Adherence to Pharmacological Treatment in Chronic Venous Disease: Results of a Real-World, Prospective, Observational Cohort Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CEAP | Clinical (C), Etiological (E), Anatomical (A), and Pathophysiological (P) |
| CVeD | Chronic Venous Disease |
| ICD | Ischemic Coronary Disease |
| MMAS-8 | Morisky Medication Adherence Scale—8 |
| MPFF | Micronized Purified Flavonoid Fraction |
| NAMMDR | National Agency of Medicine and Medical Devices in Romania |
| NBCMMD | National Bioethics Committee for Medicine and Medical Devices |
| T2DM | Type 2 Diabetes Mellitus |
| VADs | Venoactive drugs |
| VAS | Visual Analog Scale |
References
- Nicolaides, A.N.; Labropoulos, N. Burden and suffering in chronic venous disease. Adv Ther. 2019, 36 (Suppl 1.), 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Png, C.Y.M.; Sumpio, B.J.; DeCarlo, C.S.; Dua, A. Defining the human and health care costs of chronic venous insufficiency. Semin. Vasc. Surg. 2021, 34, 59–64. [Google Scholar] [CrossRef]
- Onida, S.; Davies, A.H. Predicted burden of venous disease. Phlebology 2016, 31 (Suppl. 1), 74–79. [Google Scholar] [CrossRef]
- Nicolaides, A.; Kakkos, S.; Baekgaard, N.; Comerota, A.; de Maeseneer, M.; Eklof, B.; Giannoukas, A.D.; Lugli, M.; Maleti, O.; Myers, K.; et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Part I. Int. Angiol. 2018, 37, 181–254. [Google Scholar] [CrossRef] [PubMed]
- Benn, S.; Moore, Z.; Patton, D.; O’Connor, T.; Nugent, L.; Harkin, D.; Avsar, P. What is the prevalence of chronic venous disease among health care workers? A scoping review. Int. Wound J. 2023, 20, 3821–3839. [Google Scholar] [CrossRef] [PubMed]
- Homs-Romero, E.; Verdú, J.; Blanch, J.; Rascón-Hernán, C.; Martí-Lluch, R. Validity of Chronic Venous Disease Diagnoses and Epidemiology Using Validated Electronic Health Records from Primary Care: A Real-World Data Analysis. J. Nurs. Scholarsh. 2021, 53, 296–305. [Google Scholar] [CrossRef]
- Maggioli, A. Chronic venous disorders: Pharmacological and clinical aspects of micronized purified flavonoid fraction. Phlebolymphology 2016, 23, 82–91. [Google Scholar]
- Behar, A.; Lagrue, G.; Cohen-Boulakia, F.; Baillet, J. Capillary filtration in idiopathic cyclic edema–effects of MPFF at a dose of 500 mg. Nuklearmedizin 1988, 27, 105–107. Available online: https://pubmed.ncbi.nlm.nih.gov/3043383/ (accessed on 24 February 2025). [PubMed]
- Tsoukanov, Y.T.; Tsoukanov, A.Y.; Nikolaychuk, A. Great saphenous vein transitory reflux in patients with symptoms related to chronic venous disorders, but without visible signs (C0s), and its correction with MPFF treatment. Phlebolymphology 2015, 22, 18–24. [Google Scholar]
- Eklöf, B.; Rutherford, R.B.; Bergan, J.J.; Carpentier, P.H.; Gloviczki, P.; Kistner, R.L.; Meissner, M.H.; Moneta, G.L.; Myers, K.; Padberg, F.T.; et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J. Vasc. Surg. 2004, 40, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Skovlund, E.; Shrestha, A.; Mjølstad, B.P.; Åsvold, B.O.; Sen, A. Impact of a community health worker led intervention for improved blood pressure control in urban Nepal: An open-label cluster randomised controlled trial. Lancet Reg. Health-Southeast Asia 2024, 29, 10046. [Google Scholar] [CrossRef]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003; Available online: https://iris.who.int/handle/10665/42682 (accessed on 24 February 2025).
- Katsenis, K. Micronized purified flavonoid fraction (MPFF): A review of its pharmacological effects, therapeutic efficacy, and benefits in the management of chronic venous insufficiency. Curr. Vasc. Pharmacol. 2005, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gohel, M.S.; Davies, A.H. Pharmacological agents in the treatment of venous disease: An update of the available evidence. Curr. Vasc. Pharmacol. 2009, 7, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Cyrino, F.Z.; Blanc-Guilemaud, V.; Bouskela, E. Time course of microvalve pathophysiology in high pressure low flow model of venous insufficiency and the role of micronized purified flavonoid fraction. Int. Angiol. 2021, 40, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Gracas, C.D.S.M.; Cyrino, F.Z.; De Carvalho, J.J.; Blanc-Guillemaud, V.; Bouskela, E. Protective effects of micronized purified flavonoid fraction (MPFF) on a novel experimental model of chronic venous hypertension. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 694–702. [Google Scholar] [CrossRef]
- Bouskela, E.; De Almeida Cyrino, G.; Zely, F.; Blanc-Guillemaud, V.; Lucien, A. Evaluation of microvalve alterations and assessment of MPFF treatment in an experimental model of venous hypertension. In Proceedings of the 21st Annual Meeting of the European Venous Forum, Virtual, 24–26 June 2021. Book of Abstracts, 17. [Google Scholar]
- Bergan, J.J.; Schmid-Schönbein, G.W.; Smith Coleridge, P.D.; Nicolaides, A.; Boisseau, M.R.; Eklof, B. Chronic venous disease. N. Engl. J. Med. 2006, 355, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Kakkos, S.K.; Nicolaides, A.N. Efficacy of micronized purified flavonoid fraction (Daflon®) on improving individual symptoms, signs, and quality of life in patients with chronic venous disease: A systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int. Angiol. 2018, 37, 143–154. [Google Scholar] [CrossRef]
- Ramelet, A.A.; Boisseau, M.R.; Allegra, C.; Nicolaides, A.; Jaeger, K.; Carpentier, P. Veno-active drugs in the management of chronic venous disease. An international consensus statement: Current medical position, prospective views, and final resolution. Clin. Hemorheol. Microcirc. 2005, 33, 309–319. [Google Scholar]
- Bouskela, E.; Lugli, M.; Nicolaides, A. New Perspectives on Micronised Purified Flavonoid Fraction in Chronic Venous Disease: From Microvalves to Clinical Effectiveness. Adv Ther. 2022, 39, 4413–4422. [Google Scholar] [CrossRef] [PubMed]
- Amiel, M.; Barbe, R.; Revel, D. Etude de la relation dose/effect de MPFF at a dose of 500 mg par pléthysmographie chez l’homme. J. Int. Med. 1987, 88, 19–21. [Google Scholar]
- Rabe, E.; Guex, J.J.; Puskas, A.; Scuderi, A.; Fernandez Quesada, F.; VCP Coordinators. Epidemiology of chronic venous disorders in geographically diverse populations: Results from the Vein Consult Program. Int. Angiol. 2012, 31, 105–115. [Google Scholar] [PubMed]
- Bogachev, V.; Arribas, J.M.J.; Baila, S.; Dominguez, J.U.; Walter, J.; Maharaj, D. Management and evaluation of treatment adherence and effectiveness in chronic venous disorders: Results of the international study VEIN Act Program. Drugs Ther. Perspect. 2019, 35, 396–404. [Google Scholar] [CrossRef]
- Mezalek, Z.T.; Feodor, T.; Chernukha, L.; Chen, Z.; Rueda, A.; Sánchez, I.E.; Ochoa, A.J.G.; Chirol, J.; Blanc-Guillemaud, V.; Lohier-Durel, C.; et al. VEIN STEP: A Prospective, Observational, International Study to Assess Effectiveness of Conservative Treatments in Chronic Venous Disease. Adv. Ther. 2023, 40, 5016–5036, Erratum in Adv Ther. 2024, 41, 464–465; Erratum in Adv Ther. 2024, 41, 2540–2541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Branisteanu, D.E.; Feodor, T.; Baila, S.; Mitea, I.A.; Vittos, O. Impact of chronic venous disease on quality of life: Results of vein alarm study. Exp. Ther. Med. 2019, 17, 1091–1096. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shvartz, E.; Reibold, R.C.; White, R.T.; Gaume, J.G. Hemodynamic responses in orthostasis following 5 hours of sitting. Aviat. Space Environ. Med. 1982, 53, 226–231. Available online: https://pubmed.ncbi.nlm.nih.gov/7187215/ (accessed on 24 February 2025). [PubMed]
- Mclaughlin, M.; Atkin, A.J.; Starr, L.; Hall, A.; Wolfenden, L.; Sutherland, R.; Wiggers, J.; Ramirez, A.; Hallal, P.; Pratt, M.; et al. Sedentary. Behaviour Council Global Monitoring Initiative Working Group. Worldwide surveillance of self-reported sitting time: A scoping review. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.; Ramelet, A.A. Pharmacological treatment of primary chronic venous disease: Rationale, results and unanswered questions. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Coleridge-Smith, P.; Lok, C.; Ramelet, A.A. Venous leg ulcer: A meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 198–208. [Google Scholar] [CrossRef]
- Maggioli, A.; Carpentier, P. Efficacy of MPFF 1000 mg oral suspension on CVD C0s-C1-related symptoms and quality of life. Int. Angiol. 2019, 38, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, P.; Van Bellen, B.; Karetova, D.; Hanafiah, H.; Enriquez-Vega, E.; Kirienko, A.; Dzupina, A.; Sabovic, M.; Gutierrez, L.R.; Subwongcharoen, S.; et al. Clinical efficacy, and safety of a new 1000-mg suspension versus twice-daily 500-mg tablets of MPFF in patients with symptomatic chronic venous disorders: A randomized controlled trial. Int. Angiol. 2017, 36, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Kirienko, A.; Radak, D. Clinical acceptability study of once-daily versus twice-daily micronized purified flavonoid fraction in patients with symptomatic chronic venous disease: A randomized controlled trial. Int. Angiol. 2016, 35, 399–405. [Google Scholar] [PubMed]
- Moon, S.J.; Lee, W.Y.; Hwang, J.S.; Hong, Y.P.; Morisky, D.E. Accuracy of a screening tool for medication adherence: A systematic review and meta-analysis of the Morisky Medication Adherence Scale-8. PLoS ONE 2017, 12, e0187139. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.U.; La Caze, A.; Cottrell, N. What are validated self-report adherence scales really measuring? A systematic review. Br. J. Clin. Pharmacol. 2013, 77, 427–445. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira-Filho, A.D.; Morisky, D.E.; Felizardo Neves, S.J.; Costa, A.C.; de Lyra Junior, D.P. The 8-item Morisky Medication Adherence Scale: Validation of a Brazilian–Portuguese version in hypertensive adults. Res. Soc. Adm. Pharm. 2014, 10, 554–561. [Google Scholar] [CrossRef]
- DiBonaventura, M.; Wintfeld, N.; Huang, J.; Goren, A. The association between nonadherence and glycated hemoglobin among type 2 diabetes patients using basal insulin analogs. Dovepress J. Patient Prefer. Adherence 2014, 8, 873–882. [Google Scholar] [CrossRef]
- Glasser, S.P.; Vitolins, M.; Rocco, M.V.; Harmon Still, C.; Cofield, S.S.; Haley, W.E.; Goff, D. Is Medication Adherence Predictive of Cardiovascular Outcomes and Blood Pressure Control? The Systolic Blood Pressure Intervention Trial (SPRINT). Am. J. Hypertens. 2022, 35, 182–191. [Google Scholar] [CrossRef]
- Kwan, Y.H.; Weng, S.D.; Fang Loh, D.H. Measurement Properties of Existing Patient-Reported Outcome Measures on Medication Adherence: Systematic Review. J. Med. Internet Res. 2020, 22, e19179. [Google Scholar] [CrossRef] [PubMed]
- Tosin, M.H.S.; de Oliveira, B.G.; Goetz, C.G. Rating Scales for Medication Adherence in Parkinson’s Disease: A Systematic Review for Critique and Recommendations. Int. Park. Mov. Disord. Soc. Mov. Disord. Clin. Pract. 2023, 10, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Lavsa, S.M.; Holzworth, A.; Ansani, N.T. Selection of a validated scale for measuring medication adherence. J. Am. Pharm. Assoc. 2011, 51, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Svarstad, B.L.; Chewning, B.A.; Sleath, B.L.; Claesson, C. The brief medication questionnaire: A tool for screening patient adherence and barriers to adherence. Patient Educ. Couns. 1999, 37, 113–124. [Google Scholar] [CrossRef]
- Ge, L.; Heng, B.H.; Yap, C.W. Understanding reasons and determinants of medication non-adherence in community-dwelling adults: A cross-sectional study comparing young and older age groups. BMC Health Serv Res. 2023, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; De Maeseneer, M.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352, Erratum in J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 288. [Google Scholar] [CrossRef] [PubMed]
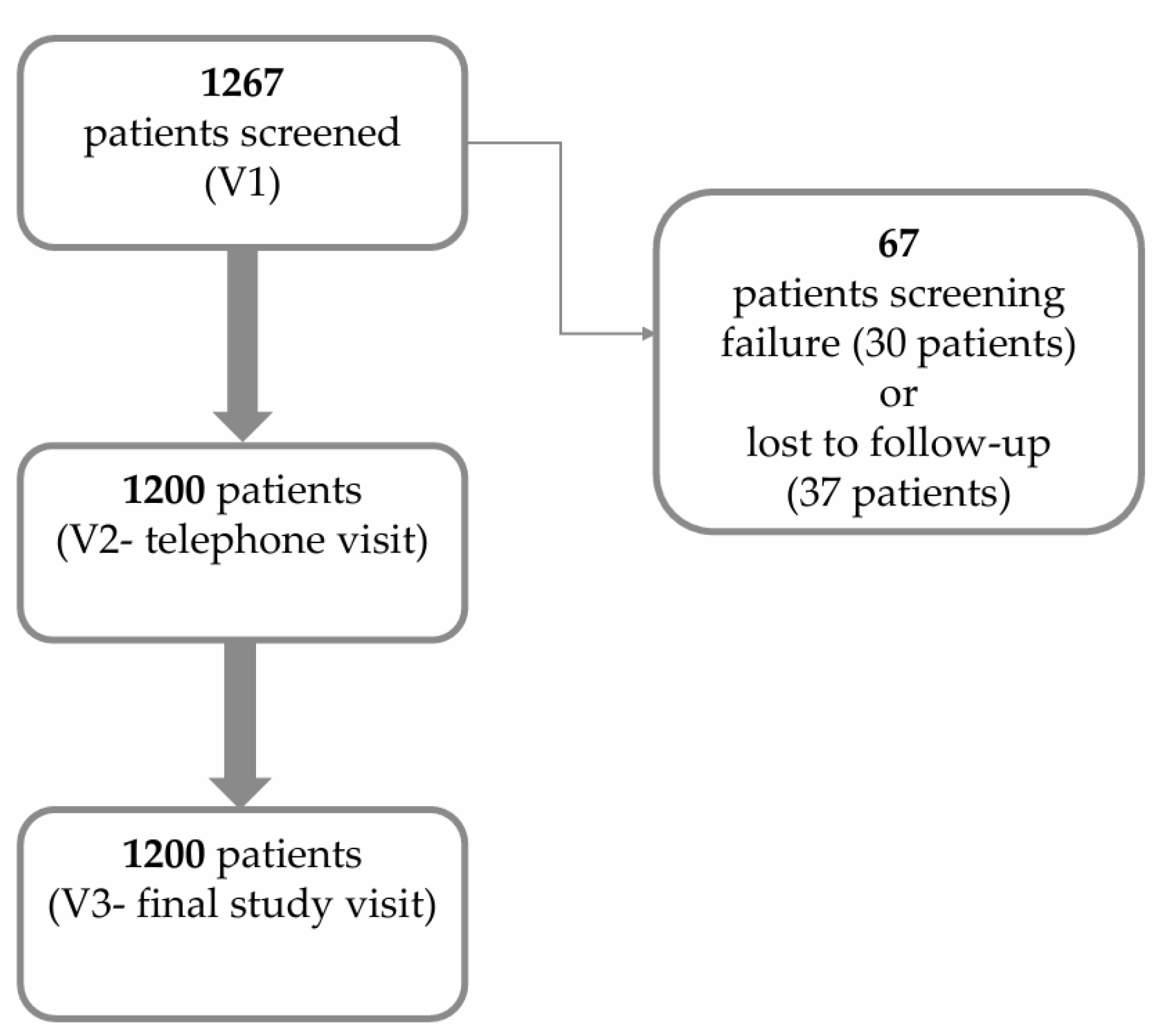
| Gender, N (%) | N = 1200 |
| Female, | 858 (71.5%) |
| Male | 342 (28.5%) |
| Age, N (%) | N = 1200 |
| <30 years | 8 (0.7%) |
| 30–50 years | 210 (17.5%) |
| 51–70 years | 607 (50.5%) |
| >70 years | 375 (31.3%) |
| Weight (kg), Mean ± SD | 80.4 ± 15.4 |
| Height (cm), Mean ± SD | 162.3 ± 6.5 |
| BMI (kg/m2), Mean ± SD | 28.9 ± 5.1 |
| BMI, n (%) | N = 1200 |
| <18.5 (underweight) | 8 (0.7%) |
| 18.5–25 (normal weight) | 263 (21.9%) |
| 25–30 (overweight) | 485 (40.4%) |
| 30–40 (first grade obesity) | 413 (34.4%) |
| >40 (second grade obesity) | 31 (2.6%) |
| Physical activity, n (%) | N = 1200 |
| Sedentary | 439 (36.6%) |
| Moderate physical activity | 542 (45.2%) |
| Intermediate physical activity | 206 (17.1%) |
| Intense physical activity | 13 (1.1%) |
| Prolonged sitting position, N (%) | N = 742 |
| <5 h/day | 424 (57.1%) |
| 5–10 h/day | 318 (42.9%) |
| Prolonged standing position, N (%) | N = 758 |
| <5 h/day | 371 (48.9%) |
| 5–10 h/day | 387 (51.1%) |
| Smoker, n (%) (N = 1200) | 192 (16.0%) |
| CVeD family history, N (%) | N = 1200 |
| One parent | 523 (43.6%) |
| Both parents | 111 (9.2%) |
| Grade 2 relatives | 50 (4.2%) |
| No medical history of CVeD | 516 (43.0%) |
| Risk factors for thrombosis, N (%) | N = 1200 |
| Accidental/surgical injuries | 231 (19.3%) |
| Prolonged immobilization in bed (postpartum or surgical) | 49 (4.1%) |
| Long journeys at altitude or in a sitting position | 107 (8.9%) |
| Thrombophlebitis | 266 (22.2%) |
| Blood clotting disorders | 40 (3.3%) |
| None of the above | 591 (49.2%) |
| Concomitant diseases, N (%) | N = 1200 |
| Hypertension | 845 (70.4%) |
| Ischemic Coronary Disease (ICD) | 337 (28.1%) |
| Type 2 Diabetes Mellitus (T2DM) | 192 (16.0%) |
| Obesity | 444 (37.0%) |
| Dyslipidemia | 576 (48.0%) |
| Others | 366 (30.5%) |
| Female population | N = 858 |
| Number of births (%- reported to female study population), n (%) | |
| 0 | 85 (9.9%) |
| 1 | 301 (35.1%) |
| 2 | 348 (40.6%) |
| >2 | 124 (14.5%) |
| Use of hormone replacement therapy (%- reported to female study population), n (%) | 10 (1.2%) |
| Use of birth control pills (%- reported to female study population), n (%) | 16 (1.9%) |
| CEAP Class (N = 1200) | V1 | |
|---|---|---|
| n | % | |
| C0s | 6 | 0.5% |
| C1 | 77 | 6.4% |
| C2 | 271 | 22.6% |
| C3 | 464 | 38.7% |
| C4a | 190 | 15.8% |
| C4b | 88 | 7.3% |
| C5 | 64 | 5.3% |
| C6 | 40 | 3.4% |
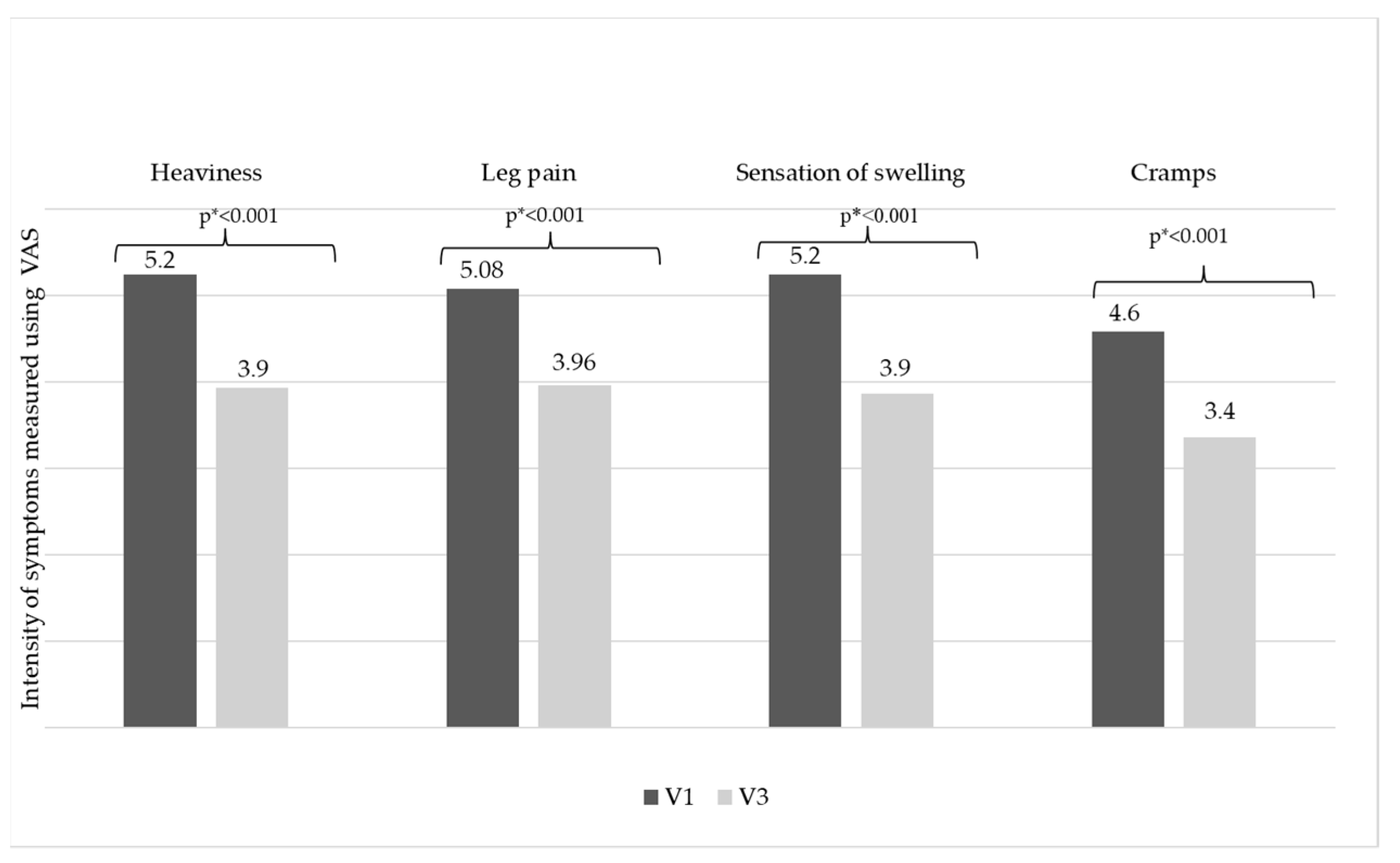
| CVeD Symptoms, n (%) | V1 | V3 |
|---|---|---|
| Heaviness | 1096 (91.3%) | 1002 (83.5%) |
| Leg pain | 1029 (85.8%) | 937 (78.1%) |
| Sensation of swelling | 1023 (85.3%) | 906 (75.5%) |
| Cramps | 875 (72.9%) | 715 (59.6%) |
| Time when CVeD symptoms appear at highest intensity, N = 1200 | V1 | |
| n | % | |
| At the end of the day | 948 | 79.0% |
| After long periods of orthostatic position | 686 | 57.2% |
| During the night | 353 | 29.4% |
| After a long period of sitting | 234 | 19.5% |
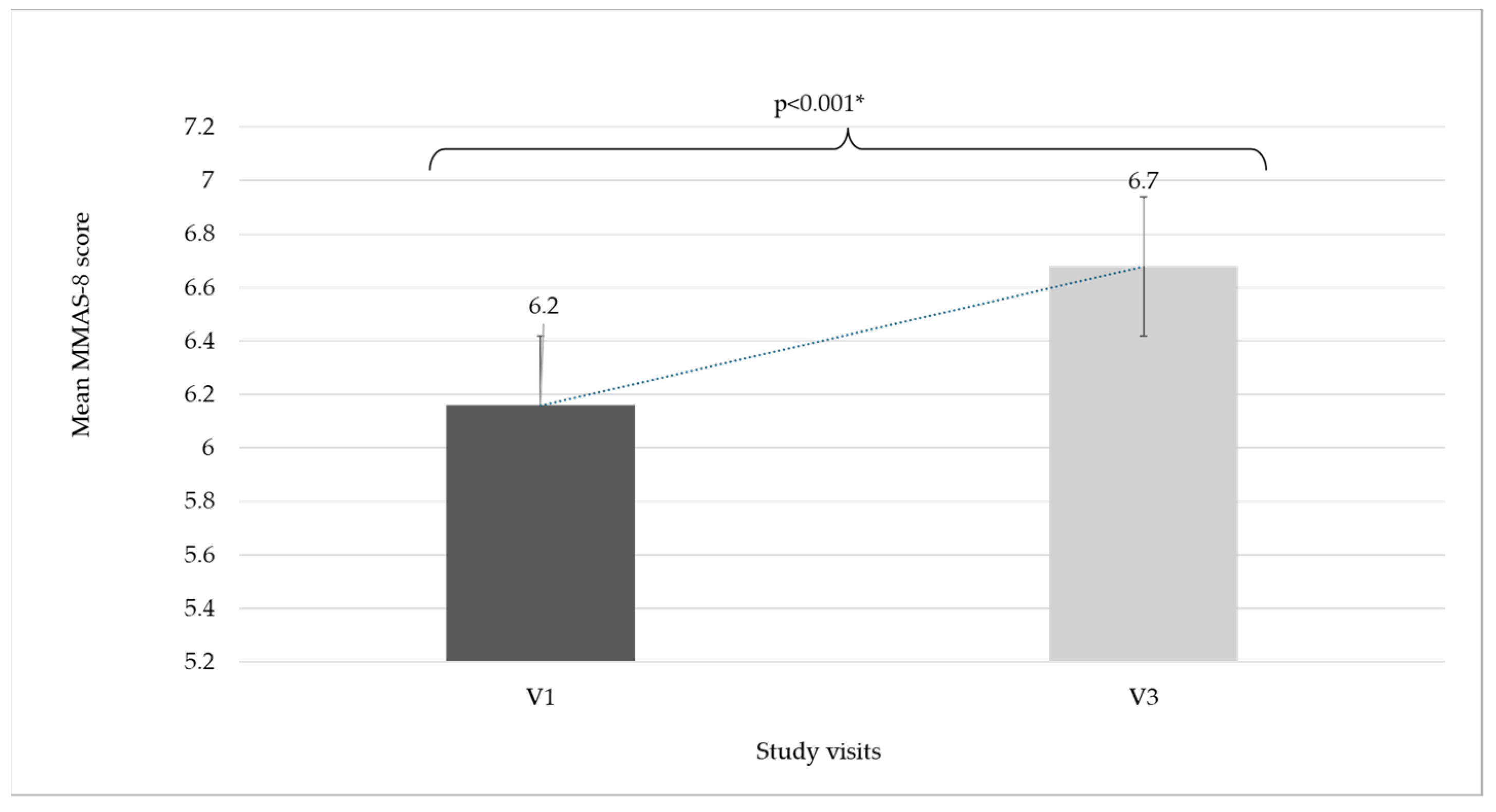
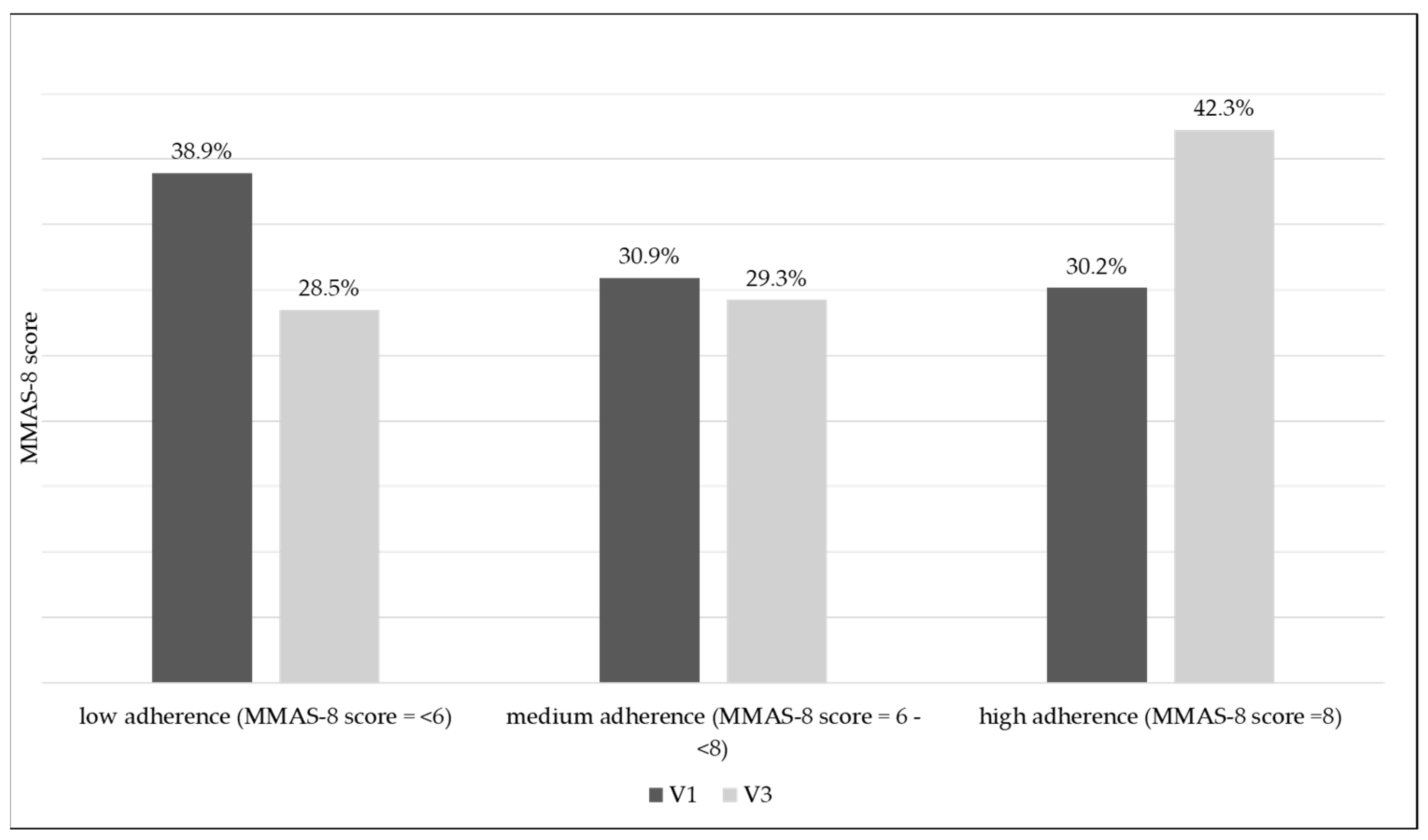
| Treatment Adherence According to MMAS-8 | V1 | V3 | p-Value (V1–V3) * | ||
|---|---|---|---|---|---|
| n (%) | Mean ± SD | n (%) | Mean ± SD | ||
| low (MMAS-8 score < 6) | 467 (38.9%) | 4.1 ± 1.3 | 342 (28.5%) | 4.4 ± 1.4 | p < 0.001 |
| medium (MMAS-8 score 6–8) | 371 (30.9%) | 7.0 ± 0.5 | 351 (29.3%) | 7.0 ± 0.5 | p < 0.001 |
| high (MMAS-8 score > 8) | 362 (30.2%) | 8.0 + 0.0 | 507 (42.3%) | 8.0 + 0.0 | p < 0.001 |
| Mean MMAS score | 6.2 ± 1.9 | 6.7 ± 1.7 | p < 0.001 | ||
| CEAP class | V1 | V3 | p-value (V1–V3) * | ||
| n (%) | MMAS-8 score (Mean ± SD) | n (%) | MMAS-8 score (Mean ± SD) | ||
| C0 | 6 (0.5%) | 5.8 ± 1.9 | 8 (0.7%) | 6.3 ± 2.2 | 0.269 |
| C1 | 77 (6.4%) | 6.2 ± 2.0 | 89 (7.4%) | 6.6 ± 2.0 | 0.001 |
| C2 | 271 (22.6%) | 6.4 ± 1.8 | 326 (27.2%) | 6.9 ± 1.6 | <0.001 |
| C3 | 464 (38.7%) | 6.3 ± 1.8 | 425 (35.4%) | 6.7 ± 1.7 | <0.001 |
| C4a | 190 (15.8%) | 6.2 ± 1.8 | 192 (16.0%) | 6.6 ± 1.6 | <0.001 |
| C4b | 88 (7.3%) | 5.3 ± 2.0 | 61 (5.1%) | 6.1 ± 2.0 | <0.001 |
| C5 | 64 (5.3%) | 6.1 ± 1.9 | 67 (5.6%) | 6.5 ± 1.6 | 0.001 |
| C6 | 40 (3.4%) | 5.3 ± 2.3 | 32 (2.7%) | 6.7 ± 1.7 | <0.001 |
| Direct and Indirect Questions and Answers | V1 | V3 | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Patients’ answers to the question asked verbally during the visit by the investigator regarding compliance with venoactive treatment recommendations, n (%) * | 1142 (95.2%) | 38 (3.2%) | 1156 (96.3%) | 21 (1.8%) |
| Patients answers to the question asked in the MMAS-8 questionnaire: Do you sometimes forget to take your medication(s)? n (%) | 465 (38.8%) | 735 (61.3%) | 375 (31.3%) | 825 (68.8%) |
| Reasons for Skipping the Treatment (V3) Declared by Patient | n | % |
|---|---|---|
| Forgot to take the medicine on certain days | 98 | 74.2% |
| Ran out of medicine | 11 | 8.3% |
| Takes too much medicine | 9 | 6.8% |
| Does not take every day, only when needed | 3 | 2.3% |
| Another reason | 11 | 8.3% |
| Patient and Physician Satisfaction with Conservative Treatment Result | Patient Satisfaction | Physician Satisfaction |
|---|---|---|
| 0—Dissatisfied | 2 (0.2%) | 3 (0.3%) |
| 1—Rather dissatisfied | 20 (1.7%) | 18 (1.5%) |
| 2—Rather satisfied | 325 (27.1%) | 271 (22.6%) |
| 3—Very satisfied | 574 (47.8%) | 588 (49.0%) |
| 4—Extremely satisfied | 279 (23.3%) | 320 (26.7%) |
| Patient motivation to follow recommended treatment | N | % |
| 0—Not motivated | 2 | 0.2% |
| 2—Rather unmotivated | 27 | 2.3% |
| 4—Rather motivated | 155 | 12.9% |
| 6—Motivated | 313 | 26.1% |
| 8—Very motivated | 427 | 35.6% |
| 10—Extremely motivated | 276 | 23.0% |
| CEAP Classification | Patient Receiving Compression Therapy at V1 (n = 796) | ||||
|---|---|---|---|---|---|
| CEAP Class | n | % (Reported to Total Study Population, N = 1200) | N | % (Reported to Total Study Population, N = 1200) | % (Reported to CEAP Class) |
| C0 | 6 | 0.50% | 1 | 0.08% | 16.67% |
| C1 | 77 | 6.42% | 21 | 1.75% | 27.27% |
| C2 | 271 | 22.58% | 152 | 12.67% | 56.09% |
| C3 | 464 | 38.67% | 314 | 26.17% | 67.67% |
| C4a | 190 | 15.83% | 150 | 12.50% | 78.95% |
| C4b | 88 | 7.33% | 67 | 5.58% | 76.14% |
| C5 | 64 | 5.33% | 51 | 4.25% | 79.69% |
| C6 | 40 | 3.33% | 40 | 3.33% | 100.00% |
| Total | 1200 | 100% | 796 | 66.33% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branisteanu, D.E.; Munteanu, A.E.; Dolofan, B.M.; Popescu, E.G.; Vittos, O. Adherence to Pharmacological Treatment in Chronic Venous Disease: Results of a Real-World, Prospective, Observational Cohort Study. Life 2025, 15, 377. https://doi.org/10.3390/life15030377
Branisteanu DE, Munteanu AE, Dolofan BM, Popescu EG, Vittos O. Adherence to Pharmacological Treatment in Chronic Venous Disease: Results of a Real-World, Prospective, Observational Cohort Study. Life. 2025; 15(3):377. https://doi.org/10.3390/life15030377
Chicago/Turabian StyleBranisteanu, Daciana Elena, Alice Elena Munteanu, Bogdan Mihai Dolofan, Elena Gabriela Popescu, and Oana Vittos. 2025. "Adherence to Pharmacological Treatment in Chronic Venous Disease: Results of a Real-World, Prospective, Observational Cohort Study" Life 15, no. 3: 377. https://doi.org/10.3390/life15030377
APA StyleBranisteanu, D. E., Munteanu, A. E., Dolofan, B. M., Popescu, E. G., & Vittos, O. (2025). Adherence to Pharmacological Treatment in Chronic Venous Disease: Results of a Real-World, Prospective, Observational Cohort Study. Life, 15(3), 377. https://doi.org/10.3390/life15030377







