Antimicrobial Investigation of Phthalimide and N-Phthaloylglycine Esters: Activity, Mechanism of Action, Synergism and Ecotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Procedure for Synthesis of Potassium Salts of Phthalimide and N-Phthaloylglycine
2.1.2. General Procedure for Synthesis of Esters (2a–d)
- 4-Chlorobutyl benzoate (2a) in 85% yield; colorless liquid; full data were found to be identical with the ones described in Ref. [19].
- 4-Chlorobutyl 4-methylbenzoate (2b) in 81% yield; colorless liquid; full data were found to be identical with the ones described in Ref. [19].
- 4-Chlorobutyl 4-methoxybenzoate (2c) in 80% yield; yellow liquid; full data were found to be identical with the ones described in Ref. [18].
- 4-Chlorobutyl 4-chlorobenzoate (2d) in 78% yield; yellow liquid; full data were found to be identical with the ones described in Ref. [19].
2.1.3. General Procedure for Synthesis of Phthalimide and N-Phthaloylglycine Esters (3a–d and 4a–d)
- 4-(1,3-Dioxoisoindolin-2-yl)butyl benzoate (3a, C19H17NO4) in 91% yield; Rf: 0.43 (hexane:ethyl acetate 7:3); white solid; m.p.: 98–100 °C (Ref. [20] 97–98 °C); 1H NMR (400 MHz, CDCl3): δ = 8.02 (dd, J = 8.1, 1.0 Hz, 2H, HAr), 7.84 (dd, J = 5.5, 3.1 Hz, 2H, HAr), 7.72–7.69 (m, 2H, HAr), 7.54 (t, J = 7.5 Hz, 1H, HAr), 7.42 (t, J = 7.6 Hz, 2H, HAr), 4.35 (t, J = 6.8 Hz, 2H, CH2), 3.77 (t, J = 6.8 Hz, 2H, CH2), 1.87–1.84 (m, 4H, 2 × CH2) ppm; 13C NMR (100 MHz, CDCl3): δ = 168.5, 166.6 (2 × C=O), 134.4, 134.0, 133.0, 132.2, 130.3, 129.6, 128.4, 123.7, 123.3 (9 × CAr), 64.4, 37.7, 26.3, 25.4 (4 × CH2) ppm; IR (KBr): ν = 3068 (HAr), 2965, 2938, 2868 (Halkanic), 1771, 1705 (C=O), 1602, 1462 (C=C), 1273, 1105, 1040 (C–O/N), 868, 705 (Ar) cm−1; HRMS (ESI): calcd for C19H17NNaO4 ([M + Na]+) 346.1050, found 346.1052.
- 4-(1,3-Dioxoisoindolin-2-yl)butyl 4-methylbenzoate (3b, C20H19NO4) in 89% yield; Rf: 0.48 (hexane:ethyl acetate 7:3); white solid; m.p.: 121–122 °C (Ref. [21] yellow liquid); 1H NMR (500 MHz, CDCl3): δ = 7.91 (d, J = 8.4 Hz, 2H, HAr), 7.84 (dd, J = 5.3, 3.1 Hz, 2H, HAr), 7.71 (dd, J = 5.5, 3.1 Hz, 2H, HAr), 7.21 (d, J = 8.1 Hz, 2H, HAr), 4.33 (t, J = 6.8 Hz, 2H, CH2), 3.77 (t, J = 6.8 Hz, 2H, CH2), 2.39 (s, 3H, CH3), 1.87–1.81 (m, 4H, 2 × CH2) ppm; 13C NMR (125 MHz, CDCl3): δ = 168.5, 166.7 (2 × C=O), 143.6, 134.0, 132.2, 129.7, 129.1, 127.6 (6 × CAr), 64.2, 37.7, 26.3, 25.5 (4 × CH2), 21.7 (CH3) ppm; IR (KBr): ν = 3062 (HAr), 2968, 2919, 2871 (Halkanic), 1770, 1710 (C=O), 1607, 1470 (C=C), 1280, 1122, 1059 (C–O/N), 755, 726 (Ar) cm−1; HRMS (ESI): calcd for C20H19NNaO4 ([M + Na]+) 360.1205, found 360.1206.
- 4-(1,3-Dioxoisoindolin-2-yl)butyl 4-methoxybenzoate (3c, C20H19NO5) in 93% yield; Rf: 0.48 (hexane:ethyl acetate 7:3); white solid; m.p.: 110–112 °C; 1H NMR (500 MHz, CDCl3): δ = 7.97 (d, J = 6.9 Hz, 2H, HAr), 7.83 (d, 2H, J = 8.4 Hz, HAr), 7.71 (d, J = 3.1 Hz, 2H, HAr), 6.89 (d, J = 8.9 Hz, 2H, HAr), 4.31 (t, J = 6.1 Hz, 2H, CH2), 3.84 (s, 3H, CH3) 3.76 (t, J = 6.9 Hz, 2H, CH2), 1.85–1.83 (m, 4H, 2 × CH2) ppm; 13C NMR (125 MHz, CDCl3): δ = 168.5, 166.4 (2 × C=O), 163.4, 134.0, 132.2, 131.6, 123.3, 122.8, 113.6 (7 × CAr), 64.1 (CH2), 55.5 (CH3), 37.7 (CH2), 26.3 (CH2), 25.5 (CH2) ppm; IR (KBr): ν = 3019 (HAr), 2980, 2923, 2896 (Halkanic), 1773, 1701 (C=O), 1606, 1511 (C=C), 1253, 1161, 1127, 1055 (C–O/N), 852, 720 (Ar) cm−1; HRMS (ESI): calcd for C20H19NNaO5 ([M + Na]+) 376.1155, found 376.1157.
- 4-(1,3-Dioxoisoindolin-2-yl)butyl 4-chlorobenzoate (3d, C19H16ClNO4) in 95% yield; Rf: 0.42 (hexane:ethyl acetate 7:3); white solid; m.p.: 98 °C; 1H NMR (400 MHz, CDCl3): δ = 7.95 (d, J = 8.8 Hz, 2H, HAr), 7.84 (dd, J = 5.5, 3.1 Hz, 2H, HAr), 7.71 (dd, J = 5.4, 3.1 Hz, 2H, HAr), 7.39 (d, J = 8.8 Hz, 2H, HAr), 4.34 (t, J = 6.1 Hz, 2H, CH2), 3.76 (t, 2H, J = 6.1 Hz, CH2), 1.84–1.83 (m, 4H, 2 × CH2) ppm; 13C NMR (100 MHz, CDCl3): δ = 168.5, 165.8 (2 × C=O), 139.4, 134.1, 132.2, 131.1, 128.8, 123.3 (6 × CAr), 64.6, 37.6, 26.2, 25.4 (4 × CH2) ppm; IR (KBr): ν = 3064 (HAr), 2968, 2923, 2868 (Halkanic), 1773, 1713 (C=O), 1607, 1462 (C=C), 1285, 1122, 1055 (C–O/N), 759, 722 (Ar) cm−1; HRMS (ESI): calcd for C19H16ClNNaO4 ([M + Na]+) 380.0660, found 380.0662.
- 4-(2-(1,3-Dioxoisoindolin-2-yl)acetoxy)butyl benzoate (4a, C21H19NO6) in 83% yield; Rf: 0.43 (hexane:ethyl acetate 2:1); white solid; m.p.: 59–60 °C; 1H NMR (500 MHz, CDCl3): δ = 8.02 (d, J = 7.1 Hz, 2H, HAr), 7.88 (dd, J = 5.4, 3.0 Hz, 2H, HAr), 7.73 (dd, J = 5.5, 3.0 Hz, 2H, HAr), 7.56 (t, J = 7.4 Hz, 1H, HAr), 7.44 (t, J = 7.7 Hz, 2H, HAr), 4.45 (s, 2H, CH2), 4.33 (t, J = 5.9 Hz, 2H, CH2), 4.25 (t, J = 5.9 Hz, 2H, CH2), 1.85–1.81 (m, 4H, CH2) ppm; 13C NMR (125 MHz, CDCl3): δ = 167.6, 167.4, 166.6 (3 × C=O), 134.3, 133.0, 132.1, 129.6, 128.5, 123.7 (6 × CAr), 65.4, 64.4, 39.0, 25.4, 25.4 (5 × CH2) ppm; IR (ATR): ν = 3053, 3030 (HAr), 2964, 2943, 2897 (Halkanic), 1770, 1743, 1705 (C=O), 1600, 1467 (C=C), 1263, 1247, 1193, 1111, 1072, 1039 (C–O/N), 798 (Ar) cm−1; HRMS (ESI): calcd for C21H19NNaO6 ([M + Na]+) 404.1105, found 404.1100.
- 4-(2-(1,3-Dioxoisoindolin-2-yl)acetoxy)butyl 4-methylbenzoate (4b, C22H21NO6) in 75% yield; Rf: 0.41 (hexane:ethyl acetate 2:1); beige solid; m.p.: 100–101 °C; 1H NMR (400 MHz, CDCl3): δ = 7.91 (d, J = 8.3 Hz, 2H, HAr), 7.88 (dd, J = 5.5, 3.1 Hz, 2H, HAr), 7.73 (dd, J = 5.5, 3.1 Hz, 2H, HAr), 7.23 (d, J = 8.3 Hz, 2H, HAr), 4.45 (s, 2H, CH2), 4.31 (t, J = 6.0 Hz, 2H, CH2), 4.24 (t, J = 6.0 Hz, 2H, CH2), 2.40 (s, 3H, CH3), 1.83–1.80 (m, 4H, CH2) ppm; 13C NMR (100 MHz, CDCl3): δ = 167.5, 167.3, 166.7 (3 × C=O), 143.7, 134.3, 132.1, 129.7, 129.2, 127.6, 123.7 (7 × CAr), 65.5, 64.2, 39.0, 25.4, 25.4 (5 × CH2), 21.8 (CH3) ppm; IR (KBr): ν = 3104 (HAr), 2953, 2934, 2853 (Halkanic), 1755, 1713 (C=O), 1607, 1417 (C=C), 1288, 1207, 1114, (C–O/N), 759, 719 (Ar) cm−1; HRMS (ESI): calcd for C21H19NNaO6 ([M + Na]+) 418.1261, found 418.1242.
- 4-(2-(1,3-Dioxoisoindolin-2-yl)acetoxy)butyl 4-methoxybenzoate (4c, C22H21NO7) in 98% yield; Rf: 0.40 (hexane:ethyl acetate 2:1); white solid; m.p.: 98 °C; 1H NMR (400 MHz, CDCl3): δ = 7,98–7.96 (m, 2H, HAr), 7.88 (dd, J = 5.5, 3.0 Hz, 2H, HAr), 7.73 (dd, J = 5.5, 3.1 Hz, 2H, HAr), 6.93–6.90 (m, 2H, HAr), 4.45 (s, 2H, CH2), 4.30 (t, J = 5.5 Hz, 2H, CH2), 4.24 (t, J = 4.1 Hz, 2H, CH2), 3.86 (s, 3H, CH3), 1.81 (m, 4H, CH2) ppm; 13C NMR (100 MHz, CDCl3): δ = 167.6, 167.4, 166.4 (3 × C=O), 163.4, 134.3, 132.1, 131.7, 123.7, 122.7, 113.7 (7 × CAr), 65.5 (CH2), 64.1 (CH2), 55.5 (CH3), 39.0 (CH2), 25.4 (CH2) ppm; IR (KBr): ν = 2956, 2837 (Halkanic), 1771, 1721 (C=O), 1607, 1509 (C=C), 1262, 1231, 1162, 1121, 1029 (C–O/N), 771, 709 (Ar) cm−1; HRMS (ESI): calcd for C22H21NNaO7 ([M + Na]+) 434.1210, found 434.1193.
- 4-(2-(1,3-Dioxoisoindolin-2-yl)acetoxy)butyl 4-chlorobenzoate (4d, C21H18ClNO6) in 85% yield; Rf: 0.43 (hexane:ethyl acetate 2:1); white solid; m.p.: 74–77 °C; 1H NMR (400 MHz, CDCl3): δ = 7.97–7.95 (m, 2H, HAr), 7.88 (dd, J = 5.5, 3.0 Hz, 2H, HAr), 7.74 (dd, J = 5.5, 3.0 Hz, 2H, HAr), 7.43–7.40 (m, 2H, HAr), 4.46 (s, 2H, CH2), 4.33 (t, J = 6.1 Hz, 2H, CH2), 4.25 (t, J = 6.1 Hz, 2H, CH2), 1.83 (m, 4H, CH2) ppm; 13C NMR (100 MHz, CDCl3): δ = 167.4, 167.2, 165.6 (3 × C=O), 139.4, 134.2, 132.0, 130.9, 128.7, 128.6, 123.6 (7 × CAr), 65.3, 64.5, 38.9, 25.3, 25.2 (5 × CH2) ppm; IR (ATR): ν = 3082, 3047 (HAr), 2974, 2951, 2891 (Halkanic), 1743, 1710 (C=O), 1591, 1467 (C=C), 1294, 1193, 1112, 1087, 1016 (C–O/N), 858, 798, 756, 732, 686 (Ar) cm−1; HRMS (ESI): calcd for C21H18ClNNaO6 ([M + Na]+) 438.0715, found 438.0718.
2.2. In Silico/Vitro Antimicrobial Evaluation
2.3. Antifungal Mechanism of Action
2.4. Synergy Checkerboard Assay
2.5. In Silico/Vitro Toxicity Evaluation
2.6. Molecular Docking Study
3. Results
3.1. Chemistry
3.2. In Silico/Vitro Antimicrobial Evaluation
3.3. Antifungal Mechanism of Action
3.4. Synergy Checkerboard Assay
3.5. In Silico/Vitro Toxicity Evaluation
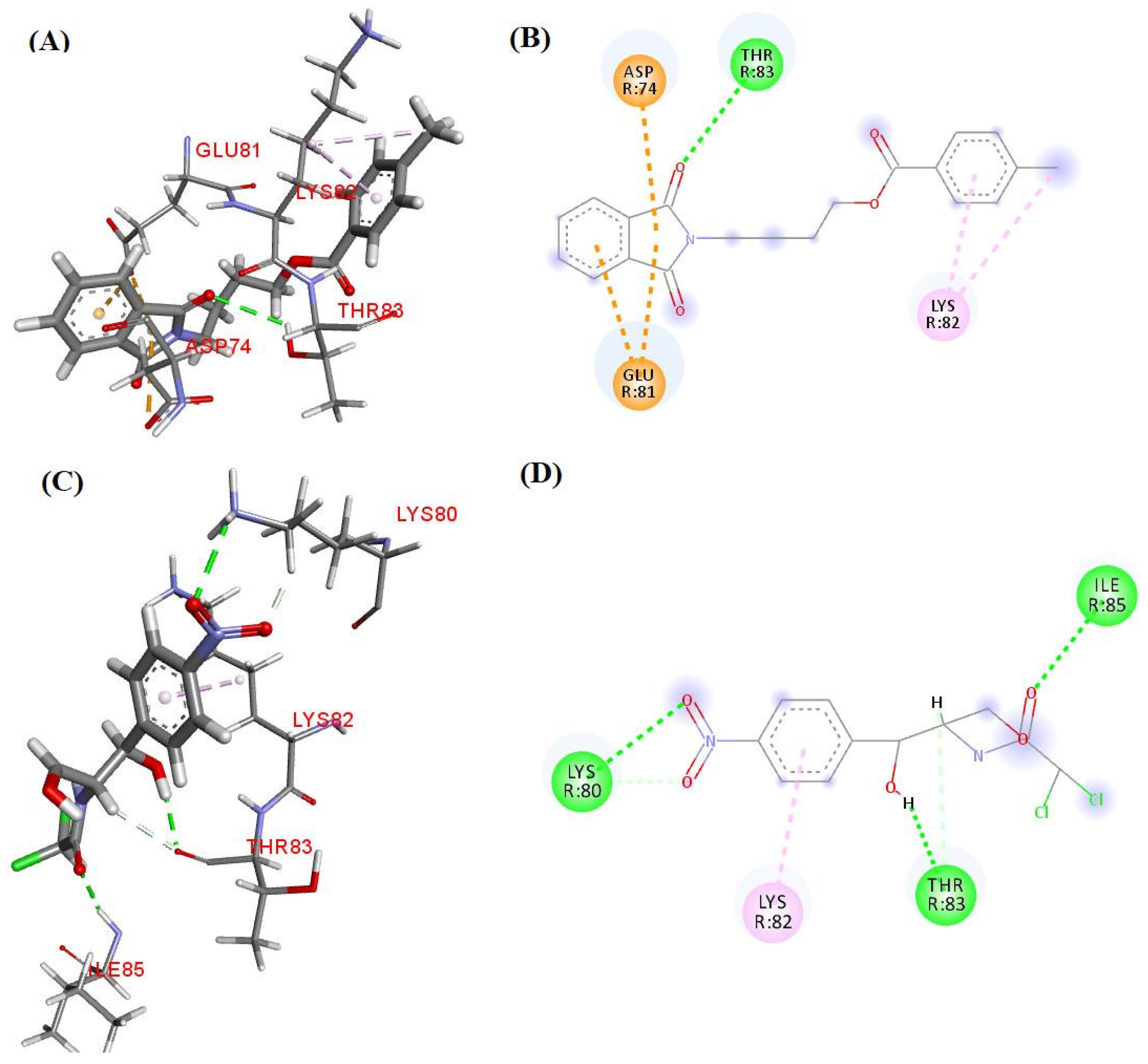
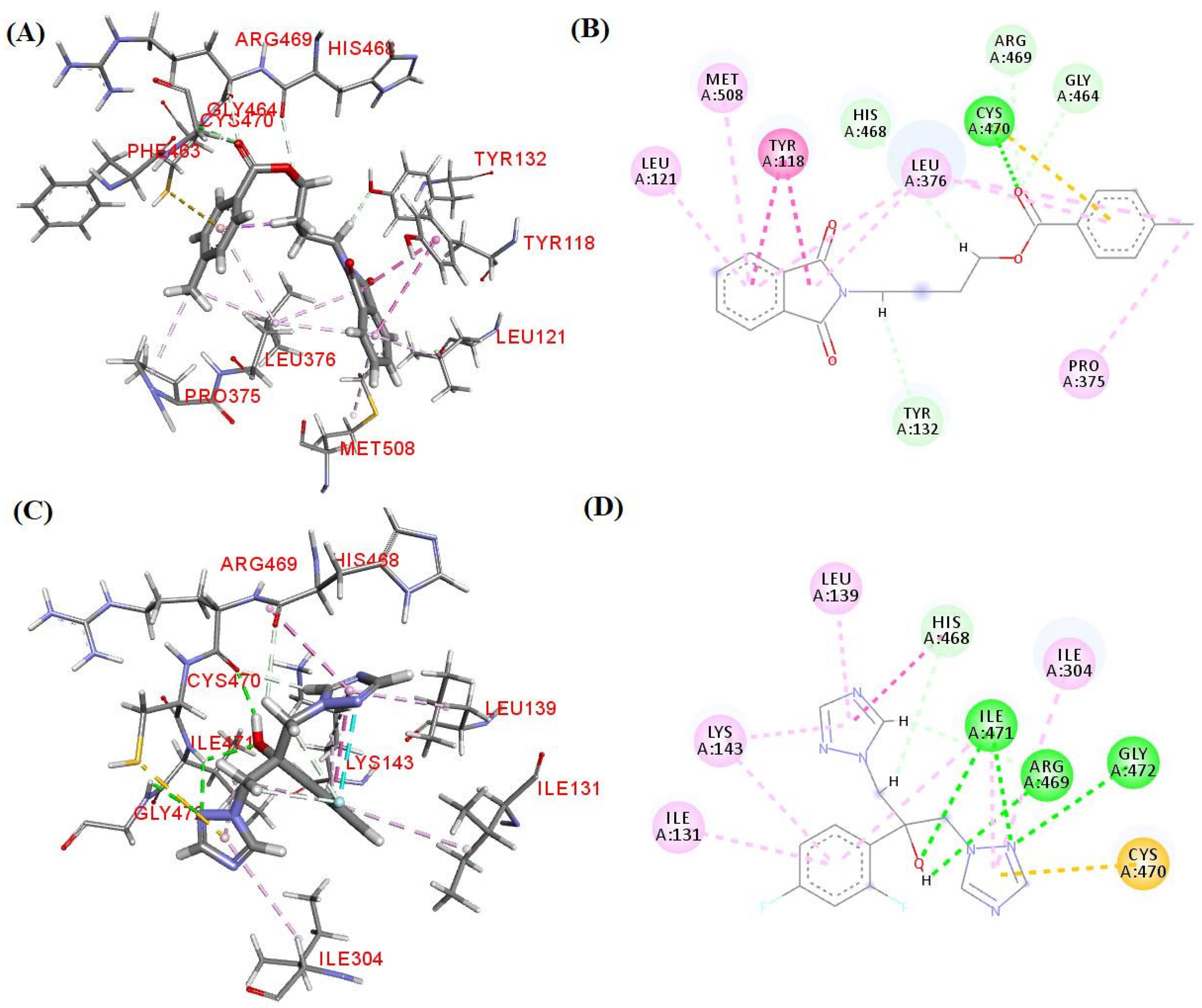
3.6. Molecular Docking Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global AMR R&D Hub. Available online: https://globalamrhub.org/publications/incentivising-the-development-of-new-antibacterial-treatments-2023/ (accessed on 2 January 2025).
- Rusic, D.; Vilovic, M.; Bukic, J.; Leskur, D.; Perisin, A.S.; Kumric, M.; Martinovic, D.; Petric, A.; Modun, D.; Bozic, J. Implications of COVID-19 pandemic on the emergence of antimicrobial resistance: Adjusting the response to future outbreaks. Life 2021, 11, 220. [Google Scholar] [CrossRef]
- Lu, H.; Hong, T.; Jiang, Y.; Whiteway, M.; Zhang, S. Candidiasis from cutaneous to systemic, new perspectives of potential targets and therapeutic strategies. Adv. Drug Deliv. Rev. 2023, 199, 114960. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 2 January 2025).
- Bertagnolio, S.; Dobreva, Z.; Centner, C.M.; Olaru, I.D.; Donà, D.; Burzo, S.; Huttner, B.D.; Chaillon, A.; Gebreselassie, N.; Wi, T.; et al. WHO global research priorities for antimicrobial resistance in human health. Lancet Microbe 2024, 5, 100902. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, S.; Alfieri, A.; Pace, M.C.; Sansone, P.; Pota, V.; Fittipaldi, C.; Fiore, M.; Passavanti, M.B. Blood stream infections from MDR bacteria. Life 2021, 11, 575. [Google Scholar] [CrossRef]
- Riccardi, N.; Rotulo, G.A.; Castagnola, E. Definition of opportunistic infections in immunocompromised children on the basis of etiologies and clinical features: A Summary for practical purposes. Curr. Pediatr. Rev. 2019, 15, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shrivastava, M.; Whiteway, M.; Jiang, Y. Candida albicans targets that potentially synergize with fluconazole. Crit. Rev. Microbiol. 2021, 47, 323–337. [Google Scholar] [CrossRef]
- Shaik, S.; Lee, J.-H.; Kim, Y.-G.; Lee, J. Antifungal, anti-biofilm, and anti-hyphal properties of N-substituted phthalimide derivatives against Candida species. Front. Cell. Infect. Microbiol. 2024, 14, 1414618. [Google Scholar] [CrossRef]
- Jelali, H.; Mansour, L.; Deniau, E.; Sauthier, M.; Hamdi, N. An efficient synthesis of phthalimides and their biological activities. Polycycl. Aromat. Compd. 2020, 42, 1806–1813. [Google Scholar] [CrossRef]
- Homsi, A.; Kasideh, A. Synthesis of some N-phthalimide derivatives and evaluation their biological activity. Pharm. Chem. J. 2015, 2, 33–41. [Google Scholar]
- Padda, I.S.; Bhatt, R.; Parmar, M. Apremilast. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572078/ (accessed on 2 January 2025).
- Engelhardt, M.; Wäsch, R.; Reinhardt, H.; Kleber, M. Pomalidomide. In Small Molecules in Oncology; Martens, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 201, pp. 359–372. [Google Scholar] [CrossRef]
- Kim, J.H.; Scialli, A.R. Thalidomide: The tragedy of birth defects and the effective treatment of disease. Toxicol. Sci. 2011, 122, 1–6. [Google Scholar] [CrossRef]
- Alves, F.; Oliveira, R.; Souza, H.; Lima, P.; Farias, F.; Marzari, B.; Coelho, H.; Luis, J.; Athayde-Filho, P.; Fiss, G. Cleaner approach and antimicrobial screening of 2-selenoacetanilides: Emergence of potential agents against co-infection. J. Sulfur Chem. 2025, 46, 212–225. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Souza, H.D.S.; Alves, F.S.; Sousa, A.P.; Lima, P.S.V.; Huang, M.-F.N.; Cordeiro, L.V.; Diniz-Neto, H.; Lima, E.O.; Trindade, E.O.; et al. Synthesis, in silico study and antimicrobial evaluation of new diesters derived from phthaloylglycine. J. Braz. Chem. Soc. 2020, 31, 953–962. [Google Scholar] [CrossRef]
- Olmedo, D.A.; Vásquez, Y.; Morán, J.A.; De Léon, E.G.; Caballero-George, C.; Solís, P.N. Understanding the Artemia salina (brine shrimp) test: Pharmacological significance and global impact. Comb. Chem. High Throughput Screen. 2024, 27, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Enthaler, S.; Weidauer, M. Synthesis of δ- and ε-cyanoesters by zinc-catalyzed ring-opening of cyclic ethers with acid chlorides and subsequent cyanation. Catal. Lett. 2012, 142, 168–175. [Google Scholar] [CrossRef]
- Trindade, E.O.; Dutra, T.F.; Brandão, M.C.R.; Diniz Neto, H.; Lima, E.O.; Lira, B.F.; Athayde-Filho, P.F.; Barbosa-Filho, J.M. Synthesis, in silico study and antimicrobial activity of new piperine derivatives containing substituted δ-esters. J. Braz. Chem. Soc. 2020, 31, 2590–2602. [Google Scholar] [CrossRef]
- Bergmann, E.D.; Kaluszyner, A. Reaction products of primary β-hydroxyamines with carbonyl compounds: XVI condensation of carbonyl compounds with 4-hydroxy- and 4-mercaptobutylamine. Rec. Trav. Chim. 1959, 78, 331–336. [Google Scholar] [CrossRef]
- Guan, W.; Lu, D.; Yang, X.; Deng, W.; Xiang, J.; Kambe, N.; Qiu, R. CF3SO2Na-mediated five-component carbonylation of triarylboroxines with TMSCF3 and THF/LiOH/NaI to give aroyloxyalkyl iodides. J. Org. Chem. 2022, 87, 9635–9644. [Google Scholar] [CrossRef]
- Scotti, M.T.; Herrera-Acevedo, C.; Menezes, R.P.B.; Martin, H.-J.; Muratov, E.N.; Silva, A.I.S.; Albuquerque, E.F.; Calado, L.F.; Coy-Barrera, E.; Scotti, L. MolPredictX: Online biological activity predictions by machine learning models. Mol. Inf. 2022, 41, 2200133. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Tenth Edition; CLSI Document M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; Available online: https://clsi.org/media/1632/m07a10_sample.pdf (accessed on 2 January 2025).
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Third Edition; CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Available online: https://clsi.org/media/1461/m27a3_sample.pdf (accessed on 2 January 2025).
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Frost, D.J.; Brandt, K.D.; Cugier, D.; Goldman, R. A whole-cell Candida albicans assay for the detection of inhibitors towards fungal cell wall synthesis and assembly. J. Antibiot. 1995, 48, 306–310. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.P.; Souza, H.D.S.; Almeida-Júnior, A.; Silva, M.F.R.; Cordeiro, L.V.; Lima, E.O.L.; Fiss, G.F.; Athayde-Filho, P.F. Novel esters derived from 4-hydroxychalcones as potential sunscreens with antimicrobial action. Synth. Commun. 2024, 54, 973–991. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: a new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Hansen, J.L.; Moore, P.B.; Steitz, T.A. Structures of five antibiotics bound at the peptidyl transferase center of the large ribosomal subunit. J. Mol. Biol. 2003, 330, 1061–1075. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Friggeri, L.; Wawrzak, Z.; Qi, A.; Hoekstra, W.J.; Schotzinger, R.J.; York, J.D.; Guengerich, F.P.; Lepesheva, G.I. Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J. Biol. Chem. 2017, 292, 6728–6743. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Onodera, K.; Satou, K.; Hirota, H. Evaluations of molecular docking programs for virtual screening. J. Chem. Inf. Model. 2007, 47, 1609–1618. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Linday, J.; Beattie, K.A.; Birmingham, S.; Saker, M.L.; Törökné, A.K.; Codd, G.A. Toxicity of cylindrospermopsin to the brine shrimp Artemia salina: Comparisons with protein synthesis inhibitors and microcystins. Toxicon 2002, 40, 1115–1120. [Google Scholar] [CrossRef]
- Silveira, B.M.; Novack, K.M.; Marcondes, H.C.; Santos, V.M.R. Preparation, characterization, and biological activity against Artemia salina of news copolymer PMMA-g-PEG derivatives incorporated with fluconazole. Macromol. Symp. 2018, 378, 1700066. [Google Scholar] [CrossRef]
- Nguta, J.M.; Mbaria, J.M.; Gakuya, D.W.; Gathumbi, P.K.; Kabasa, J.D.; Kiama, S.G. Biological screening of Kenyan medicinal plants using Artemia salina L. (Artemiidae). Pharmacologyonline 2011, 2, 458–478. [Google Scholar]
- Sedaghati, M.; Akbari, R.; Hagghi, L.L.; Yousefi, S.; Mesbahi, T.; Delfi, M. Survey of probable synergism between melittin and ciprofloxacin, rifampicin, and chloramphenicol against multidrug-resistant Pseudomonas aeruginosa. Front. Microbiol. 2024, 15, 1480299. [Google Scholar] [CrossRef]
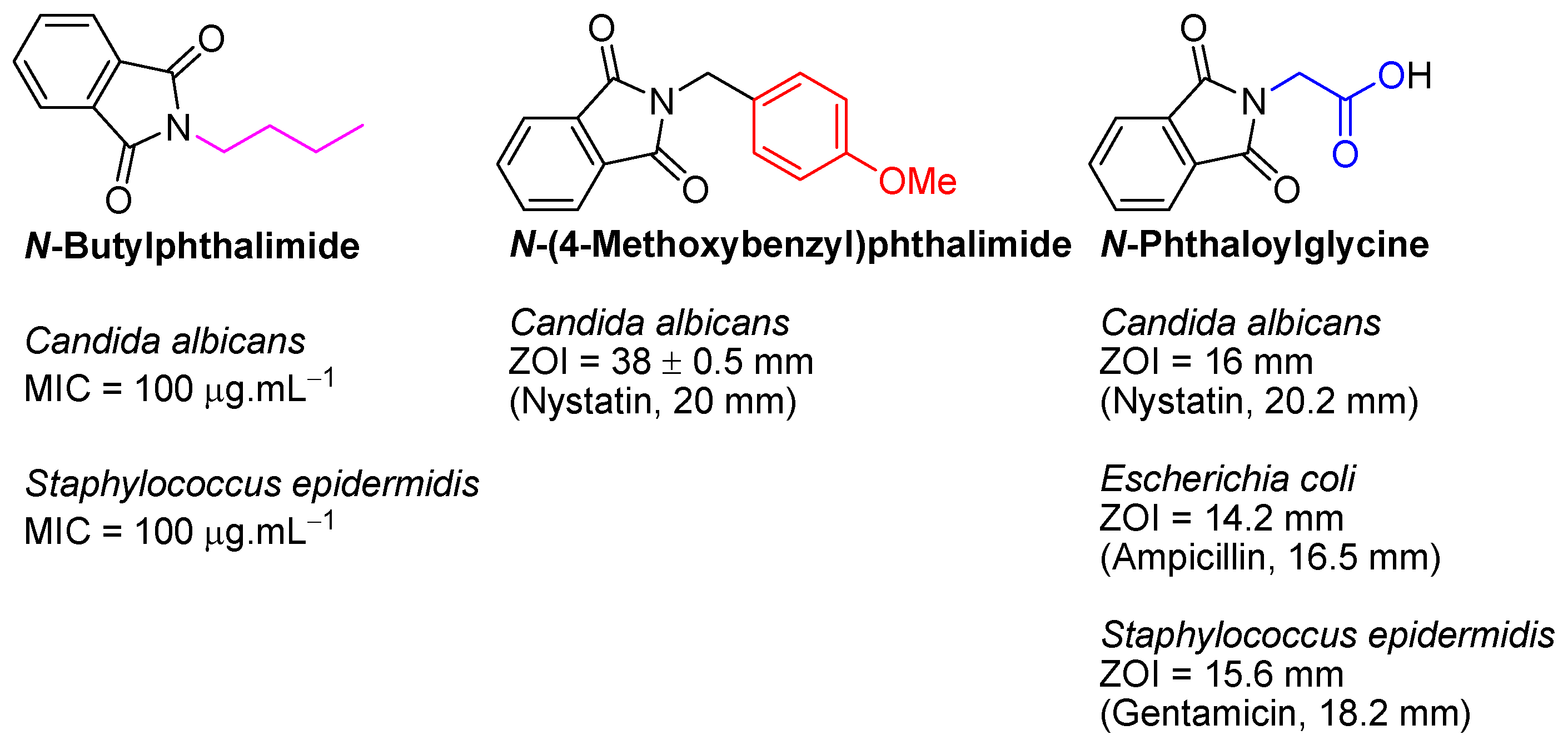

| Compound | R | Bacteria MIC/MBC * | Fungi MIC/MFC * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Gram-(+) | Gram-(−) | Yeast | Filamentous | ||||||
| S. aureus | E. coli | P. aeruginosa | C. tropicalis | A. flavus | A. niger | ||||
| ATCC 25923 | LM 01 | ATCC 25922 | ATCC 25853 | ATCC 13803 | LM 303 | ATCC 4603 | ATCC 6275 | ||
| 3a | H | 1024/− | 1024/− | 1024/− | 1024/− | 1024/− | 1024/− | 1024/− | 1024/− |
| 3b | Me | 128/512 | 128/512 | 256/512 | 128/512 | 128/512 | 256/512 | 1024/− | 1024/− |
| 3c | OMe | 512/− | 512/− | 512/− | 512/− | 256/1024 | 256/1024 | 1024/1024 | 1024/1024 |
| 3d | Cl | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 4a | H | 256/1024 | 256/1024 | 256/1024 | 512/1024 | 256/512 | 256/512 | −/− | −/− |
| 4b | Me | 1024/− | 1024/− | 1024/− | 1024/− | 1024/− | 1024/− | −/− | −/− |
| 4c | OMe | 512/− | 512/− | 512/− | 1024/− | 512/− | 512/− | −/− | −/− |
| 4d | Cl | 1024/− | 1024/− | 1024/− | 1024/− | 1024/− | 1024/− | −/− | −/− |
| Chloramphenicol | NA | 32/128 | 32/128 | 64/128 | 64/128 | NA | NA | NA | NA |
| Fluconazole | NA | NA | NA | NA | NA | 256/512 | 256/512 | 256/1024 | 512/1024 |
| Culture medium | NA | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Compound | R | Yeast Fungus C. albicans | |||
|---|---|---|---|---|---|
| In Silico | In Vitro MIC/MFC * | ||||
| Predicted Outcome | Probability Active (%) | ATCC 76485 | LM 65 | ||
| 3a | H | Inactive | 40 | 1024/− | 1024/− |
| 3b | Me | Active | 60 | 128/512 | 128/512 |
| 3c | OMe | Active | 100 | 256/1024 | 256/1024 |
| 3d | Cl | Active | 100 | −/− | −/− |
| 4a | H | Inactive | 20 | 256/512 | 256/512 |
| 4b | Me | Inactive | 20 | 1024/− | 1024/− |
| 4c | OMe | Inactive | 20 | 512/− | 512/− |
| 4d | Cl | Active | 60 | 1024/− | 1024/− |
| Fluconazole | NA | Active | 100 | 128/512 | 256/512 |
| Culture medium | NA | NA | NA | −/− | −/− |
| Compound | C. albicans ATCC 76485 | |||
|---|---|---|---|---|
| Sorbitol | Ergosterol | |||
| Absence | Presence | Absence | Presence | |
| 3b | 128 | 128 | 128 | 1024 |
| Caspofungin | 0.5 | 2 | NA | NA |
| Fluconazole | NA | NA | 128 | 512 |
| Culture medium | −/− | −/− | −/− | −/− |
| Strains | FICA | FICB | FICI | Outcome |
|---|---|---|---|---|
| S. aureus ATCC 25923 | 0.25 | 1.0 | 1.25 | Indifference |
| P. aeruginosa ATCC 25853 | 0.25 | 0.25 | 0.5 | Synergy |
| Compound | R | Toxicity | Lipophilicity | ||||
|---|---|---|---|---|---|---|---|
| In Silico | In Vitro | In Silico | |||||
| Ames | Hepato | Skin Sensitization | Minnow Log LC50 | Artemia salina LC50 * | Log P | ||
| 3b | Me | Yes | Yes | No | −1.895 | 239.000 | 3.228 |
| 3c | OMe | Yes | Yes | No | −1.784 | 224.095 | 2.928 |
| 4a | H | Yes | Yes | No | 0.455 | 1028.33 | 2.463 |
| 4c | OMe | No | Yes | No | −0.497 | >>1000 | 2.471 |
| Chloramphenicol | NA | Yes | No | No | 1.892 | >20 [36] | 0.909 |
| Fluconazole | NA | No | Yes | No | 3.872 | 802.28 [37] | 0.735 |
| PDB ID | Macromolecule | Species | PDB Ligand | Resolution | RMSD |
|---|---|---|---|---|---|
| 1NJI | 50S ribosomal subunit | Haloarcula marismortui | CLM_9001 | 3.00 Å | 2.21 Å |
| 5TZ1 | Sterol 14-alpha demethylase (CYP51) | Candida albicans | VT1_602 | 2.00 Å | 1.87 Å |
| Compound | 50S Ribosomal Subunit | Sterol 14-Alpha Demethylase (CYP51) |
|---|---|---|
| 3b | −92.69 | −107.25 |
| Chloramphenicol | −73.13 | − |
| Fluconazole | − | −113.86 |
| CLM_9001 | −73.13 | − |
| VT1_602 | − | −112.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, F.S.; Sousa, A.P.; Almeida-Júnior, A.; Lima, P.S.V.; Silva, M.F.R.; Galvão, J.L.F.M.; Lima, E.O.; Souza, H.D.S.; Luis, J.A.S.; Athayde-Filho, P.F.; et al. Antimicrobial Investigation of Phthalimide and N-Phthaloylglycine Esters: Activity, Mechanism of Action, Synergism and Ecotoxicity. Life 2025, 15, 518. https://doi.org/10.3390/life15040518
Alves FS, Sousa AP, Almeida-Júnior A, Lima PSV, Silva MFR, Galvão JLFM, Lima EO, Souza HDS, Luis JAS, Athayde-Filho PF, et al. Antimicrobial Investigation of Phthalimide and N-Phthaloylglycine Esters: Activity, Mechanism of Action, Synergism and Ecotoxicity. Life. 2025; 15(4):518. https://doi.org/10.3390/life15040518
Chicago/Turabian StyleAlves, Francinara S., Abraão P. Sousa, Alexandre Almeida-Júnior, Priscila S. V. Lima, Marcelo F. R. Silva, José L. F. M. Galvão, Edeltrudes O. Lima, Helivaldo D. S. Souza, José A. S. Luis, Petrônio F. Athayde-Filho, and et al. 2025. "Antimicrobial Investigation of Phthalimide and N-Phthaloylglycine Esters: Activity, Mechanism of Action, Synergism and Ecotoxicity" Life 15, no. 4: 518. https://doi.org/10.3390/life15040518
APA StyleAlves, F. S., Sousa, A. P., Almeida-Júnior, A., Lima, P. S. V., Silva, M. F. R., Galvão, J. L. F. M., Lima, E. O., Souza, H. D. S., Luis, J. A. S., Athayde-Filho, P. F., & Fiss, G. F. (2025). Antimicrobial Investigation of Phthalimide and N-Phthaloylglycine Esters: Activity, Mechanism of Action, Synergism and Ecotoxicity. Life, 15(4), 518. https://doi.org/10.3390/life15040518







