Tangeretin Unravels Metabolic Dysfunction-Associated Fatty Liver Disease in Rats by Enhancing the IRS/Akt Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Induction and Experimental Designs
2.3. Fasting Blood Glucose and Oral Glucose Tolerance Test (OGTT)
2.4. Fasting Serum Insulin and HOMA-IR Index Assessment
2.5. Adiponectin Level Measurement
2.6. Lipid Profile Assessments
2.7. Serum Alanine Transaminase (AST) and Aspartate Transaminase (ALT) Activity Measurement
2.8. Assay of Superoxide (O2•−) Production
2.9. Measurement of Malondialdehyde (MDA) Levels in Plasma and Liver Tissue
2.10. Assay of Catalase (CAT) Enzyme Activity
2.11. Tumor Necrosis Factor-α (TNF-α) and Interleukin-6 (IL-6) Assay
2.12. Histological Examination
2.13. Western Blot Analysis
2.14. Statistical Analysis
3. Results
3.1. Effect of Tangeretin on Body and Organ Weight
3.2. Role of Tangeretin on Metabolic Parameters
3.3. Role of Tangeretin on Lipid Profiles
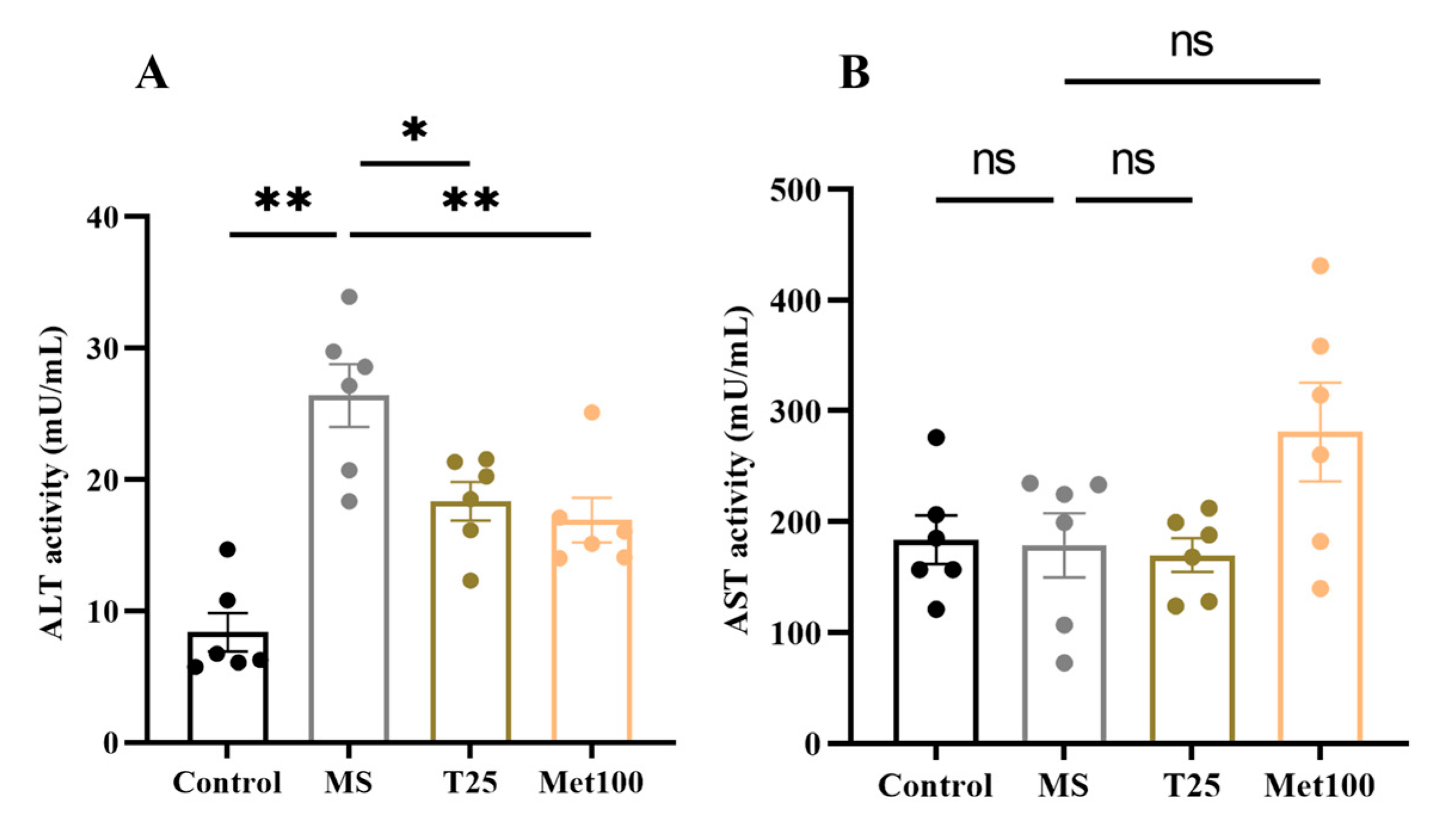
3.4. Effect of Tangeretin on Liver Function
3.5. Effect of Tangeretin on Liver Oxidative Stress in Metabolic Syndrome Rats
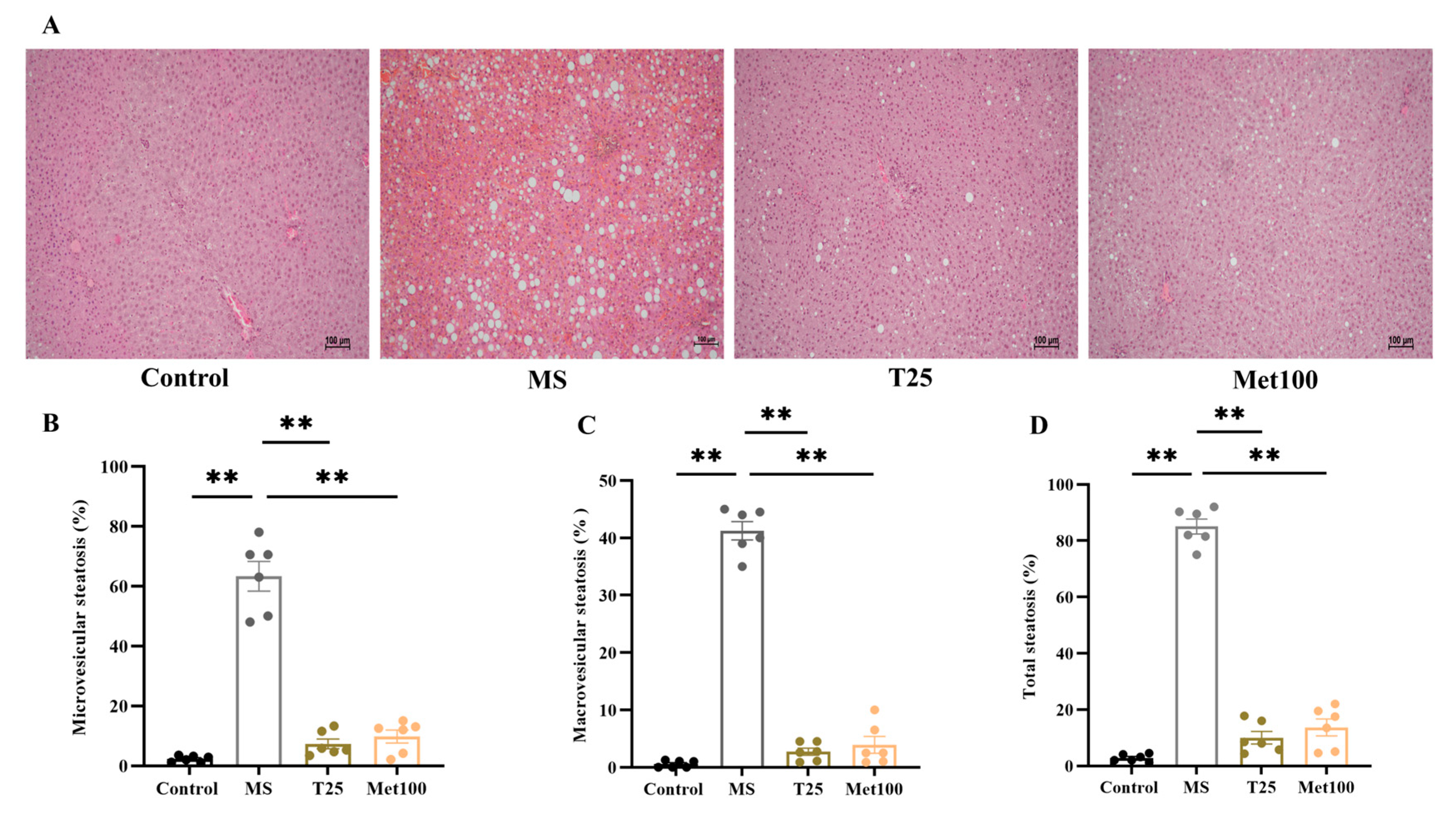
3.6. Effect of Tangeretin on Lipid Droplets Accumulation in Hepatocytes
3.7. Effect of Tangeretin on IRS-1 and Akt Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | Protein Kinase B |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| ATP | Adenosine Triphosphate |
| AUC | Area under the curve |
| FFAs | Free fatty acid |
| IL-6 | Interleukin 6 |
| HDL-c | High-density lipoprotein cholesterol |
| HFD | High-fat diet |
| HOMA-IR | Homeostatic model assessment of insulin resistance |
| IRS-1 | Insulin receptor substrate 1 |
| LDL-c | Low-density lipoprotein cholesterol |
| MAFLD | Metabolic dysfunction-associated fatty liver disease |
| MDA | Malondialdehyde |
| Met | Metformin |
| Mets | Metabolic syndrome |
| NAFLD | Non-alcoholic fatty liver disease |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nrf2 | Nuclear factor erythroid-derived 2-like 2 |
| O2•− | Superoxide anion |
| OGTT | Oral glucose tolerance test |
| PI3K | Phosphoinositide 3-kinase |
| TC | Total cholesterol |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor-α |
References
- Haffner, S.M. The Metabolic Syndrome: Inflammation, Diabetes Mellitus, and Cardiovascular Disease. Am. J. Cardiol. 2006, 97, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Armandi, A.; Rosso, C.; Caviglia, G.P.; Bugianesi, E. Insulin Resistance across the Spectrum of Nonalcoholic Fatty Liver Disease. Metabolites 2021, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Sangro, P.; de la Torre Aláez, M.; Sangro, B.; D’Avola, D. Metabolic dysfunction-associated fatty liver disease (MAFLD): An update of the recent advances in pharmacological treatment. J. Physiol. Biochem. 2023, 79, 869–879. [Google Scholar] [CrossRef]
- Lima, T.d.R.; Voltarelli, F.A.; Freire, L.S.; da Silva, F.A.; de Almeida, P.C.; Ávila, E.T.P.; de França, S.A.; Pereira, M.P.; Damazo, A.S.; Navalta, J.W. High-fat diet and fructose drink introduced after weaning rats, induces a better human obesity model than very high-fat diet. J. Food Biochem. 2021, 45, e13671. [Google Scholar] [CrossRef]
- Zakaria, Z.; Othman, Z.A.; Bagi Suleiman, J.; Jalil, N.A.C.; Ghazali, W.S.W.; Mohamed, M. Protective and Therapeutic Effects of Orlistat on Metabolic Syndrome and Oxidative Stress in High-Fat Diet-Induced Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) in Rats: Role on Nrf2 Activation. Vet. Sci. 2021, 8, 274. [Google Scholar] [CrossRef]
- Bugianesi, E.; Gastaldelli, A.; Vanni, E.; Gambino, R.; Cassader, M.; Baldi, S.; Ponti, V.; Pagano, G.; Ferrannini, E.; Rizzetto, M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: Sites and mechanisms. Diabetologia 2005, 48, 634–642. [Google Scholar] [CrossRef]
- Ziemke, F.; Mantzoros, C.S. Adiponectin in insulin resistance: Lessons from translational research1234. Am. J. Clin. Nutr. 2010, 91, 258S–261S. [Google Scholar] [CrossRef]
- Almalki, N.A.R.; Al-Abbasi, F.A.; Moglad, E.; Afzal, M.; Al-Qahtani, S.D.; Alzarea, S.I.; Imam, F.; Sayyed, N.; Kazmi, I. Protective activity of hirsutidin in high-fat intake and streptozotocin-induced diabetic rats: In silico and in vivo study. Heliyon 2024, 10, e38625. [Google Scholar] [CrossRef]
- Xu, Z.J.; Fan, J.G.; Ding, X.D.; Qiao, L.; Wang, G.L. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig. Dis. Sci. 2010, 55, 931–940. [Google Scholar] [CrossRef]
- Miao, J.; Guo, L.; Cui, H.; Wang, L.; Zhu, B.; Lei, J.; Li, P.; Jia, J.; Zhang, Z. Er-Chen Decoction Alleviates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Rats through Remodeling Gut Microbiota and Regulating the Serum Metabolism. Evid.-Based Complement. Altern. Med. 2022, 2022, 6221340. [Google Scholar] [CrossRef] [PubMed]
- Coccia, F.; Testa, M.; Guarisco, G.; Di Cristofano, C.; Silecchia, G.; Leonetti, F.; Gastaldelli, A.; Capoccia, D. Insulin resistance, but not insulin response, during oral glucose tolerance test (OGTT) is associated to worse histological outcome in obese NAFLD. Nutr. Metab. Cardiovasc. Dis. NMCD 2020, 30, 106–113. [Google Scholar] [CrossRef]
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the Regulation of Hepatic Metabolism by Insulin. Trends Endocrinol. Metab. TEM 2017, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Sztolsztener, K.; Chabowski, A. Hepatic-Metabolic Activity of α-Lipoic Acid-Its Influence on Sphingolipid Metabolism and PI3K/Akt/mTOR Pathway in a Rat Model of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients 2024, 16, 1501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Hahn, M.; Webb, M.; Shibolet, O.; Kariv, R.; Tirosh, O. Serum Malondialdehyde is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women. Antioxidants 2020, 9, 578. [Google Scholar] [CrossRef]
- Ratziu, V.; Goodman, Z.; Sanyal, A. Current efforts and trends in the treatment of NASH. J. Hepatol. 2015, 62, S65–S75. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Ghasemipour Afshar, E. Tangeretin: A mechanistic review of its pharmacological and therapeutic effects. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190191. [Google Scholar] [CrossRef]
- Hung, W.L.; Chang, W.S.; Lu, W.C.; Wei, G.J.; Wang, Y.; Ho, C.T.; Hwang, L.S. Pharmacokinetics, bioavailability, tissue distribution and excretion of tangeretin in rat. J. Food Drug Anal. 2018, 26, 849–857. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, R.; Chen, J.; Cao, J.; Xiao, J.; Li, X.; Sun, C. Tangeretin maintains antioxidant activity by reducing CUL3 mediated NRF2 ubiquitination. Food Chem. 2021, 365, 130470. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, H.; Li, M.; Ding, M. A possible underlying mechanism behind the cardioprotective efficacy of tangeretin on isoproterenol triggered cardiotoxicity via modulating PI3K/Akt signaling pathway in a rat model. J. Food Biochem. 2020, 44, e13368. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, J.; Zhou, Z.; Li, D. Tangeretin inhibits streptozotocin-induced cell apoptosis via regulating NF-κB pathway in INS-1 cells. J. Cell. Biochem. 2019, 120, 3286–3293. [Google Scholar] [CrossRef] [PubMed]
- Meephat, S.; Prasatthong, P.; Rattanakanokchai, S.; Bunbupha, S.; Maneesai, P.; Pakdeechote, P. Diosmetin attenuates metabolic syndrome and left ventricular alterations via the suppression of angiotensin II/AT1 receptor/gp91phox/p-NF-κB protein expression in high-fat diet fed rats. Food Funct. 2021, 12, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Prasatthong, P.; Meephat, S.; Rattanakanokchai, S.; Khamseekaew, J.; Bunbupha, S.; Prachaney, P.; Maneesai, P.; Pakdeechote, P. Galangin Resolves Cardiometabolic Disorders through Modulation of AdipoR1, COX-2, and NF-κB Expression in Rats Fed a High-Fat Diet. Antioxidants 2021, 10, 769. [Google Scholar] [CrossRef]
- Calco, G.N.; Proskocil, B.J.; Jacoby, D.B.; Fryer, A.D.; Nie, Z. Metformin prevents airway hyperreactivity in rats with dietary obesity. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 321, L1105–L1118. [Google Scholar] [CrossRef]
- Arivazhagan, L.; Subramanian, S.P. Tangeretin, a citrus flavonoid attenuates oxidative stress and protects hepatocellular architecture in rats with 7, 12—Dimethylbenz(a)anthracene induced experimental mammary carcinoma. J. Funct. Foods 2015, 15, 339–353. [Google Scholar] [CrossRef]
- Cicek, B.; Danisman, B.; Bolat, I.; Kiliclioglu, M.; Kuzucu, M.; Suleyman, H.; Tsarouhas, K.; Tsatsakis, A.; Taghizadehghalehjoughi, A. Effect of tangeretin on cisplatin-induced oxido-inflammatory brain damage in rats. J. Cell. Mol. Med. 2024, 28, e18565. [Google Scholar] [CrossRef]
- Guo, J.; Chen, J.; Ren, W.; Zhu, Y.; Zhao, Q.; Zhang, K.; Su, D.; Qiu, C.; Zhang, W.; Li, K. Citrus flavone tangeretin is a potential insulin sensitizer targeting hepatocytes through suppressing MEK-ERK1/2 pathway. Biochem. Biophys. Res. Commun. 2020, 529, 277–282. [Google Scholar] [CrossRef]
- Li, X.; Xie, P.; Hou, Y.; Chen, S.; He, P.; Xiao, Z.; Zhan, J.; Luo, D.; Gu, M.; Lin, D. Tangeretin Inhibits Oxidative Stress and Inflammation via Upregulating Nrf-2 Signaling Pathway in Collagen-Induced Arthritic Rats. Pharmacology 2019, 104, 187–195. [Google Scholar] [CrossRef]
- Luangaram, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P. Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food Chem. Toxicol. 2007, 45, 448–455. [Google Scholar] [CrossRef]
- Poasakate, A.; Maneesai, P.; Rattanakanokchai, S.; Bunbupha, S.; Tong-Un, T.; Pakdeechote, P. Genistein Prevents Nitric Oxide Deficiency-Induced Cardiac Dysfunction and Remodeling in Rats. Antioxidants 2021, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Maneesai, P.; Potue, P.; Khamseekaew, J.; Sangartit, W.; Rattanakanokchai, S.; Poasakate, A.; Pakdeechote, P. Kaempferol protects against cardiovascular abnormalities induced by nitric oxide deficiency in rats by suppressing the TNF-α pathway. Eur. J. Pharmacol. 2023, 960, 176112. [Google Scholar] [CrossRef] [PubMed]
- Prasatthong, P.; Meephat, S.; Rattanakanokchai, S.; Bunbupha, S.; Prachaney, P.; Maneesai, P.; Pakdeechote, P. Hesperidin ameliorates signs of the metabolic syndrome and cardiac dysfunction via IRS/Akt/GLUT4 signaling pathway in a rat model of diet-induced metabolic syndrome. Eur. J. Nutr. 2021, 60, 833–848. [Google Scholar] [CrossRef]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a General NAFLD Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef]
- Muhammad, N.; Lembede, B.W.; Erlwanger, K.H. Neonatal zingerone protects against the development of high-fructose diet-induced metabolic syndrome in adult Sprague-Dawley rats. J. Dev. Orig. Health Dis. 2021, 12, 671–679. [Google Scholar] [CrossRef]
- Zabielski, P.; Hady, H.R.; Chacinska, M.; Roszczyc, K.; Gorski, J.; Blachnio-Zabielska, A.U. The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci. Rep. 2018, 8, 7249. [Google Scholar] [CrossRef]
- Tappy, L. Metabolism of sugars: A window to the regulation of glucose and lipid homeostasis by splanchnic organs. Clin. Nutr. 2021, 40, 1691–1698. [Google Scholar] [CrossRef]
- Mortera, R.R.; Bains, Y.; Gugliucci, A. Fructose at the crossroads of the metabolic syndrome and obesity epidemics. FBL 2019, 24, 186–211. [Google Scholar] [CrossRef]
- Tappy, L. Fructose metabolism and noncommunicable diseases: Recent findings and new research perspectives. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 214–222. [Google Scholar] [CrossRef]
- Gunawan, S.; Munika, E.; Wulandari, E.T.; Ferdinal, F.; Purwaningsih, E.H.; Wuyung, P.E.; Louisa, M.; Soetikno, V. 6-gingerol ameliorates weight gain and insulin resistance in metabolic syndrome rats by regulating adipocytokines. Saudi Pharm. J. 2023, 31, 351–358. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Vatner, D.F.; Goedeke, L.; Hirabara, S.M.; Zhang, Y.; Perry, R.J.; Shulman, G.I. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 32584–32593. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, L.; Qiu, C. Tangeretin protects mice from diet-induced metabolic inflammation via activating adipose lactate accumulation and macrophage M2 polarization. Biochem. Biophys. Res. Commun. 2022, 630, 16–23. [Google Scholar] [CrossRef]
- Nery, M.; Ferreira, P.S.; Gonçalves, D.R.; Spolidorio, L.C.; Manthey, J.A.; Cesar, T.B. Physiological effects of tangeretin and heptamethoxyflavone on obese C57BL/6J mice fed a high-fat diet and analyses of the metabolites originating from these two polymethoxylated flavones. Food Sci. Nutr. 2021, 9, 1997–2009. [Google Scholar] [CrossRef]
- Kurowska, E.M.; Manthey, J.A. Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia. J. Agric. Food Chem. 2004, 52, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, C.; Wang, Q.; Liu, X.; Zhang, J.; Zhang, H.; Fei, W.; Zhao, H.; Ren, L. Oxymatrine Alleviates High-Fat-High-Fructose-Induced Fatty Liver in Rats: Understanding the Molecular Mechanism Through an Untargeted Metabonomics Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 4013–4024. [Google Scholar] [CrossRef]
- Jarukamjorn, K.; Jearapong, N.; Pimson, C.; Chatuphonprasert, W. A High-Fat, High-Fructose Diet Induces Antioxidant Imbalance and Increases the Risk and Progression of Nonalcoholic Fatty Liver Disease in Mice. Scientifica 2016, 2016, 5029414. [Google Scholar] [CrossRef]
- Ke, Z.; Tan, S.; Li, H.; Jiang, S.; Li, Y.; Chen, R.; Li, M. Tangeretin improves hepatic steatosis and oxidative stress through the Nrf2 pathway in high fat diet-induced nonalcoholic fatty liver disease mice. Food Funct. 2022, 13, 2782–2790. [Google Scholar] [CrossRef]
- Chua, D.; Low, Z.S.; Cheam, G.X.; Ng, A.S.; Tan, N.S. Utility of Human Relevant Preclinical Animal Models in Navigating NAFLD to MAFLD Paradigm. Int. J. Mol. Sci. 2022, 23, 14762. [Google Scholar] [CrossRef]
- Alla, N.; Palatheeya, S.; Challa, S.R.; Kakarla, R. Tangeretin confers neuroprotection, cognitive and memory enhancement in global cerebral ischemia in rats. 3 Biotech 2023, 14, 9. [Google Scholar] [CrossRef]
- Chen, Q.; Gu, Y.; Tan, C.; Sundararajan, B.; Li, Z.; Wang, D.; Zhou, Z. Comparative effects of five polymethoxyflavones purified from Citrus tangerina on inflammation and cancer. Front. Nutr. 2022, 9, 963662. [Google Scholar] [CrossRef]
- Michael, M.D.; Kulkarni, R.N.; Postic, C.; Previs, S.F.; Shulman, G.I.; Magnuson, M.A.; Kahn, C.R. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 2000, 6, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Titchenell, P.M.; Chu, Q.; Monks, B.R.; Birnbaum, M.J. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nat. Commun. 2015, 6, 7078. [Google Scholar] [CrossRef] [PubMed]
- Leavens, K.F.; Birnbaum, M.J. Insulin signaling to hepatic lipid metabolism in health and disease. Crit. Rev. Biochem. Mol. Biol. 2011, 46, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J.; Ban, Q.; Zhang, M.; Ban, B. Effects of metformin on the glucose regulation, lipid levels and gut microbiota in high-fat diet with streptozotocin induced type 2 diabetes mellitus rats. Endocrine 2024, 86, 163–172. [Google Scholar] [CrossRef]
- Yasmin, T.; Rahman, M.M.; Khan, F.; Kabir, F.; Nahar, K.; Lasker, S.; Islam, M.D.; Hossain, M.M.; Hasan, R.; Rana, S.; et al. Metformin treatment reverses high fat diet- induced non-alcoholic fatty liver diseases and dyslipidemia by stimulating multiple antioxidant and anti-inflammatory pathways. Biochem. Biophys. Rep. 2021, 28, 101168. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef]
- Kim, M.S.; Hur, H.J.; Kwon, D.Y.; Hwang, J.-T. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Mol. Cell. Endocrinol. 2012, 358, 127–134. [Google Scholar] [CrossRef]
- Stojnić, B.; Serrano, A.; Sušak, L.; Palou, A.; Bonet, M.L.; Ribot, J. Protective Effects of Individual and Combined Low Dose Beta-Carotene and Metformin Treatments against High-Fat Diet-Induced Responses in Mice. Nutrients 2021, 13, 3607. [Google Scholar] [CrossRef]




| Parameters | Control | MS | T25 | Met100 |
|---|---|---|---|---|
| Initial BW (g) | 205.66 ± 3.67 | 204.88 ± 3.83 | 204.36 ± 4.05 | 199.53 ± 4.19 |
| Final BW (g) | 605.70 ± 10.69 | 947.12 ± 33.04 a | 824.00 ± 33.40 ab | 810.91 ± 35.00 ab |
| Food intake (g/day) | 29.86 ± 0.37 | 26.93 ± 0.74 | 27.44 ± 0.78 | 27.48 ± 0.95 |
| Calorie intake (Kcal/day) | 111.77 ± 1.41 | 144.09 ± 3.43 a | 145.26 ± 3.68 a | 147.37 ± 4.39 a |
| Liver weight (g) | 14.73 ± 0.47 | 26.62 ± 1.13 a | 20.83 ± 1.18 b | 21.56 ± 1.19 b |
| Liver/BW ratio (%) | 2.32 ± 0.05 | 2.83 ± 0.06 a | 2.47 ± 0.07 b | 2.54 ± 0.05 b |
| Visceral adipose tissue weight (g) | 15.27 ± 1.05 | 71.98 ± 4.39 a | 43.98 ± 5.7 ab | 36.55 ± 7.73 ab |
| Visceral adipose tissue/BW (%) | 2.51 ± 0.15 | 7.70 ± 0.35 a | 5.14 ± 0.34 ab | 4.65 ± 0.82 ab |
| Epididymal adipose tissue weight (g) | 10.86 ± 0.22 | 26.44 ± 20.21 a | 20.21 ± 1.30 ab | 20.45 ± 2.73 ab |
| Epididymal adipose tissue/BW (%) | 1.77 ± 0.04 | 3.08 ± 00.8 a | 2.40 ± 0.02 ab | 2.58 ± 0.25 ab |
| Parameters | Control | MS | T25 | Met100 |
|---|---|---|---|---|
| Serum total cholesterol (mmol/L) | 17.97 ± 1.41 | 38.42 ± 3.70 a | 19.13 ± 4.67 b | 18.66 ± 9.15 b |
| Serum triglyceride (mmol/L) | 0.99 ± 0.35 | 5.08 ± 0.32 a | 1.05 ± 0.19 b | 3.58 ± 0.33 ab |
| Serum HDL-c (mmol/L) | 38.88 ± 7.10 | 14.58 ± 1.64 a | 41.11 ± 5.45 b | 51.38 ± 3.52 b |
| Serum free fatty acid (μM) | 201.81 ± 2.36 | 619.91± 60.21 a | 202.73 ± 49.88 b | 183.56 ± 29.27 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuenyong, I.; Potue, P.; Maneesai, P.; Khamseekaew, J.; Manimmanakorn, A.; Iampanichakul, M.; Pakdeechote, P. Tangeretin Unravels Metabolic Dysfunction-Associated Fatty Liver Disease in Rats by Enhancing the IRS/Akt Pathway. Life 2025, 15, 491. https://doi.org/10.3390/life15030491
Yuenyong I, Potue P, Maneesai P, Khamseekaew J, Manimmanakorn A, Iampanichakul M, Pakdeechote P. Tangeretin Unravels Metabolic Dysfunction-Associated Fatty Liver Disease in Rats by Enhancing the IRS/Akt Pathway. Life. 2025; 15(3):491. https://doi.org/10.3390/life15030491
Chicago/Turabian StyleYuenyong, Ittipon, Prapassorn Potue, Putcharawipa Maneesai, Juthamas Khamseekaew, Apiwan Manimmanakorn, Metee Iampanichakul, and Poungrat Pakdeechote. 2025. "Tangeretin Unravels Metabolic Dysfunction-Associated Fatty Liver Disease in Rats by Enhancing the IRS/Akt Pathway" Life 15, no. 3: 491. https://doi.org/10.3390/life15030491
APA StyleYuenyong, I., Potue, P., Maneesai, P., Khamseekaew, J., Manimmanakorn, A., Iampanichakul, M., & Pakdeechote, P. (2025). Tangeretin Unravels Metabolic Dysfunction-Associated Fatty Liver Disease in Rats by Enhancing the IRS/Akt Pathway. Life, 15(3), 491. https://doi.org/10.3390/life15030491







