Quantifying Impairments in the Subacute Phase of Whiplash Associated Disorders—A Cross-Sectional Study
Abstract
1. Introduction
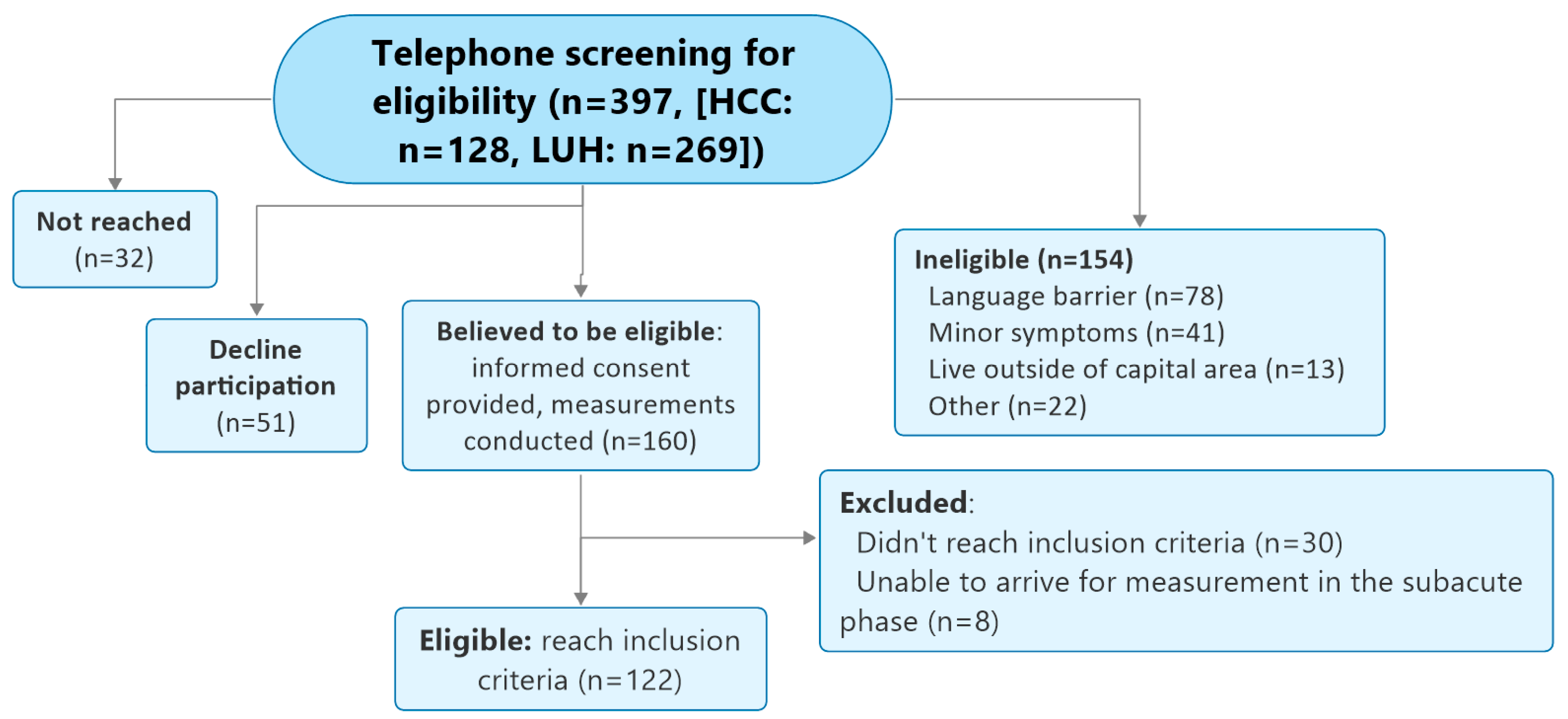
2. Materials and Methods
- The Butterfly Test (previously known as the Fly test) for cervical movement control is a reliable and valid test that has been proven to be fast and easy to use in a clinical setting [57] that can discriminate between healthy individuals and those with neck pain [19,40,58]. It consists of three different, unpredictable trajectories with increasing difficulty (easy, medium, and difficult, Figure 2) determined by the geometry of the movement tasks, the velocity of the target, and the length of the trajectories, as described by Oddsdottir et al. [58]; however, the trajectories used in this study were approximately three times smaller than in previous research, with range of motion 10° in each direction (diameter: 20°). Participants were instructed to track the trajectories by following a small red circle as accurately as possible, aiming at keeping the cursor within the red circle, and by using head and neck movement to manipulate an on-screen cursor. Metrics included (1) the Amplitude Accuracy (AA): the absolute average distance (radius) in arbitrary length units between the cursor that represents the head position and the target, where a lesser value represents a better score; and (2) the percentage of time the cursor spends: (a) in a mathematically determined, invisible free zone around the target (Time on Target, TOT); (b) ahead of the target (Overshoot, OS); or (c) behind the target (Undershoot, US). Each trajectory was repeated three times.
- The Whole Cervical Range of Motion Test for maximum active ROM in all 3 planes measured in degrees. Each movement (flexion–extension (sagittal), left/right rotations (transverse), and left/right lateral flexions (frontal)) was repeated three times, and the maximum value for each direction in each plane was used for analysis for maximum total movement in each plane.
- The Head Neck Relocation Test (HNRT), which assesses head–neck position sense, is derived from the cervical Joint Position Error Test (cJPT) [59]. The cJPT is a validated tool capable of distinguishing between healthy individuals and those with neck pain [60,61] as well as between healthy individuals and those with acute or subacute WADs [62]. The test was conducted both in the sagittal plane (flexion/extension) and the transverse plane (left/right rotation). Participants were blindfolded to eliminate visual input and asked to locate their perceived neutral head position. Once identified, they were instructed to move their head as far as comfortably possible in the specified direction and then return to what they believed was the original neutral position. Participants verbally indicated when they felt like they had returned to neutral by saying ‘ok’, at which point the examiner recorded the position with a spacebar click. If necessary, the examiner repositioned the participant to the original neutral position between the trials. The outcome measure was the absolute error, defined as the angular difference in degrees between the actual and perceived neutral positions.
3. Results
3.1. Between Group Differences
3.2. Specific Analyses of WAD Participants
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WAD | Whiplash-Associated Disorder |
| TA | Traffic accident |
| cROM | Cervical range of motion |
| CNS | Central nervous system |
| CS | Central sensitization |
| NDI | Neck Disability Index |
| LUH | Landspitali University Hospital |
| HCC | Health Care Center |
| VAS | Visual Analog Scale |
| DHI | Dizziness Handicap Inventory |
| CSI | Central Sensitization Inventory |
| HNRT | Head–Neck Relocation Test |
| JPE | Joint position error |
| cJPT | Cervical Joint Position Test |
| ANOVA | Analysis of variance |
| OS | Overshoot |
| US | Undershoot |
| TOT | Time on target |
| AA | Amplitude accuracy |
References
- Spitzer, W.O.; Skovron, M.L.; Salmi, L.R.; Cassidy, J.D.; Duranceau, J.; Suissa, S.; Zeiss, E. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: Redefining “whiplash” and its management. Spine 1995, 20, 1S–73S. [Google Scholar]
- Jull, G.; Kenardy, J.; Hendrikz, J.; Cohen, M.; Sterling, M. Management of acute whiplash: A randomized controlled trial of multidisciplinary stratified treatments. Pain 2013, 154, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, E.L.; Randhawa, K.; Torres, P.; Yu, H.; Verville, L.; Hartvigsen, J.; Côté, P.; Haldeman, S. The Global Spine Care Initiative: A systematic review of individual and community-based burden of spinal disorders in rural populations in low- and middle-income communities. Eur. Spine J. 2018, 27, 802–815. [Google Scholar] [CrossRef]
- Walton, D.M.; Macdermid, J.C.; Giorgianni, A.A.; Mascarenhas, J.C.; West, S.C.; Zammit, C.A. Risk factors for persistent problems following acute whiplash injury: Update of a systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2013, 43, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Daenen, L.; Nijs, J.; Raadsen, B.; Roussel, N.; Cras, P.; Dankaerts, W. Cervical motor dysfunction and its predictive value for long-term recovery in patients with acute whiplash-associated disorders: A systematic review. J. Rehabil. Med. 2013, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, A.; Vasseljen, O. Altered motor control patterns in whiplash and chronic neck pain. BMC Musculoskelet. Disord. 2008, 9, 90. [Google Scholar] [CrossRef]
- Stenneberg, M.; Rood, M.; Bie, R.; Schmitt, M.; Cattrysse, E.; Scholten-Peeters, G. To which degree differs active cervical range of motion between patients with neck pain, whiplash and healthy controls? A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2016, 98, 1407–1434. [Google Scholar] [CrossRef]
- Mazaheri, M.; Abichandani, D.; Kingma, I.; Treleaven, J.; Falla, D. A meta-analysis and systematic review of changes in joint position sense and static standing balance in patients with whiplash-associated disorder. PLoS ONE 2021, 16, e0249659. [Google Scholar] [CrossRef]
- Treleaven, J. Dizziness, Unsteadiness, Visual Disturbances, and Sensorimotor Control in Traumatic Neck Pain. J. Orthop. Sports Phys. Ther. 2017, 47, 492–502. [Google Scholar] [CrossRef]
- Gil, C.; Decq, P. How similar are whiplash and mild traumatic brain injury? A systematic review. Neurochirurgie 2021, 67, 238–243. [Google Scholar] [CrossRef]
- Elliott, J.M.; Smith, A.C.; Hoggarth, M.A.; Albin, S.R.; Weber, K.A., 2nd; Haager, M.; Fundaun, J.; Wasielewski, M.; Courtney, D.M.; Parrish, T.B. Muscle fat infiltration following whiplash: A computed tomography and magnetic resonance imaging comparison. PLoS ONE 2020, 15, e0234061. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Yang, L.; Li, Y.; Liu, T.; Liu, Y. Cervical Proprioception Impairment in Neck Pain-Pathophysiology, Clinical Evaluation, and Management: A Narrative Review. Pain Ther. 2021, 10, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Falla, D.; Jull, G.; Hodges, P.W. Feedforward activity of the cervical flexor muscles during voluntary arm movements is delayed in chronic neck pain. Exp. Brain Res. 2004, 157, 43–48. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, D.I. Kinesthetic sensibility. Physiol. Rev. 1978, 58, 763–820. [Google Scholar] [CrossRef]
- Stillman, B.C. Making Sense of Proprioception: The meaning of proprioception, kinaesthesia and related terms. Physiotherapy 2002, 88, 667–676. [Google Scholar] [CrossRef]
- Vasavada, A.N.; Danaraj, J.; Siegmund, G.P. Head and neck anthropometry, vertebral geometry and neck strength in height-matched men and women. J. Biomech. 2008, 41, 114–121. [Google Scholar] [CrossRef]
- Alalawi, A.; Luque-Suarez, A.; Fernandez-Sanchez, M.; Tejada-Villalba, R.; Navarro-Martin, R.; Devecchi, V.; Gallina, A.; Falla, D. Perceived pain and disability but not fear of movement are associated with altered cervical kinematics in people with acute neck pain following a whiplash injury. Musculoskelet. Sci. Pract. 2022, 62, 102633. [Google Scholar] [CrossRef]
- Kristjansson, E.; Hardardottir, L.; Asmundardottir, M.; Gudmundsson, K. A new clinical test for cervicocephalic kinesthetic sensibility: “the fly”. Arch. Phys. Med. Rehabil. 2004, 85, 490–495. [Google Scholar] [CrossRef]
- Kristjansson, E.; Björnsdottir, S.V.; Oddsdottir, G.L. The long-term course of deficient cervical kinaesthesia following a whiplash injury has a tendency to seek a physiological homeostasis. A prospective study. Man. Ther. 2016, 22, 196–201. [Google Scholar] [CrossRef]
- Ragnarsdottir, H.; Peterson, G.; Gislason, M.K.; Oddsdottir, G.L.; Peolsson, A. The effect of a neck-specific exercise program on cervical kinesthesia for patients with chronic whiplash-associated disorders: A case-control study. BMC Musculoskelet. Disord. 2024, 25, 346. [Google Scholar] [CrossRef]
- De Rosario, H.; Vivas, M.J.; Sinovas, M.I.; Page, Á. Relationship between neck motion and self-reported pain in patients with whiplash associated disorders during the acute phase. Musculoskelet. Sci. Pract. 2018, 38, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterwijck, J.; Nijs, J.; Meeus, M.; Paul, L. Evidence for central sensitization in chronic whiplash: A systematic literature review. Eur. J. Pain 2013, 17, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, I.; Ickmans, K.; Cagnie, B.; Nijs, J.; De Pauw, R.; Noten, S.; Meeus, M. Cognitive Performance Is Related to Central Sensitization and Health-related Quality of Life in Patients with Chronic Whiplash-Associated Disorders and Fibromyalgia. Pain Physician 2015, 18, E389–E401. [Google Scholar]
- Daenen, L.; Nijs, J.; Roussel, N.; Wouters, K.; Van Loo, M.; Cras, P. Dysfunctional pain inhibition in patients with chronic whiplash-associated disorders: An experimental study. Clin. Rheumatol. 2013, 32, 23–31. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Morlion, B.; Perrot, S.; Dahan, A.; Dickenson, A.; Kress, H.G.; Wells, C.; Bouhassira, D.; Mohr Drewes, A. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur. J. Pain 2018, 22, 216–241. [Google Scholar] [CrossRef]
- Sterling, M.; Jull, G.; Vicenzino, B.; Kenardy, J. Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain 2003, 104, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Sterling, M.; Hendrikz, J.; Kenardy, J.; Kristjansson, E.; Dumas, J.P.; Niere, K.; Cote, J.; deSerres, S.; Rivest, K.; Jull, G. Assessment and validation of prognostic models for poor functional recovery 12 months after whiplash injury: A multicentre inception cohort study. Pain 2012, 153, 1727–1734. [Google Scholar] [CrossRef]
- Sterling, M.; Hendrikz, J.; Kenardy, J. Similar factors predict disability and posttraumatic stress disorder trajectories after whiplash injury. Pain 2011, 152, 1272–1278. [Google Scholar] [CrossRef]
- Farrell, S.F.; Armfield, N.R.; Kristjansson, E.; Niere, K.; Christensen, S.W.M.; Sterling, M. Trajectories of cold but not mechanical sensitivity correspond with disability trajectories after whiplash injury. Pain 2024. [Google Scholar] [CrossRef]
- Kasch, H.; Qerama, E.; Bach, F.W.; Jensen, T.S. Reduced cold pressor pain tolerance in non-recovered whiplash patients: A 1-year prospective study. Eur. J. Pain 2005, 9, 561–569. [Google Scholar] [CrossRef]
- Nijs, J.; George, S.Z.; Clauw, D.J.; Fernández-de-Las-Peñas, C.; Kosek, E.; Ickmans, K.; Fernández-Carnero, J.; Polli, A.; Kapreli, E.; Huysmans, E.; et al. Central sensitisation in chronic pain conditions: Latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021, 3, e383–e392. [Google Scholar] [CrossRef]
- King, W. Acute Pain, Subacute Pain, and Chronic Pain. In Encyclopedia of Pain; Gebhart, G.F., Schmidt, R.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 60–63. [Google Scholar]
- Treleaven, J.; Jull, G.; Sterling, M. Dizziness and unsteadiness following whiplash injury: Characteristic features and relationship with cervical joint position error. J. Rehabil. Med. 2003, 35, 36–43. [Google Scholar] [CrossRef]
- Sremakaew, M.; Jull, G.; Treleaven, J.; Uthaikhup, S. Effectiveness of adding rehabilitation of cervical related sensorimotor control to manual therapy and exercise for neck pain: A randomized controlled trial. Musculoskelet. Sci. Pract. 2023, 63, 102690. [Google Scholar] [CrossRef] [PubMed]
- Sarig Bahat, H.; Takasaki, H.; Chen, X.; Bet-Or, Y.; Treleaven, J. Cervical kinematic training with and without interactive VR training for chronic neck pain—A randomized clinical trial. Man. Ther. 2015, 20, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Tejera, D.M.; Beltran-Alacreu, H.; Cano-de-la-Cuerda, R.; Leon Hernández, J.V.; Martín-Pintado-Zugasti, A.; Calvo-Lobo, C.; Gil-Martínez, A.; Fernández-Carnero, J. Effects of Virtual Reality versus Exercise on Pain, Functional, Somatosensory and Psychosocial Outcomes in Patients with Non-specific Chronic Neck Pain: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 5950. [Google Scholar] [CrossRef] [PubMed]
- Nusser, M.; Knapp, S.; Kramer, M.; Krischak, G. Effects of virtual reality-based neck-specific sensorimotor training in patients with chronic neck pain: A randomized controlled pilot trial. J. Rehabil. Med. 2021, 53, jrm00151. [Google Scholar] [CrossRef]
- Rezaei, I.; Razeghi, M.; Ebrahimi, S.; Kayedi, S.; Rezaeian Zadeh, A. A Novel Virtual Reality Technique (Cervigame®) Compared to Conventional Proprioceptive Training to Treat Neck Pain: A Randomized Controlled Trial. J. Biomed. Phys. Eng. 2019, 9, 355–366. [Google Scholar] [CrossRef]
- Grassini, S. Virtual Reality Assisted Non-Pharmacological Treatments in Chronic Pain Management: A Systematic Review and Quantitative Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4071. [Google Scholar] [CrossRef]
- Kristjansson, E.; Oddsdottir, G.L. “The Fly”: A new clinical assessment and treatment method for deficits of movement control in the cervical spine: Reliability and validity. Spine 2010, 35, E1298–E1305. [Google Scholar] [CrossRef]
- Sarig Bahat, H.; Croft, K.; Carter, C.; Hoddinott, A.; Sprecher, E.; Treleaven, J. Remote kinematic training for patients with chronic neck pain: A randomised controlled trial. Eur. Spine J. 2018, 27, 1309–1323. [Google Scholar] [CrossRef]
- de Vries, J.; Ischebeck, B.K.; Voogt, L.P.; van der Geest, J.N.; Janssen, M.; Frens, M.A.; Kleinrensink, G.J. Joint position sense error in people with neck pain: A systematic review. Man. Ther. 2015, 20, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Sarig Bahat, H.; Chen, X.; Reznik, D.; Kodesh, E.; Treleaven, J. Interactive cervical motion kinematics: Sensitivity, specificity and clinically significant values for identifying kinematic impairments in patients with chronic neck pain. Man. Ther. 2015, 20, 295–302. [Google Scholar] [CrossRef]
- Treleaven, J.; Dillon, M.; Fitzgerald, C.; Smith, C.; Wright, B.; Sarig-Bahat, H. Change in a clinical measure of cervical movement sense following four weeks of kinematic training. Musculoskelet. Sci. Pract. 2021, 51, 102312. [Google Scholar] [CrossRef]
- Ritchie, C.; Hendrikz, J.; Jull, G.; Elliott, J.; Sterling, M. External validation of a clinical prediction rule to predict full recovery and ongoing moderate/severe disability following acute whiplash injury. J. Orthop. Sports Phys. Ther. 2015, 45, 242–250. [Google Scholar] [CrossRef]
- Ritchie, C.; Hendrikz, J.; Kenardy, J.; Sterling, M. Derivation of a clinical prediction rule to identify both chronic moderate/severe disability and full recovery following whiplash injury. Pain 2013, 154, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsdóttir, H.; Briem, K.; Oddsdóttir, G.L. Effects of a Novel Web-Based Sensorimotor Exercise Program for Patients With Subacute Whiplash-Associated Disorders: Protocol for a Randomized Clinical Trial. Phys. Ther. 2023, 103, pzad063. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H. The Neck Disability Index: State-of-the-art, 1991–2008. J. Manip. Physiol. Ther. 2008, 31, 491–502. [Google Scholar] [CrossRef]
- Cleland, J.A.; Childs, J.D.; Whitman, J.M. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch. Phys. Med. Rehabil. 2008, 89, 69–74. [Google Scholar] [CrossRef]
- Hoving, J.L.; O’Leary, E.F.; Niere, K.R.; Green, S.; Buchbinder, R. Validity of the neck disability index, Northwick Park neck pain questionnaire, and problem elicitation technique for measuring disability associated with whiplash-associated disorders. Pain 2003, 102, 273–281. [Google Scholar] [CrossRef]
- Pietrobon, R.; Coeytaux, R.R.; Carey, T.S.; Richardson, W.J.; DeVellis, R.F. Standard scales for measurement of functional outcome for cervical pain or dysfunction: A systematic review. Spine 2002, 27, 515–522. [Google Scholar] [CrossRef]
- Pool, J.J.; Ostelo, R.W.; Hoving, J.L.; Bouter, L.M.; de Vet, H.C. Minimal clinically important change of the Neck Disability Index and the Numerical Rating Scale for patients with neck pain. Spine 2007, 32, 3047–3051. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.P.; Newman, C.W. The development of the Dizziness Handicap Inventory. Arch. Otolaryngol.—Head Neck Surg. 1990, 116, 424–427. [Google Scholar] [CrossRef]
- Mutlu, B.; Serbetcioglu, B. Discussion of the Dizziness Handicap Inventory. J. Vestib. Res. 2013, 23, 271–277. [Google Scholar] [CrossRef]
- Hendriks, E.; Voogt, L.; Lenoir, D.; Coppieters, I.; Ickmans, K. Convergent Validity of the Central Sensitization Inventory in Chronic Whiplash-Associated Disorders; Associations with Quantitative Sensory Testing, Pain Intensity, Fatigue, and Psychosocial Factors. Pain Med. 2020, 21, 3401–3412. [Google Scholar] [CrossRef] [PubMed]
- Neblett, R.; Hartzell, M.M.; Williams, M.; Bevers, K.R.; Mayer, T.G.; Gatchel, R.J. Use of the Central Sensitization Inventory (CSI) as a treatment outcome measure for patients with chronic spinal pain disorder in a functional restoration program. Spine J. 2017, 17, 1819–1829. [Google Scholar] [CrossRef]
- Kristjansson, E.; Gislason, M. The Butterfly Method for Cervical Movement Sense: Construct Validity and Clinical Applicability. Austin J. Musculoskelet. Disord. 2024, 1, 1063–1070. [Google Scholar]
- Oddsdottir, G.L.; Kristjansson, E.; Gislason, M.K. Database of movement control in the cervical spine. Reference normal of 182 asymptomatic persons. Man. Ther. 2013, 18, 206–210. [Google Scholar] [CrossRef]
- Feipel, V.; Salvia, P.; Klein, H.; Rooze, M. Head Repositioning Accuracy in Patients With Whiplash-Associated Disorders. Spine 2006, 31, E51–E58. [Google Scholar] [CrossRef]
- Revel, M.; Andre-Deshays, C.; Minguet, M. Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch. Phys. Med. Rehabil. 1991, 72, 288–291. [Google Scholar]
- de Zoete, R.M.J.; Osmotherly, P.G.; Rivett, D.A.; Farrell, S.F.; Snodgrass, S.J. Sensorimotor Control in Individuals With Idiopathic Neck Pain and Healthy Individuals: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 1257–1271. [Google Scholar] [CrossRef]
- Sterling, M.; Jull, G.; Vicenzino, B.; Kenardy, J.; Darnell, R. Development of motor system dysfunction following whiplash injury. Pain 2003, 103, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Pettorossi, V.E.; Schieppati, M. Neck Proprioception Shapes Body Orientation and Perception of Motion. Front. Hum. Neurosci. 2014, 8, 895. [Google Scholar] [CrossRef]
- Campbell, D.; Murphy, B.A.; Burkitt, J.; La Delfa, N.; Sanmugananthan, P.; Ambalavanar, U.; Yielder, P. Cervico-Ocular and Vestibulo-Ocular Reflexes in Subclinical Neck Pain and Healthy Individuals: A Cross-Sectional Study. Brain Sci. 2023, 13, 1603. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.; Nilsson, D.; Trygg, J.; Peolsson, A. Neck-specific exercise improves impaired interactions between ventral neck muscles in chronic whiplash: A randomized controlled ultrasound study. Sci. Rep. 2018, 8, 9649. [Google Scholar] [CrossRef]
- Kristjansson, E.; Gislason, M.K. Women with late whiplash syndrome have greatly reduced load-bearing of the cervical spine. In-vivo biomechanical, cross-sectional, lateral radiographic study. Eur. J. Phys. Rehabil. Med. 2018, 54, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.; Nilsson, D.; Trygg, J.; Falla, D.; Dedering, Å.; Wallman, T.; Peolsson, A. Novel insights into the interplay between ventral neck muscles in individuals with whiplash-associated disorders. Sci. Rep. 2015, 5, 15289. [Google Scholar] [CrossRef]
- Peolsson, A.; Peterson, G.; Trygg, J.; Nilsson, D. Multivariate analysis of ultrasound-recorded dorsal strain sequences: Investigation of dynamic neck extensions in women with chronic whiplash associated disorders. Sci. Rep. 2016, 6, 30415. [Google Scholar] [CrossRef]
- Karlsson, A.; Leinhard, O.D.; Åslund, U.; West, J.; Romu, T.; Smedby, Ö.; Zsigmond, P.; Peolsson, A. An Investigation of Fat Infiltration of the Multifidus Muscle in Patients With Severe Neck Symptoms Associated With Chronic Whiplash-Associated Disorder. J. Orthop. Sports Phys. Ther. 2016, 46, 886–893. [Google Scholar] [CrossRef]
- Oddsdottir, G.L.; Kristjansson, E. Two different courses of impaired cervical kinaesthesia following a whiplash injury. A one-year prospective study. Man. Ther. 2012, 17, 60–65. [Google Scholar] [CrossRef]
- Kantak, S.S.; Johnson, T.; Zarzycki, R. Linking Pain and Motor Control: Conceptualization of Movement Deficits in Patients With Painful Conditions. Phys. Ther. 2022, 102, pzab289. [Google Scholar] [CrossRef]
- Piva, S.R.; Erhard, R.E.; Childs, J.D.; Browder, D.A. Inter-tester reliability of passive intervertebral and active movements of the cervical spine. Man. Ther. 2006, 11, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Swinkels, R.A.; Swinkels-Meewisse, I.E. Normal values for cervical range of motion. Spine 2014, 39, 362–367. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.J.; Baldwin, J.N.; Ferreira, P.; Simic, M.; Vanicek, N.; Burns, J. Normative reference values for strength and flexibility of 1,000 children and adults. Neurology 2017, 88, 36–43. [Google Scholar] [CrossRef]
- Inoue, T.; Ito, K.; Ando, K.; Kobayashi, K.; Nakashima, H.; Katayama, Y.; Machino, M.; Kanbara, S.; Ito, S.; Yamaguchi, H.; et al. Age-related changes in upper and lower cervical alignment and range of motion: Normative data of 600 asymptomatic individuals. Eur. Spine J. 2020, 29, 2378–2383. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, Y.; Kato, F.; Suda, K.; Yamagata, M.; Ueta, T. Age-related changes in osseous anatomy, alignment, and range of motion of the cervical spine. Part I: Radiographic data from over 1,200 asymptomatic subjects. Eur. Spine J. 2012, 21, 1492–1498. [Google Scholar] [CrossRef]
- Pan, F.; Arshad, R.; Zander, T.; Reitmaier, S.; Schroll, A.; Schmidt, H. The effect of age and sex on the cervical range of motion—A systematic review and meta-analysis. J. Biomech. 2018, 75, 13–27. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.M.; Villaverde-Gutiérrez, C.; Mora-Sánchez, A.; Alonso-Blanco, C.; Sterling, M.; Fernández-de-Las-Peñas, C. Muscle trigger points, pressure pain threshold, and cervical range of motion in patients with high level of disability related to acute whiplash injury. J. Orthop. Sports Phys. Ther. 2012, 42, 634–641. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- De Pauw, R.; Coppieters, I.; Palmans, T.; Danneels, L.; Meeus, M.; Cagnie, B. Motor impairment in patients with chronic neck pain: Does the traumatic event play a significant role? A case-control study. Spine J. 2018, 18, 1406–1416. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Memminger, M.K.; Simeone, F.; Rath, B.; Huber, T. Clinical relevance of patient-reported outcome measures in patients who have undergone total hip arthroplasty: A systematic review. Arch. Orthop. Trauma Surg. 2024, 144, 4907–4916. [Google Scholar] [CrossRef]
- Gozzi, N.; Preatoni, G.; Ciotti, F.; Hubli, M.; Schweinhardt, P.; Curt, A.; Raspopovic, S. Unraveling the physiological and psychosocial signatures of pain by machine learning. Medicine 2024, 5, 1495–1509.e1495. [Google Scholar] [CrossRef] [PubMed]
- Peolsson, A.; Karlsson, A.; Peterson, G.; Borén, H.; Zsigmond, P.; Elliott, J.M.; Leinhard, O.D. Morphology and composition of the ventral neck muscles in individuals with chronic whiplash related disorders compared to matched healthy controls: A cross-sectional case-control study. BMC Musculoskelet. Disord. 2022, 23, 867. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.F.; Hassan, K.A.; Abdelmajeed, S.F.; Moustafa, I.M.; Silva, A.G. The Relationship Between Forward Head Posture and Neck Pain: A Systematic Review and Meta-Analysis. Curr. Rev. Musculoskelet. Med. 2019, 12, 562–577. [Google Scholar] [CrossRef] [PubMed]



| Mean (SD) | ||||
|---|---|---|---|---|
| WAD Group (n = 122) | Healthy Controls (n = 45) | |||
| Butterfly | Easy | AA | 2.8 (1.3) | 1.6 (0.3) * |
| TOT | 53.6 (17.0) | 72.9 (6.0) * | ||
| OS | 13.4 (13.2) | 7.0 (2.6) * | ||
| US | 33.1 (7.5) | 20.1 (5.0) * | ||
| Medium | AA | 5.0 (2.2) | 2.9 (0.5) * | |
| TOT | 23.3(12.5) | 39.2 (9.8) * | ||
| OS | 23.9 (9.2) | 17.5 (5.7) * | ||
| US | 52.8 (10.1) | 43.3 (7.6) * | ||
| Difficult | AA | 7.3 (2.9) | 4.5 (0.8) * | |
| TOT | 12.0 (7.0) | 20.6 (6.5) * | ||
| OS | 23.7 (7.5) | 20.9 (6.7) * | ||
| US | 64.3 (7.8) | 58.4 (7.7) * | ||
| Head Neck Relocation | Flexion | 2.8 (1.9) | 2.7 (1.8) | |
| Extension | 3.1 (2.0) | 2.9 (1.9) * | ||
| Left rot | 2.8 (2.0) | 2.0 (1.3) * | ||
| Right rot | 3.1 (1.9) | 3.1 (1.6) | ||
| Mean (SD) | ||
|---|---|---|
| WAD Group (n = 122) | Healthy Controls (n = 45) | |
| Flexion | 45.2 (13.7) | 60.1 (8.9) * |
| Extension | 50.9 (16.8) | 65.5 (11.9) * |
| Left rot | 58.0 (14.2) | 73.4 (8.4) * |
| Right rot | 57.3 (14.7) | 74.0 (7.3) * |
| Left lat flex | 31.7 (8.8) | 39.2 (8.2) * |
| Right lat flex | 31.7 (9.3) | 32.8 (8.2) * |
| NDI | 42.3 (19.3) | 3.4 (3.7) |
| VAS | 6.8 (2.1) | NA |
| CSI | 49.7 (16.8) | NA |
| DHI | 35.2 (25.3) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragnarsdóttir, H.; Oddsdóttir, G.L.; Gíslason, M.K.; Briem, K. Quantifying Impairments in the Subacute Phase of Whiplash Associated Disorders—A Cross-Sectional Study. Life 2025, 15, 562. https://doi.org/10.3390/life15040562
Ragnarsdóttir H, Oddsdóttir GL, Gíslason MK, Briem K. Quantifying Impairments in the Subacute Phase of Whiplash Associated Disorders—A Cross-Sectional Study. Life. 2025; 15(4):562. https://doi.org/10.3390/life15040562
Chicago/Turabian StyleRagnarsdóttir, Harpa, Guðný Lilja Oddsdóttir, Magnús Kjartan Gíslason, and Kristín Briem. 2025. "Quantifying Impairments in the Subacute Phase of Whiplash Associated Disorders—A Cross-Sectional Study" Life 15, no. 4: 562. https://doi.org/10.3390/life15040562
APA StyleRagnarsdóttir, H., Oddsdóttir, G. L., Gíslason, M. K., & Briem, K. (2025). Quantifying Impairments in the Subacute Phase of Whiplash Associated Disorders—A Cross-Sectional Study. Life, 15(4), 562. https://doi.org/10.3390/life15040562






