuster, D.P.; Metzler, M.; Opal, S.; Lowry, S.; Balk, R.; Abraham, E.; Levy, H.; Slotman, G.; Coyne, E.; Souza, S.; et al. Recombinant platelet-activating factor acetylhydrolase to prevent acute respiratory distress syndrome and mortality in severe sepsis: Phase IIb, multicenter, randomized, placebo-controlled, clinical trial. Crit. Care Med. 2003, 31, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Harishkumar, R.; Hans, S.; Stanton, J.E.; Grabrucker, A.M.; Lordan, R.; Zabetakis, I. Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients. Nutrients 2022, 14, 4414. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Kwong, A.C.; Madarati, H.; Kunasekaran, S.; Sparring, T.; Fox-Robichaud, A.E.; Liaw, P.C.; Kretz, C.A. Characterization of ADAMTS13 and von Willebrand factor levels in septic and non-septic ICU patients. PLoS ONE 2021, 16, e0247017. [Google Scholar] [CrossRef] [PubMed]
- Peetermans, M.; Meyers, S.; Liesenborghs, L.; Vanhoorelbeke, K.; De Meyer, S.F.; Vandenbriele, C.; Lox, M.; Hoylaerts, M.F.; Martinod, K.; Jacquemin, M.; et al. Von Willebrand factor and ADAMTS13 impact on the outcome of Staphylococcus aureus sepsis. J. Thromb. Haemost. 2020, 18, 722–731. [Google Scholar] [CrossRef]
- Yin, H.; Stojanovic-Terpo, A.; Xu, W.; Corken, A.; Zakharov, A.; Qian, F.; Pavlovic, S.; Krbanjevic, A.; Lyubimov, A.V.; Wang, Z.J.; et al. Role for platelet glycoprotein Ib-IX and effects of its inhibition in endotoxemia-induced thrombosis, thrombocytopenia, and mortality. Arter. Thromb. Vasc. Biol. 2013, 33, 2529–2537. [Google Scholar] [CrossRef]
- Farmer, H.; Xu, H.; Navar, A.M.; Nanna, M.; George, L.; Dupre, M. Chronic stress and risks for myocardial infarction in US adults. Innov. Aging. 2020, 4, 394. [Google Scholar] [CrossRef]
- Baker, K.E.; Wilson, L.M.; Sharma, R.; Dukhanin, V.; McArthur, K.; Robinson, K.A. Hormone Therapy, Mental Health, and Quality of Life Among Transgender People: A Systematic Review. J. Endocr. Soc. 2021, 5, bvab011. [Google Scholar] [CrossRef]
- Martinez-Sanchez, N.; Sweeney, O.; Sidarta-Oliveira, D.; Caron, A.; Stanley, S.A.; Domingos, A.I. The sympathetic nervous system in the 21st century: Neuroimmune interactions in metabolic homeostasis and obesity. Neuron 2022, 110, 3597–3626. [Google Scholar] [CrossRef]
- Drăgoescu, A.N.; Pădureanu, V.; Stănculescu, A.D.; Chiuțu, L.C.; Tomescu, P.; Geormăneanu, C.; Pădureanu, R.; Iovănescu, V.F.; Ungureanu, B.S.; Pănuș, A.; et al. Neutrophil to Lymphocyte Ratio (NLR)-A Useful Tool for the Prognosis of Sepsis in the ICU. Biomedicines 2021, 10, 75. [Google Scholar] [CrossRef]
- Enz Hubert, R.M.; Rodrigues, M.V.; Andreguetto, B.D.; Santos, T.M.; de Fátima Pereira Gilberti, M.; de Castro, V.; Annichino-Bizzacchi, J.M.; Dragosavac, D.; Carvalho-Filho, M.A.; De Paula, E.V. Association of the immature platelet fraction with sepsis diagnosis and severity. Sci. Rep. 2015, 5, 8019. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, J.; Guo, F.; Longhini, F.; Gao, Z.; Huang, Y.; Qiu, H. Combination of C-reactive protein, procalcitonin and sepsis-related organ failure score for the diagnosis of sepsis in critical patients. Ann. Intensive Care 2016, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Kountchev, J.; Bijuklic, K.; Bellmann, R.; Wiedermann, C.J.; Joannidis, M. Reduction of D-dimer levels after therapeutic administration of antithrombin in acquired antithrombin deficiency of severe sepsis. Crit. Care 2005, 9, R596. [Google Scholar] [CrossRef] [PubMed]
- Mesters, R.M.; Mannucci, P.M.; Coppola, R.; Keller, T.; Ostermann, H.; Kienast, J. Factor VIIa and antithrombin III activity during severe sepsis and septic shock in neutropenic patients. Blood 1996, 88, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.J.; Taneja, A.; Niccum, D.; Kumar, G.; Jacobs, E.; Nanchal, R. The association of serum bilirubin levels on the outcomes of severe sepsis. J. Intensive Care Med. 2015, 30, 23–29. [Google Scholar] [CrossRef]
- Davis, J.S.; Yeo, T.W.; Piera, K.A.; Woodberry, T.; Celermajer, D.S.; Stephens, D.P.; Anstey, N.M. Angiopoietin-2 is increased in sepsis and inversely associated with nitric oxide-dependent microvascular reactivity. Crit. Care 2010, 14, R89. [Google Scholar] [CrossRef]
- Trancă, S.; Petrișor, C.; Hagău, N.; Ciuce, C. Can APACHE II, SOFA, ISS, and RTS Severity Scores be used to Predict Septic Complications in Multiple Trauma Patients? J. Crit. Care Med. (Univ. Med. Farm. Din Targu-Mures) 2016, 2, 124–130. [Google Scholar] [CrossRef]
- Caserta, S.; Kern, F.; Cohen, J.; Drage, S.; Newbury, S.F.; Llewelyn, M.J. Circulating Plasma microRNAs can differentiate Human Sepsis and Systemic Inflammatory Response Syndrome (SIRS). Sci. Rep. 2016, 6, 28006. [Google Scholar] [CrossRef]
- Kumpf, O.; Giamarellos-Bourboulis, E.J.; Koch, A.; Hamann, L.; Mouktaroudi, M.; Oh, D.-Y.; Latz, E.; Lorenz, E.; Schwartz, D.A.; Ferwerda, B.; et al. Influence of genetic variations in TLR4 and TIRAP/Mal on the course of sepsis and pneumonia and cytokine release: An observational study in three cohorts. Crit. Care 2010, 14, R103. [Google Scholar] [CrossRef]
- Thomas-Rüddel, D.O.; Fröhlich, H.; Schwarzkopf, D.; Bloos, F.; Riessen, R. Sepsis and underlying comorbidities in intensive care unit patients. Med. Klin. Intensivmed. Notfallmed. 2024, 119, 123–128. [Google Scholar] [CrossRef]
- Tang, A.; Shi, Y.; Dong, Q.; Wang, S.; Ge, Y.; Wang, C.; Gong, Z.; Zhang, W.; Chen, W. Prognostic differences in sepsis caused by gram-negative bacteria and gram-positive bacteria: A systematic review and meta-analysis. Crit. Care 2023, 27, 467. [Google Scholar] [CrossRef]
- Morrell, C.N.; Aggrey, A.A.; Chapman, L.M.; Modjeski, K.L. Emerging roles for platelets as immune and inflammatory cells. Blood 2014, 123, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Staley, E.M.; Cao, W.; Pham, H.P.; Kim, C.H.; Kocher, N.K.; Zheng, L.; Gangaraju, R.; Lorenz, R.G.; Williams, L.A.; Marques, M.B.; et al. Clinical factors and biomarkers predict outcome in patients with immune-mediated thrombotic thrombocytopenic purpura. Haematologica 2019, 104, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Peigne, V.; Azoulay, E.; Coquet, I.; Mariotte, E.; Darmon, M.; Legendre, P.; Adoui, N.; Marfaing-Koka, A.; Wolf, M.; Schlemmer, B.; et al. The prognostic value of ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13) deficiency in septic shock patients involves interleukin-6 and is not dependent on disseminated intravascular coagulation. Crit. Care 2013, 17, R273. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.H.; Wang, L.; Yeow, A.Y.K.; Poh, H.; Li, K.; Yeow, J.J.L.; Tan, G.Y.H. Artificial intelligence in sepsis early prediction and diagnosis using unstructured data in healthcare. Nat. Commun. 2021, 12, 711. [Google Scholar] [CrossRef]
- Fischetti, L.; Zhong, Z.; Pinder, C.L.; Tregoning, J.S.; Shattock, R.J. The synergistic effects of combining TLR ligand based adjuvants on the cytokine response are dependent upon p38/JNK signalling. Cytokine 2017, 99, 287–296. [Google Scholar] [CrossRef]
- Arora, J.; Mendelson, A.A.; Fox-Robichaud, A. Sepsis: Network pathophysiology and implications for early diagnosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 324, R613–R624. [Google Scholar] [CrossRef]
- Wang, K.; Lu, D.; Wang, F. Subphenotypes of platelet count trajectories in sepsis from multi-center ICU data. Sci. Rep. 2024, 14, 20187. [Google Scholar] [CrossRef]
- Venkata, C.; Kashyap, R.; Farmer, J.C.; Afessa, B. Thrombocytopenia in adult patients with sepsis: Incidence, risk factors, and its association with clinical outcome. J. Intensive Care 2013, 1, 9. [Google Scholar] [CrossRef]
- Vardon-Bounes, F.; Ruiz, S.; Gratacap, M.P.; Garcia, C.; Payrastre, B.; Minville, V. Platelets Are Critical Key Players in Sepsis. Int. J. Mol. Sci. 2019, 20, 3494. [Google Scholar] [CrossRef]
- Ali, N.; Auerbach, H.E. New-onset acute thrombocytopenia in hospitalized patients: Pathophysiology and diagnostic approach. J. Community Hosp. Intern. Med. Perspect. 2017, 7, 157–167. [Google Scholar] [CrossRef]
- Qu, M.; Liu, Q.; Zhao, H.G.; Peng, J.; Ni, H.; Hou, M.; Jansen, A.J.G. Low platelet count as risk factor for infections in patients with primary immune thrombocytopenia: A retrospective evaluation. Ann. Hematol. 2018, 97, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Liu, Y.; Barty, R.; Cook, R.; Rochwerg, B.; Iorio, A.; Warkentin, T.E.; Heddle, N.M.; Arnold, D.M. The association between platelet transfusions and mortality in patients with critical illness. Transfusion 2019, 59, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.R.; Garrigue, D.; Nougue, H.; Meyer, A.; Boutonnet, M.; Meaudre, E.; Culver, A.; Gaertner, E.; Audibert, G.; Vigué, B.; et al. Impact of platelet transfusion on outcomes in trauma patients. Crit. Care 2022, 26, 49. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef]
- Claushuis, T.A.M.; van Vught, L.A.; Scicluna, B.P.; Wiewel, M.A.; Klein Klouwenberg, P.M.C.; Hoogendijk, A.J.; Ong, D.S.Y.; Cremer, O.L.; Horn, J.; Franitza, M.; et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood 2016, 127, 3062–3072. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Ma, J.; Bai, S.G.; Fu, S.Z. Predictive value of thrombocytopenia for bloodstream infection in patients with sepsis and septic shock. World J. Crit. Care Med. 2024, 13, 88540. [Google Scholar] [CrossRef]
- Nandi, M.; Jackson, S.K.; Macrae, D.; Shankar-Hari, M.; Tremoleda, J.L.; Lilley, E. Rethinking animal models of sepsis-working towards improved clinical translation whilst integrating the 3Rs. Clin. Sci. 2020, 134, 1715–1734. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
| Section | Key Findings | Mechanisms | Clinical Relevance | Future Directions |
|---|---|---|---|---|
| Platelet transfusions [71,76,82,83,84,85,87,88] | They are commonly used but have mixed outcomes in sepsis. Higher transfusion thresholds may reduce mortality, but risks include volume overload, thrombosis, and immune dysfunction. | They may exacerbate inflammation and coagulation. Platelet activation and microvascular dysfunction contribute to adverse effects. | Platelet transfusions are a mainstay for severe thrombocytopenia but require careful patient selection and monitoring to avoid complications. | Conduct RCTs to define optimal transfusion strategies and identify patient subgroups that benefit most. |
| Intravenous immunoglobulins (IVIG) [90,91,92,93,94,95,96,98,99,100] | IVIG boosts platelet counts in sepsis by modulating immune responses, potentially reducing immune-mediated destruction and improving outcomes. | IVIG inhibits autoantibody-mediated platelet clearance and modulates Fc receptor interactions. It reduces inflammation and enhances platelet survival. | IVIG is effective in immune-mediated thrombocytopenia and may benefit septic patients with similar mechanisms. | Evaluate IVIG in larger randomized trials for SAT and explore combination therapies. |
| Recombinant human thrombopoietin (rhTPO) [72,101,102,103,104,105,106] | rhTPO enhances platelet production and reduces transfusion dependency in sepsis. It improves platelet counts and reduces ICU stays, but its cost-effectiveness needs further evaluation. | rhTPO stimulates megakaryocyte differentiation and platelet production in the bone marrow. It does not alter platelet function or morphology. | rhTPO is a promising therapy for SAT, particularly in patients with severe thrombocytopenia and high disease severity. | Investigate cost-effectiveness and long-term outcomes of rhTPO in sepsis. Explore its use in combination with other therapies. |
| Recombinant human IL-11 [107,108,109,110] | IL-11 has thrombopoietic effects and improves platelet counts in sepsis. It reduces IL-6 levels and sepsis markers, but its role in SAT requires further investigation. | IL-11 stimulates megakaryopoiesis and platelet production. It also modulates inflammatory responses by reducing pro-inflammatory cytokines. | IL-11 shows potential in improving platelet counts and reducing inflammation in sepsis, but evidence is limited. | Conduct RCTs to validate IL-11’s efficacy and safety in SAT. |
| Recombinant human IL-6 [111,112,115,116,136,137] | IL-6 inhibition (e.g., tocilizumab) shows potential in managing sepsis and thrombocytopenia, but its dual role in platelet consumption and inflammation complicates its use. | IL-6 drives cytokine storms and platelet consumption in sepsis. Tocilizumab inhibits IL-6 receptors, reducing inflammation but may also cause thrombocytopenia. | IL-6 inhibition is a promising strategy for severe sepsis, but its effects on platelets require careful monitoring. | Further research into IL-6 inhibition’s safety and efficacy in sepsis, particularly in patients with thrombocytopenia. |
| TLR4 inhibition [117,118,120,121,129,138] | TLR4 activation contributes to sepsis progression and thrombocytopenia. TLR4 inhibitors (e.g., TAK-242) show promise in reducing inflammation and improving outcomes in sepsis. | TLR4 recognizes LPS and triggers NF-κB signaling, leading to pro-inflammatory cytokine release and platelet activation. TLR4 inhibition reduces inflammation and platelet consumption. | TLR4 inhibitors may improve outcomes in sepsis by reducing inflammation and thrombocytopenia. | Conduct clinical trials to evaluate TLR4 inhibitors’ efficacy in sepsis and their impact on thrombocytopenia. |
| PAF inhibition [130,131,132] | PAF mediates thrombocytopenia and inflammation in sepsis. PAF inhibitors (e.g., TCV-309) reduce thrombocytopenia and improve outcomes in animal models. | PAF activates inflammatory and pro-thrombotic pathways, contributing to platelet consumption and organ damage. PAF inhibition reduces these effects. | PAF inhibitors show therapeutic potential in sepsis, particularly for reducing thrombocytopenia and inflammation. | Investigate PAF inhibitors in clinical trials for sepsis, and explore their use in combination with other therapies. |
| vWF-binding [133,134,135] | Imbalance between vWF and ADAMTS13 in sepsis contributes to thrombosis and thrombocytopenia. Targeting vWF-mediated adhesion (e.g., GPIb-IX inhibitors) shows therapeutic potential. | Elevated vWF and reduced ADAMTS13 activity promote platelet adhesion and microthrombosis. Inhibiting vWF-GPIb interactions reduces platelet consumption and thrombosis. | Targeting vWF-mediated adhesion may reduce thrombocytopenia and thrombosis in sepsis. | Develop and test vWF inhibitors in clinical trials for sepsis-associated thrombocytopenia. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setarehaseman, A.; Mohammadi, A.; Maitta, R.W. Thrombocytopenia in Sepsis. Life 2025, 15, 274. https://doi.org/10.3390/life15020274
Setarehaseman A, Mohammadi A, Maitta RW. Thrombocytopenia in Sepsis. Life. 2025; 15(2):274. https://doi.org/10.3390/life15020274
Chicago/Turabian StyleSetarehaseman, Alireza, Abbas Mohammadi, and Robert W. Maitta. 2025. "Thrombocytopenia in Sepsis" Life 15, no. 2: 274. https://doi.org/10.3390/life15020274
APA StyleSetarehaseman, A., Mohammadi, A., & Maitta, R. W. (2025). Thrombocytopenia in Sepsis. Life, 15(2), 274. https://doi.org/10.3390/life15020274






