Age-Dependent Changes in Taurine, Serine, and Methionine Release in the Frontal Cortex of Awake Freely-Moving Rats: A Microdialysis Study
Abstract
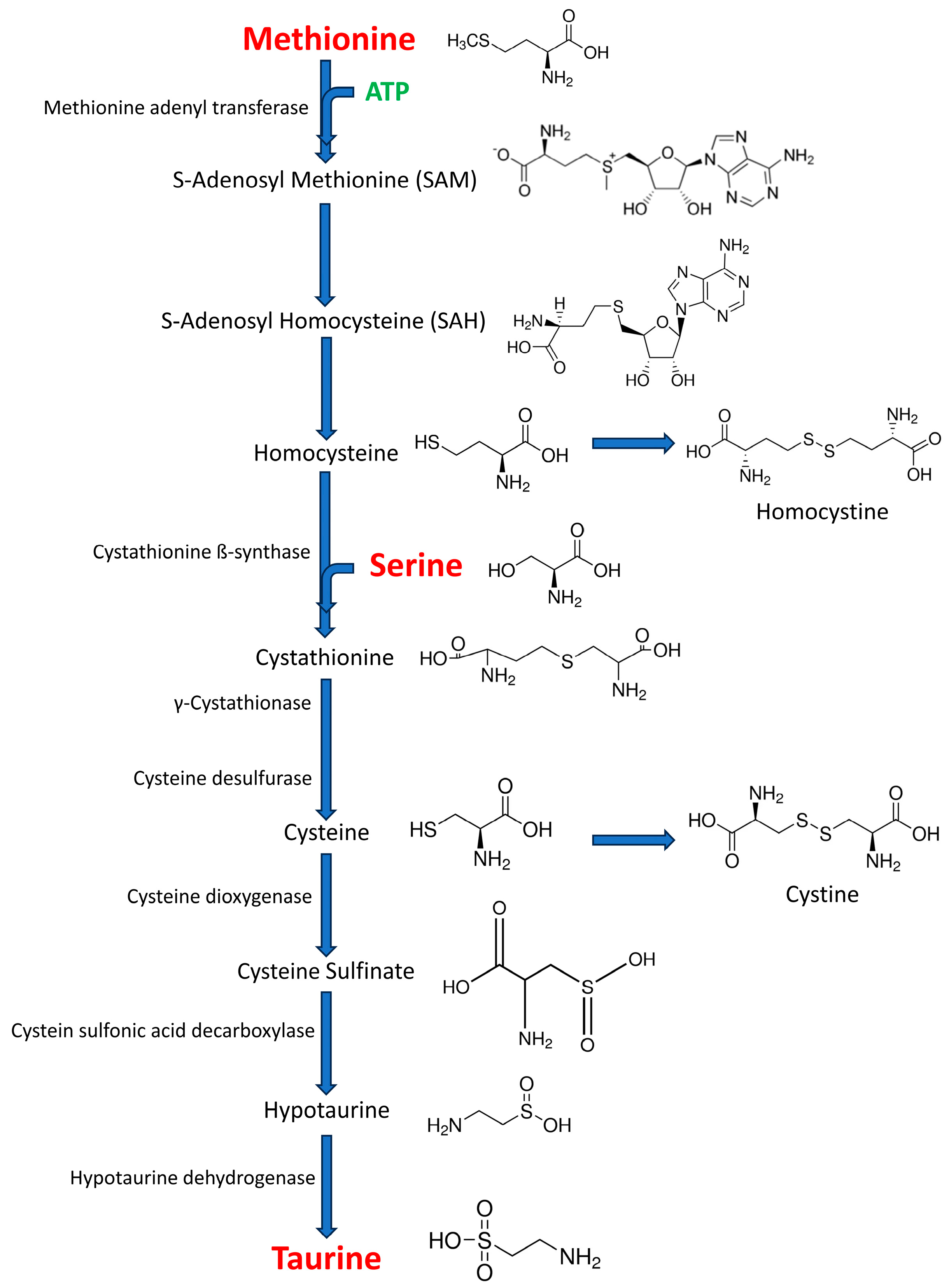
1. Introduction
2. Materials and Methods
2.1. Animals
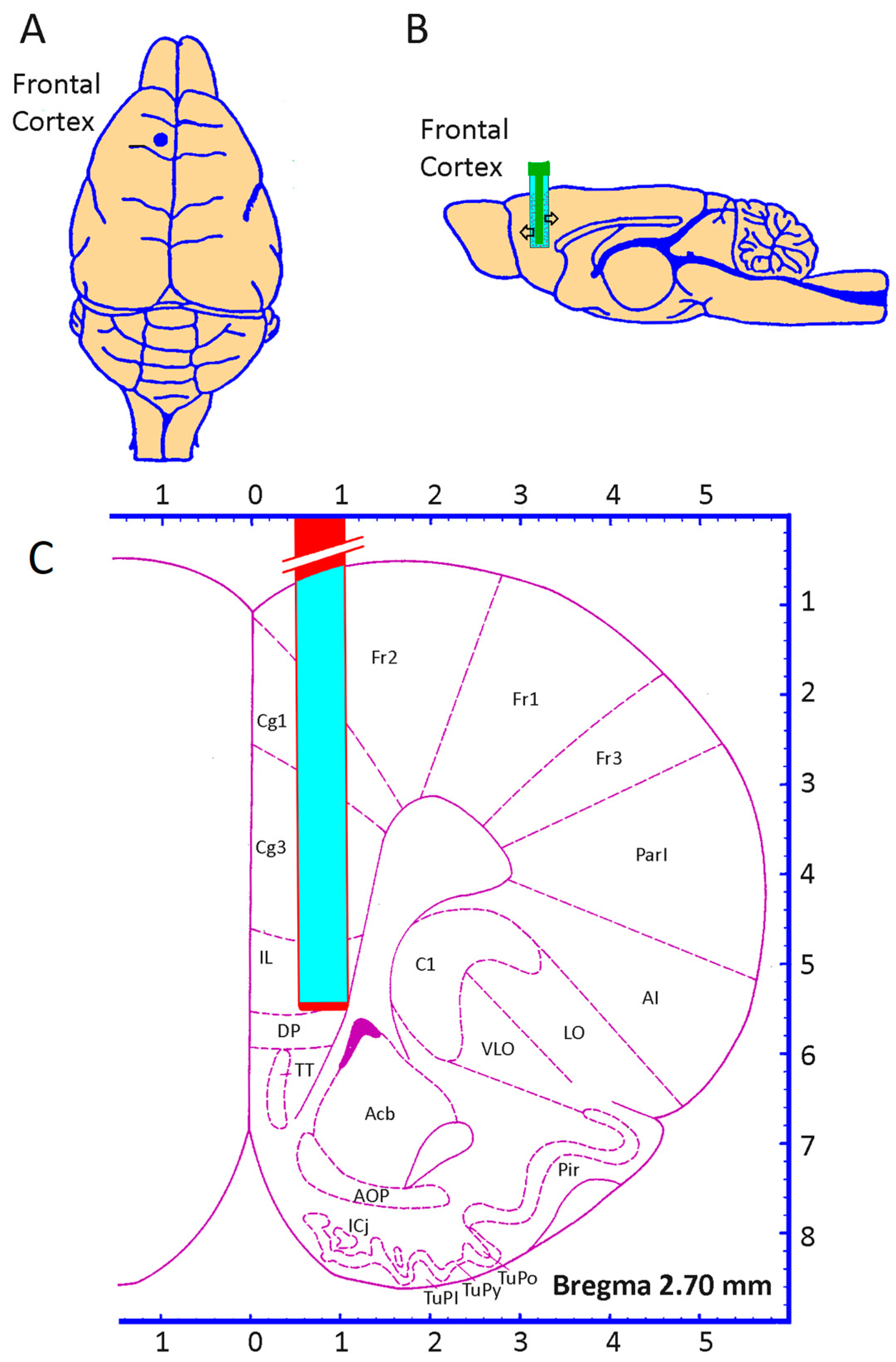
2.2. Microdialysis Procedure
2.3. Amino Acids Determination
2.4. Statistical Analysis
3. Results
3.1. Basal Release of Methionine, Serine, and Taurine
3.2. Methionine, Serine, and Taurine Release Evoked by 100 mM K+
3.3. TSM Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Berliocchi, L.; Li, Z.; Rasmussen, L.J. Interactions between mitochondrial dysfunction and other hallmarks of aging: Paving a path toward interventions that promote healthy old age. Aging Cell 2024, 23, e13942. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chi, X.; Cai, Y.; Ji, Z.; Wang, S.; Ren, J.; Liu, G.H. Decoding Aging Hallmarks at the Single-Cell Level. Annu. Rev. Biomed. Data Sci. 2023, 6, 129–152. [Google Scholar] [CrossRef]
- Rezaei, H.; Wang, H.W.; Tian, W.; Zhao, J.; Najibi, A.; Retana-Marquez, S.; Rafiei, E.; Rowhanirad, A.; Sabouri, S.; Kiafar, M.; et al. Long-term taurine supplementation regulates brain mitochondrial dynamics in mice. Basic Clin. Pharmacol. Toxicol. 2025, 136, e14101. [Google Scholar] [CrossRef] [PubMed]
- Asadi Shahmirzadi, A.; Edgar, D.; Liao, C.Y.; Hsu, Y.M.; Lucanic, M.; Asadi Shahmirzadi, A.; Wiley, C.D.; Gan, G.; Kim, D.E.; Kasler, H.G.; et al. Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab. 2020, 32, 447–456 e446. [Google Scholar] [CrossRef]
- Imai, S.I.; Guarente, L. It takes two to tango: NAD(+) and sirtuins in aging/longevity control. NPJ Aging Mech. Dis. 2016, 2, 16017. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Berglund, E.D.; Coate, K.C.; He, T.T.; Katafuchi, T.; Xiao, G.; Potthoff, M.J.; Wei, W.; Wan, Y.; et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 2012, 1, e00065. [Google Scholar] [CrossRef] [PubMed]
- El Idrissi, A.; Shen, C.H.; L’Amoreaux, W.J. Neuroprotective role of taurine during aging. Amino Acids 2013, 45, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gollapalli, K.; Mangiola, S.; Schranner, D.; Yusuf, M.A.; Chamoli, M.; Shi, S.L.; Lopes Bastos, B.; Nair, T.; Riermeier, A.; et al. Taurine deficiency as a driver of aging. Science 2023, 380, eabn9257. [Google Scholar] [CrossRef]
- Hebert, A.; Forquin-Gomez, M.P.; Roux, A.; Aubert, J.; Junot, C.; Heilier, J.F.; Landaud, S.; Bonnarme, P.; Beckerich, J.M. New insights into sulfur metabolism in yeasts as revealed by studies of Yarrowia lipolytica. Appl. Environ. Microbiol. 2013, 79, 1200–1211. [Google Scholar] [CrossRef]
- Lambert, I.H.; Kristensen, D.M.; Holm, J.B.; Mortensen, O.H. Physiological role of taurine--from organism to organelle. Acta Physiol. 2015, 213, 191–212. [Google Scholar] [CrossRef]
- Spriet, L.L.; Whitfield, J. Taurine and skeletal muscle function. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, R.; Camilo, M.E. Taurine: A conditionally essential amino acid in humans? An overview in health and disease. Nutr. Hosp. 2002, 17, 262–270. [Google Scholar] [PubMed]
- Huxtable, R.J. Expanding the circle 1975–1999: Sulfur biochemistry and insights on the biological functions of taurine. Adv. Exp. Med. Biol. 2000, 483, 1–25. [Google Scholar] [PubMed]
- Wu, J.Y.; Prentice, H. Role of taurine in the central nervous system. J. Biomed. Sci. 2010, 17 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.C.; Carey, R.E.; Schmidt, S.Y. Retinal degeneration associated with taurine deficiency in the cat. Science 1975, 188, 949–951. [Google Scholar] [CrossRef]
- Jacobsen, J.G.; Smith, L.H. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 1968, 48, 424–511. [Google Scholar] [CrossRef] [PubMed]
- Preising, M.N.; Gorg, B.; Friedburg, C.; Qvartskhava, N.; Budde, B.S.; Bonus, M.; Toliat, M.R.; Pfleger, C.; Altmuller, J.; Herebian, D.; et al. Biallelic mutation of human SLC6A6 encoding the taurine transporter TAUT is linked to early retinal degeneration. FASEB J. 2019, 33, 11507–11527. [Google Scholar] [CrossRef]
- Warskulat, U.; Heller-Stilb, B.; Oermann, E.; Zilles, K.; Haas, H.; Lang, F.; Haussinger, D. Phenotype of the taurine transporter knockout mouse. Methods Enzymol. 2007, 428, 439–458. [Google Scholar] [CrossRef]
- Ochoa-de la Paz, L.; Zenteno, E.; Gulias-Canizo, R.; Quiroz-Mercado, H. Taurine and GABA neurotransmitter receptors, a relationship with therapeutic potential? Expert Rev. Neurother. 2019, 19, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Oja, S.S.; International Union of Pharmacology. Taurine, biological actions and clinical perspectives. In Proceedings of the Satellite Symposium of the 9th IUPHAR Congress of Pharmacology Held at Hanasaari, Espoo/Helsinki, Finland, 6–8 August 1984; A.R. Liss: New York, NY, USA, 1985. Section of the Proceedings: xvii. p. 483. [Google Scholar]
- Oja, S.S.; Saransaari, P. Taurine as osmoregulator and neuromodulator in the brain. Metab. Brain Dis. 1996, 11, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Lazarewicz, J.W.; Noremberg, K.; Lehmann, A.; Hamberger, A. Effects of taurine on calcium binding and accumulation in rabbit hippocampal and cortical synaptosomes. Neurochem. Int. 1985, 7, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Lombardini, J.B. Effects of taurine on calcium ion uptake and protein phosphorylation in rat retinal membrane preparations. J. Neurochem. 1985, 45, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Solis, J.M.; Herranz, A.S.; Herreras, O.; Lerma, J.; Martin del Rio, R. Does taurine act as an osmoregulatory substance in the rat brain? Neurosci. Lett. 1988, 91, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wade, J.V.; Olson, J.P.; Samson, F.E.; Nelson, S.R.; Pazdernik, T.L. A possible role for taurine in osmoregulation within the brain. J. Neurochem. 1988, 51, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Prentice, H.; Wu, J.Y. Taurine and its neuroprotective role. Adv. Exp. Med. Biol. 2013, 775, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Kang, Y.S. Taurine Protects Glutamate Neurotoxicity in Motor Neuron Cells. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 887–895. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Garg, S.K.; Banerjee, R. Taurine biosynthesis by neurons and astrocytes. J. Biol. Chem. 2011, 286, 32002–32010. [Google Scholar] [CrossRef] [PubMed]
- Froger, N.; Moutsimilli, L.; Cadetti, L.; Jammoul, F.; Wang, Q.P.; Fan, Y.; Gaucher, D.; Rosolen, S.G.; Neveux, N.; Cynober, L.; et al. Taurine: The comeback of a neutraceutical in the prevention of retinal degenerations. Prog. Retin. Eye Res. 2014, 41, 44–63. [Google Scholar] [CrossRef]
- Gupta, R.C.; Win, T.; Bittner, S. Taurine analogues; a new class of therapeutics: Retrospect and prospects. Curr. Med. Chem. 2005, 12, 2021–2039. [Google Scholar] [CrossRef] [PubMed]
- Pavlopoulos, E.; Jones, S.; Kosmidis, S.; Close, M.; Kim, C.; Kovalerchik, O.; Small, S.A.; Kandel, E.R. Molecular mechanism for age-related memory loss: The histone-binding protein RbAp48. Sci. Transl. Med. 2013, 5, 200ra115. [Google Scholar] [CrossRef] [PubMed]
- Santa-Maria, I.; Hernandez, F.; Moreno, F.J.; Avila, J. Taurine, an inducer for tau polymerization and a weak inhibitor for amyloid-beta-peptide aggregation. Neurosci. Lett. 2007, 429, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Corona-Trejo, A.; Gonsebatt, M.E.; Trejo-Solis, C.; Campos-Pena, V.; Quintas-Granados, L.I.; Villegas-Vazquez, E.Y.; Daniel Reyes-Hernandez, O.; Hernandez-Abad, V.J.; Figueroa-Gonzalez, G.; Silva-Adaya, D. Transsulfuration pathway: A targeting neuromodulator in Parkinson’s disease. Rev. Neurosci. 2023, 34, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.M.; Rodrigures, C.M.P.; Zhao, L.R.; Steer, C.J.; Low, W.C. Tauroursodeoxycholic Acid Improves the Survival and Function of Nigral Transplants in a Rat Model of Parkinson’s Disease. Cell Transplant. 2002, 11, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Engelborghs, S.; Marescau, B.; De Deyn, P.P. Amino acids and biogenic amines in cerebrospinal fluid of patients with Parkinson’s disease. Neurochem. Res. 2003, 28, 1145–1150. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, Y.; Tang, J.; Chen, D.; Fu, X.; Mao, M.; Mu, D. PI3K/Akt signaling pathway is required for neuroprotection of thalidomide on hypoxic-ischemic cortical neurons in vitro. Brain Res. 2010, 1357, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Hou, L.; Sun, F.; Zhang, C.; Liu, X.; Piao, F.; Zhang, D.; Li, H.; Wang, Q. Taurine protects dopaminergic neurons in a mouse Parkinson’s disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, B.; Tian, T.; Zhang, B.; Shi, G.; Zhang, C.; Li, G.; Huang, M. Taurine protects dopaminergic neurons in paraquat-induced Parkinson’s disease mouse model through PI3K/Akt signaling pathways. Amino Acids 2022, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Al Mutairi, F. Hyperhomocysteinemia: Clinical Insights. J. Cent. Nerv. Syst. Dis. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, X.; Pu, H.; Zhang, W.; An, C.; Hu, X.; Liou, A.K.; Leak, R.K.; Gao, Y.; Chen, J. Scriptaid, a novel histone deacetylase inhibitor, protects against traumatic brain injury via modulation of PTEN and AKT pathway: Scriptaid protects against TBI via AKT. Neurotherapeutics 2013, 10, 124–142. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. The prefrontal cortex--an update: Time is of the essence. Neuron 2001, 30, 319–333. [Google Scholar] [CrossRef]
- Martinez-Martos, J.M.; Iribar, M.C.; Peinado, J.M. Evoked GABA release is not mediated by N-type VDCC in the frontal cortex of awake rats: Effects of neomycin. Brain Res. Bull. 1997, 43, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Hascup, E.R.; af Bjerken, S.; Hascup, K.N.; Pomerleau, F.; Huettl, P.; Stromberg, I.; Gerhardt, G.A. Histological studies of the effects of chronic implantation of ceramic-based microelectrode arrays and microdialysis probes in rat prefrontal cortex. Brain Res. 2009, 1291, 12–20. [Google Scholar] [CrossRef]
- Fekkes, D. Automated analysis of primary amino acids in plasma by high-performance liquid chromatography. Methods Mol. Biol. 2012, 828, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Expósito, M.J.; Ruíz-Sanjuan, M.D.; Martínez-Martos, J.M. Influence of dietary fats on circulating amino acid profile in experimental breast cancer. J. Clin. Mol. Med. 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Fekkes, D.; van der Cammen, T.J.; van Loon, C.P.; Verschoor, C.; van Harskamp, F.; de Koning, I.; Schudel, W.J.; Pepplinkhuizen, L. Abnormal amino acid metabolism in patients with early stage Alzheimer dementia. J. Neural Transm. 1998, 105, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Fekkes, D.; Bode, W.T.; Zijlstra, F.J.; Pepplinkhuizen, L. Eicosanoid and amino acid metabolism in transient acute psychoses with psychedelic symptoms. Prostaglandins Leukot. Essent. Fat. Acids 1996, 54, 261–264. [Google Scholar] [CrossRef][Green Version]
- McGaunn, J.; Baur, J.A. Taurine linked with healthy aging. Science 2023, 380, 1010–1011. [Google Scholar] [CrossRef] [PubMed]
- Vidal Valero, M. Taurine supplement makes animals live longer—What it means for people is unclear. Nature 2023. [Google Scholar] [CrossRef]
- Ommati, M.M.; Rezaei, H.; Socorro, R.M.; Tian, W.; Zhao, J.; Rouhani, A.; Sabouri, S.; Ghaderi, F.; Niknahad, A.M.; Najibi, A.; et al. Pre/postnatal taurine supplementation improves neurodevelopment and brain function in mice offspring: A persistent developmental study from puberty to maturity. Life Sci. 2024, 336, 122284. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Hascoet, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Shoji, H.; Takao, K.; Hattori, S.; Miyakawa, T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol. Brain 2016, 9, 11. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Pietzner, M.; Stewart, I.D.; Raffler, J.; Khaw, K.T.; Michelotti, G.A.; Kastenmuller, G.; Wareham, N.J.; Langenberg, C. Plasma metabolites to profile pathways in noncommunicable disease multimorbidity. Nat. Med. 2021, 27, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar] [PubMed]
- Sturman, J.A.; Rassin, D.K.; Gaull, G.E.; Cote, L.J. Taurine in developing rhesus monkey brain. J. Neurochem. 1980, 35, 304–310. [Google Scholar] [CrossRef]
- Worden, J.A.; Stipanuk, M.H. A comparison by species, age and sex of cysteinesulfinate decarboxylase activity and taurine concentration in liver and brain of animals. Comp. Biochem. Physiol. B 1985, 82, 233–239. [Google Scholar] [CrossRef]
- Sturman, J.A. Taurine in development. Physiol. Rev. 1993, 73, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Chesney, R.W. Taurine: Is it required for infant nutrition? J. Nutr. 1988, 118, 6–10. [Google Scholar] [CrossRef]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Jong, C.J.; Ramila, K.C.; Ito, T.; Kramer, J. Differences Between Physiological and Pharmacological Actions of Taurine. Adv. Exp. Med. Biol. 2022, 1370, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, M.; Itoi, M.; Kobayashi, S.; Sunaga, H.; Suzuki, K.T. Changes of glutathione and taurine concentrations in lenses of rat eyes induced by galactose-cataract formation or ageing. Exp. Eye Res. 1992, 54, 49–53. [Google Scholar] [CrossRef]
- Moran, J.; Salazar, P.; Pasantes-Morales, H. Effect of tocopherol and taurine on membrane fluidity of retinal rod outer segments. Exp. Eye Res. 1987, 45, 769–776. [Google Scholar] [CrossRef]
- Saransaari, P.; Oja, S.S. Taurine release from the developing and ageing hippocampus: Stimulation by agonists of ionotropic glutamate receptors. Mech. Ageing Dev. 1997, 99, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Saransaari, P.; Oja, S.S. Enhanced taurine release in cell-damaging conditions in the developing and ageing mouse hippocampus. Neuroscience 1997, 79, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Saransaari, P.; Oja, S.S. Taurine transport in the mouse cerebral cortex during development and ageing. Adv. Exp. Med. Biol. 1992, 315, 215–220. [Google Scholar] [CrossRef]
- Suarez, L.M.; Munoz, M.D.; Martin Del Rio, R.; Solis, J.M. Taurine content in different brain structures during ageing: Effect on hippocampal synaptic plasticity. Amino Acids 2016, 48, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef]
- Paula-Lima, A.C.; De Felice, F.G.; Brito-Moreira, J.; Ferreira, S.T. Activation of GABA(A) receptors by taurine and muscimol blocks the neurotoxicity of beta-amyloid in rat hippocampal and cortical neurons. Neuropharmacology 2005, 49, 1140–1148. [Google Scholar] [CrossRef]
- Aslan Karakelle, N.; Dincer, S.; Yar Saglam, A.S. The effect of intracerebroventricular amyloid beta 1-42 application on cognitive functions in aged rats supplemented with taurine and the change of peroxisomal proteins in this process. Brain Res. Bull. 2021, 172, 89–97. [Google Scholar] [CrossRef]
- Alom, J.; Mahy, J.N.; Brandi, N.; Tolosa, E. Cerebrospinal fluid taurine in Alzheimer’s disease. Ann. Neurol. 1991, 30, 735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yuan, Y.; Tong, Q.; Jiang, S.; Xu, Q.; Ding, J.; Zhang, L.; Zhang, R.; Zhang, K. Reduced plasma taurine level in Parkinson’s disease: Association with motor severity and levodopa treatment. Int. J. Neurosci. 2016, 126, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Gervais, F.; Paquette, J.; Morissette, C.; Krzywkowski, P.; Yu, M.; Azzi, M.; Lacombe, D.; Kong, X.; Aman, A.; Laurin, J.; et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol. Aging 2007, 28, 537–547. [Google Scholar] [CrossRef]
- Sola, S.; Castro, R.E.; Laires, P.A.; Steer, C.J.; Rodrigues, C.M. Tauroursodeoxycholic acid prevents amyloid-beta peptide-induced neuronal death via a phosphatidylinositol 3-kinase-dependent signaling pathway. Mol. Med. 2003, 9, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hou, Q.; Cheung, N.S.; Li, Q.T. Neuronal cell death caused by inhibition of intracellular cholesterol trafficking is caspase dependent and associated with activation of the mitochondrial apoptosis pathway. J. Neurochem. 2006, 97, 280–291. [Google Scholar] [CrossRef]
- Wade, J.V.; Samson, F.E.; Nelson, S.R.; Pazdernik, T.L. Changes in extracellular amino acids during soman- and kainic acid-induced seizures. J. Neurochem. 1987, 49, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, E.; Jones, D.G. Dendritic spine changes in rat hippocampal pyramidal cells after postnatal lead treatment: A Golgi study. Exp. Neurol. 1982, 77, 236–239. [Google Scholar] [CrossRef]
- Lasley, S.M.; Gilbert, M.E. Lead inhibits the rat N-methyl-d-aspartate receptor channel by binding to a site distinct from the zinc allosteric site. Toxicol. Appl. Pharmacol. 1999, 159, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.S.; Wang, M.; Li, X.M.; Chen, W.H.; Chen, J.T.; Wang, H.L.; Ruan, D.Y. Influences of different developmental periods of taurine supplements on synaptic plasticity in hippocampal CA1 area of rats following prenatal and perinatal lead exposure. BMC Dev. Biol. 2007, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ma, N.; Kawanokuchi, J.; Matsuoka, K.; Oikawa, S.; Kobayashi, H.; Hiraku, Y.; Murata, M. Taurine reduces microglia activation in the brain of aged senescence-accelerated mice by increasing the level of TREM2. Sci. Rep. 2024, 14, 7427. [Google Scholar] [CrossRef]
- Maugard, M.; Vigneron, P.A.; Bolanos, J.P.; Bonvento, G. l-Serine links metabolism with neurotransmission. Prog. Neurobiol. 2021, 197, 101896. [Google Scholar] [CrossRef]
- Wang, G.H.; Jiang, Z.L.; Chen, Z.Q.; Li, X.; Peng, L.L. Neuroprotective effect of L-serine against temporary cerebral ischemia in rats. J. Neurosci. Res. 2010, 88, 2035–2045. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Dunlop, R.A.; Powell, J.T.; Banack, S.A.; Cox, P.A. L-Serine: A Naturally-Occurring Amino Acid with Therapeutic Potential. Neurotox. Res. 2018, 33, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.P.; Xu, L.H.; Yang, J.J.; Jiang, Z.L.; Zhao, G.W.; Sun, L.; Wang, G.H.; Li, X. Reduction of inflammatory responses by L-serine treatment leads to neuroprotection in mice after traumatic brain injury. Neuropharmacology 2015, 95, 1–11. [Google Scholar] [CrossRef]
- Tian, Z.; Tang, C.; Wang, Z. Neuroprotective effect of ginkgetin in experimental cerebral ischemia/reperfusion via apoptosis inhibition and PI3K/Akt/mTOR signaling pathway activation. J. Cell Biochem. 2019, 120, 18487–18495. [Google Scholar] [CrossRef]
- Levine, T.D.; Miller, R.G.; Bradley, W.G.; Moore, D.H.; Saperstein, D.S.; Flynn, L.E.; Katz, J.S.; Forshew, D.A.; Metcalf, J.S.; Banack, S.A.; et al. Phase I clinical trial of safety of L-serine for ALS patients. Amyotroph Lateral Scler Front. Degener. 2017, 18, 107–111. [Google Scholar] [CrossRef]
- Ren, T.J.; Qiang, R.; Jiang, Z.L.; Wang, G.H.; Sun, L.; Jiang, R.; Zhao, G.W.; Han, L.Y. Improvement in regional CBF by L-serine contributes to its neuroprotective effect in rats after focal cerebral ischemia. PLoS ONE 2013, 8, e67044. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qiang, R.; Yang, Y.; Jiang, Z.L.; Wang, G.H.; Zhao, G.W.; Ren, T.J.; Jiang, R.; Xu, L.H. L-serine treatment may improve neurorestoration of rats after permanent focal cerebral ischemia potentially through improvement of neurorepair. PLoS ONE 2014, 9, e93405. [Google Scholar] [CrossRef]
- Le Douce, J.; Maugard, M.; Veran, J.; Matos, M.; Jego, P.; Vigneron, P.A.; Faivre, E.; Toussay, X.; Vandenberghe, M.; Balbastre, Y.; et al. Impairment of Glycolysis-Derived l-Serine Production in Astrocytes Contributes to Cognitive Deficits in Alzheimer’s Disease. Cell Metab. 2020, 31, 503–517 e508. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Sun, Y.; Jiang, Z.; Wang, G. L-Serine, an Endogenous Amino Acid, Is a Potential Neuroprotective Agent for Neurological Disease and Injury. Front. Mol. Neurosci. 2021, 14, 726665. [Google Scholar] [CrossRef] [PubMed]
- Bardaweel, S.K.; Alzweiri, M.; Ishaqat, A.A. D-Serine in neurobiology: CNS neurotransmission and neuromodulation. Can. J. Neurol. Sci. 2014, 41, 164–176. [Google Scholar] [CrossRef]
- Wolosker, H. The Neurobiology of d-Serine Signaling. Adv. Pharmacol. 2018, 82, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Olivella, M.; Grau, C.; Armstrong, J.; Alcon, C.; Gasull, X.; Santos-Gomez, A.; Locubiche, S.; Gomez de Salazar, M.; Garcia-Diaz, R.; et al. L-Serine dietary supplementation is associated with clinical improvement of loss-of-function GRIN2B-related pediatric encephalopathy. Sci. Signal. 2019, 12, 586. [Google Scholar] [CrossRef]
- Yamamori, H.; Hashimoto, R.; Fujita, Y.; Numata, S.; Yasuda, Y.; Fujimoto, M.; Ohi, K.; Umeda-Yano, S.; Ito, A.; Ohmori, T.; et al. Changes in plasma D-serine, L-serine, and glycine levels in treatment-resistant schizophrenia before and after clozapine treatment. Neurosci. Lett. 2014, 582, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Garcia-Serrano, A.M.; Duarte, J.M.N. Taurine Supplementation as a Neuroprotective Strategy upon Brain Dysfunction in Metabolic Syndrome and Diabetes. Nutrients 2022, 14, 1292. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Iqbal, M. Taurine as a potential anti-ageing therapy: The key to reversing the ageing process? Short communication. Ann. Med. Surg. 2023, 85, 3759–3760. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cueto-Ureña, C.; Ramírez-Expósito, M.J.; Carrera-González, M.P.; Martínez-Martos, J.M. Age-Dependent Changes in Taurine, Serine, and Methionine Release in the Frontal Cortex of Awake Freely-Moving Rats: A Microdialysis Study. Life 2025, 15, 295. https://doi.org/10.3390/life15020295
Cueto-Ureña C, Ramírez-Expósito MJ, Carrera-González MP, Martínez-Martos JM. Age-Dependent Changes in Taurine, Serine, and Methionine Release in the Frontal Cortex of Awake Freely-Moving Rats: A Microdialysis Study. Life. 2025; 15(2):295. https://doi.org/10.3390/life15020295
Chicago/Turabian StyleCueto-Ureña, Cristina, María Jesús Ramírez-Expósito, María Pilar Carrera-González, and José Manuel Martínez-Martos. 2025. "Age-Dependent Changes in Taurine, Serine, and Methionine Release in the Frontal Cortex of Awake Freely-Moving Rats: A Microdialysis Study" Life 15, no. 2: 295. https://doi.org/10.3390/life15020295
APA StyleCueto-Ureña, C., Ramírez-Expósito, M. J., Carrera-González, M. P., & Martínez-Martos, J. M. (2025). Age-Dependent Changes in Taurine, Serine, and Methionine Release in the Frontal Cortex of Awake Freely-Moving Rats: A Microdialysis Study. Life, 15(2), 295. https://doi.org/10.3390/life15020295







