Risk of Permanent Corneal Injury in Microgravity: Spaceflight-Associated Hazards, Challenges to Vision Restoration, and Role of Biotechnology in Long-Term Planetary Missions
Abstract
1. Introduction
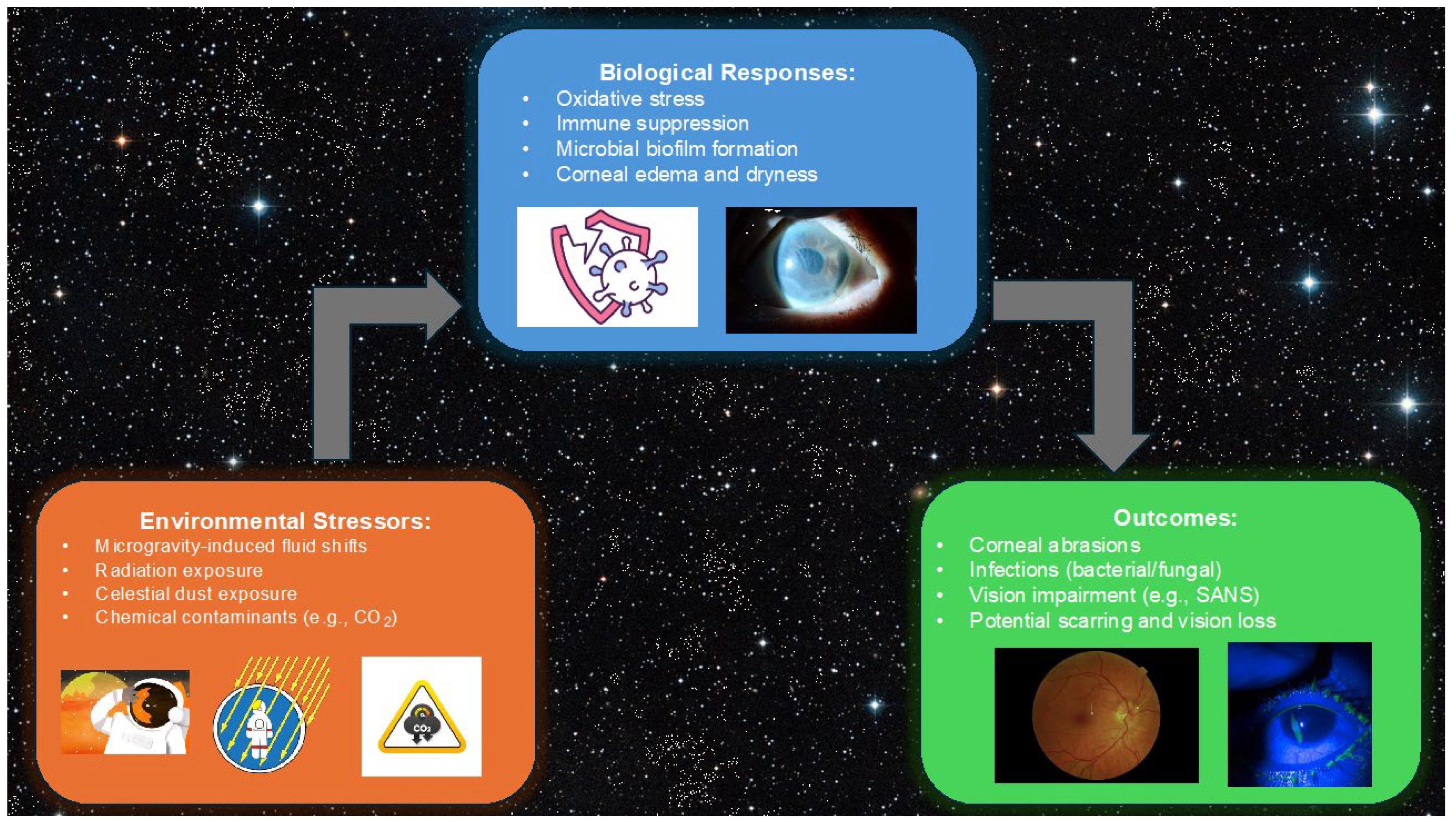
2. Spaceflight Environment and Ocular Risks
2.1. Microgravity and Its Impact on Ocular Health
2.2. Corneal Bullae Observations in Mice
2.3. Ocular Surface and Tear Film Changes
2.4. Infections and Immune Suppression
2.5. Radiation Exposure and Corneal Damage
3. Traumatic Corneal Injuries
3.1. Injury Mechanisms in Microgravity
3.2. Celestial Dust Hazards
3.3. Spaceflight Associated Neuro-Ocular Syndrome
3.4. Corneal Stromal Scarring Risk Assessment
4. Current Management Strategies for Corneal Scarring
4.1. Standard Terrestrial Treatments
4.2. Limitations in Space
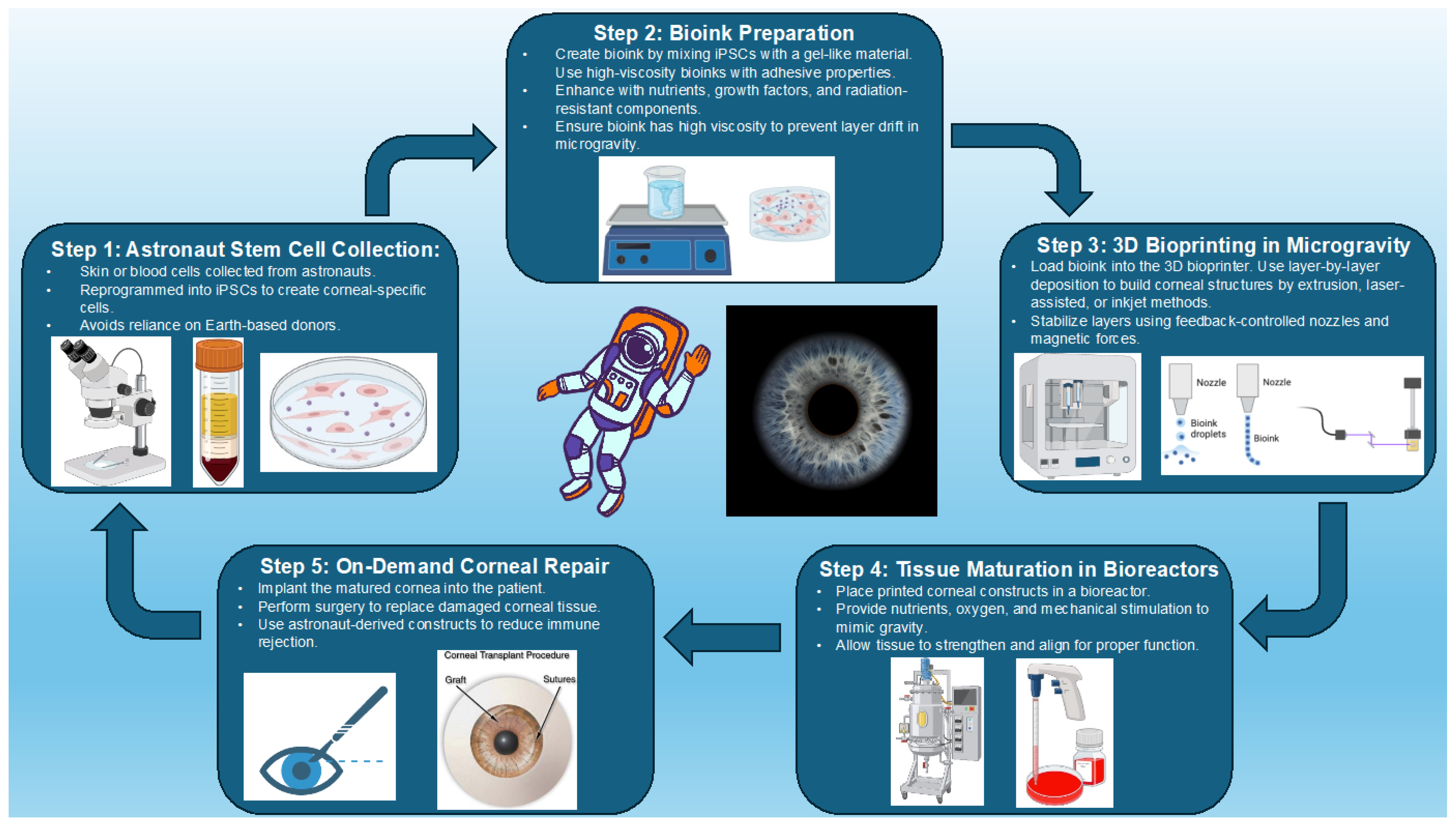
5. Three-Dimensional Bioprinting of the Cornea
5.1. Advances in 3D Bioprinting Technology
5.2. Stem Cell Integration and Tissue Engineering
5.3. Addressing Space-Specific Challenges
5.4. Future Research Directions
6. Strategic Planning and Risk Mitigation
6.1. Protective Eyewear
6.2. Artificial Tear Solutions
7. Telemedicine and AI Support Systems
7.1. Remote Medical Assistance
7.2. AI-Driven Decision Support
7.3. Contingency Plans for Planetary Missions
7.4. Astronaut Training Programs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Caddy, H.T.; Kelsey, L.J.; Parker, L.P.; Green, D.J.; Doyle, B.J. Modelling large scale artery haemodynamics from the heart to the eye in response to simulated microgravity. Npj Microgravity 2024, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Lingam, M.; Balbi, A. From Stars to Life: A Quantitative Approach to Astrobiology, 1st ed.; Cambridge University Press: Cambridge, UK, 2024. [Google Scholar] [CrossRef]
- Corydon, T.J.; Schulz, H.; Richter, P.; Strauch, S.M.; Böhmer, M.; Ricciardi, D.A.; Wehland, M.; Krüger, M.; Erzinger, G.S.; Lebert, M.; et al. Current Knowledge about the Impact of Microgravity on Gene Regulation. Cells 2023, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- NASA Astrobiology Institute. Available online: https://astrobiology.nasa.gov/nai/about/index.html (accessed on 29 March 2025).
- The Astrobiological Perspective on Life’s Origin | National Center for Science Education. Available online: https://ncse.ngo/astrobiological-perspective-lifes-origin (accessed on 29 March 2025).
- The Human Body in Space-NASA. 2 February 2021. Available online: https://www.nasa.gov/humans-in-space/the-human-body-in-space/ (accessed on 29 March 2025).
- Patel, Z.S.; Brunstetter, T.J.; Tarver, W.J.; Whitmire, A.M.; Zwart, S.R.; Smith, S.M.; Huff, J.L. Red risks for a journey to the red planet: The highest priority human health risks for a mission to Mars. Npj Microgravity 2020, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Suh, A.; Ong, J.; Gibson, C.R.; Mader, T.; Berdahl, J.; Waisberg, E.; Lee, A.G. The evaluation and management of corneal penetrating and perforating injuries during long-duration spaceflight. Eye 2024, 38, 1793–1795. [Google Scholar] [CrossRef]
- Hughson, R.L.; Irving, E.L. Spaceflight not an eye-popping experience for astronauts. J. Physiol. 2021, 599, 1011–1012. [Google Scholar] [CrossRef]
- Ong, J.; Tarver, W.; Brunstetter, T.; Mader, T.H.; Gibson, C.R.; Mason, S.S.; Lee, A. Spaceflight associated neuro-ocular syndrome: Proposed pathogenesis, terrestrial analogues, and emerging countermeasures. Br. J. Ophthalmol. 2023, 107, 895–900. [Google Scholar] [CrossRef]
- Rooney, B.V.; Crucian, B.E.; Pierson, D.L.; Laudenslager, M.L.; Mehta, S.K. Herpes Virus Reactivation in Astronauts During Spaceflight and Its Application on Earth. Front. Microbiol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Sampige, R.; Ong, J.; Waisberg, E.; Berdahl, J.; Lee, A.G. The Ocular Surface–Gut Axis in Spaceflight: Implications of Intestinal Changes in Microgravity on Tear Film Physiology. J. Clin. Transl. Ophthalmol. 2024, 2, 79–86. [Google Scholar] [CrossRef]
- Armitage, W.J. Preservation of Human Cornea. Transfus. Med. Hemother. 2011, 38, 143–147. [Google Scholar] [CrossRef]
- Feilmeier, M.; Tabin, G.; Williams, L.; Oliva, M. The use of glycerol-preserved corneas in the developing world. Middle East Afr. J. Ophthalmol. 2010, 17, 38. [Google Scholar] [CrossRef]
- Balters, L.; Reichl, S. 3D bioprinting of corneal models: A review of the current state and future outlook. J. Tissue Eng. 2023, 14, 20417314231197793. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, P.; Kini, A.; Al Othman, B.; Galdamez, L.A.; Lee, A.G. Spaceflight associated neuro-ocular syndrome. Curr. Opin. Neurol. 2020, 33, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Cornea Edema-An Overview | ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/cornea-edema (accessed on 18 March 2025).
- Bullous Keratopathy-An Overview | ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/bullous-keratopathy (accessed on 18 March 2025).
- Ax, T.; Ganse, B.; Fries, F.N.; Szentmáry, N.; De Paiva, C.S.; March De Ribot, F.; Jensen, S.O.; Seitz, B.; Millar, T.J. Dry eye disease in astronauts: A narrative review. Front. Physiol. 2023, 14, 1281327. [Google Scholar] [CrossRef]
- Zanello, S.B.; Theriot, C.A.; Ponce, C.M.P.; Chevez-Barrios, P. Spaceflight Effects and Molecular Responses in the Mouse Eye: Preliminary Observations After Shuttle Mission STS-133. Gravitational. Space Res. 2013, 1, 29–46. [Google Scholar] [CrossRef]
- Meer, E.; Grob, S.; Antonsen, E.L.; Sawyer, A. Ocular conditions and injuries, detection and management in spaceflight. Npj Microgravity 2023, 9, 37. [Google Scholar] [CrossRef]
- Tarver, W.; Brunstetter, T. Visual Impairment Intracranial Pressure (VIIP) [aka Microgravity Ocular Syndrome (MOS)]. Presented at: February 1, 2017. Available online: https://ntrs.nasa.gov/citations/20170001329 (accessed on 3 April 2025).
- Kilic Bektas, C.; Hasirci, V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater. Sci. 2020, 8, 438–449. [Google Scholar] [CrossRef]
- Noroozi, R.; Arif, Z.U.; Taghvaei, H.; Khalid, M.Y.; Sahbafar, H.; Hadi, A.; Sadeghianmaryan, A.; Chen, X. 3D and 4D Bioprinting Technologies: A Game Changer for the Biomedical Sector? Ann. Biomed. Eng. 2023, 51, 1683–1712. [Google Scholar] [CrossRef]
- Jia, S.; Bu, Y.; Lau, D.-S.A.; Lin, Z.; Sun, T.; Lu, W.W.; Lu, S.; Ruan, C.; Chan, C.-H.J. Advances in 3D bioprinting technology for functional corneal reconstruction and regeneration. Front. Bioeng. Biotechnol. 2023, 10, 1065460. [Google Scholar] [CrossRef]
- Yang, W.-J.; Yang, Y.-N.; Cao, J.; Man, Z.-H.; Yuan, J.; Xiao, X.; Xing, Y.-Q. Risk Factors for Dry Eye Syndrome: A Retrospective Case-Control Study. Optom. Vis. Sci. 2015, 9, e199–e205. [Google Scholar]
- Sujithra, H.; Shah, K.N.; Anoop, R.; Ullattil, P.K.; Pillai, G.S.; Ravindran, G.C.; Vazirani, J. Ocular surface changes in patients who have undergone head and neck radiation therapy. Indian J. Ophthalmol. 2024, 72, S669–S675. [Google Scholar] [CrossRef] [PubMed]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Brinza, D.E.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Mars’ Surface Radiation Environment Measured with the Mars Science Laboratory’s Curiosity Rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Djotyan, G.P.; Joshi, R.; Juhasz, T.; Brown, D.J.; Jester, J.V. Modeling meibum secretion: Alternatives for obstructive Meibomian Gland Dysfunction (MGD). Ocul. Surf. 2024, 31, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, C.A.; Ott, C.M.; Wilson, J.W.; Ramamurthy, R.; Pierson, D.L. Microbial Responses to Microgravity and Other Low-Shear Environments. Microbiol. Mol. Biol. Rev. 2004, 68, 345–361. [Google Scholar] [CrossRef]
- Meer, E.; Grob, S.R.; Lehnhardt, K.; Sawyer, A. Ocular complaints and diagnoses in spaceflight. Npj Microgravity 2024, 10, 1. [Google Scholar] [CrossRef]
- Lee, A.G.; Mader, T.H.; Gibson, C.R.; Tarver, W.; Rabiei, P.; Riascos, R.F.; Galdamez, L.A.; Brunstetter, T. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: A review and an update. Npj Microgravity 2020, 6, 7. [Google Scholar] [CrossRef]
- Soares, B.; Ong, J.; Waisberg, E.; Sarker, P.; Zaman, N.; Tavakkoli, A.; Lee, A.G. Imaging in spaceflight associated neuro-ocular syndrome (SANS): Current technology and future directions in modalities. Life Sci. Space Res. 2024, 42, 40–46. [Google Scholar] [CrossRef]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Feiveson, A.H.; Sams, C.F.; Pierson, D.L. Latent virus reactivation in astronauts on the international space station. Npj Microgravity 2017, 3, 11. [Google Scholar] [CrossRef]
- Ong, J.; Soares, B.; Osteicoechea, D.; Kadipasaoglu, C.M.; Waisberg, E.; Suh, A.; Sampige, R.; Nguyen, T.; Masalkhi, M.; Sarker, P.; et al. The cornea during spaceflight: A frontier in space medicine ophthalmology. Eye 2024, 38, 3207–3209. [Google Scholar] [CrossRef]
- Landry, K.S.; Morey, J.M.; Bharat, B.; Haney, N.M.; Panesar, S.S. Biofilms—Impacts on Human Health and Its Relevance to Space Travel. Microorganisms 2020, 8, 998. [Google Scholar] [CrossRef]
- Tesei, D.; Jewczynko, A.; Lynch, A.M.; Urbaniak, C. Understanding the Complexities and Changes of the Astronaut Microbiome for Successful Long-Duration Space Missions. Life 2022, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, I.; De Goüyon Matignon de Pontourade, C.M.F.; Lincke, J.-B.; Keller, I.; Zinkernagel, M.S.; Zysset-Burri, D.C. The Human Ocular Surface Microbiome and Its Associations with the Tear Proteome in Dry Eye Disease. Int. J. Mol. Sci. 2023, 24, 14091. [Google Scholar] [CrossRef] [PubMed]
- Treatment and Prevention of Herpes Simplex Virus Type 1 in Immunocompetent Adolescents and Adults-UpToDate. Available online: https://www.uptodate.com/contents/treatment-and-prevention-of-herpes-simplex-virus-type-1-in-immunocompetent-adolescents-and-adults?utm_source=chatgpt.com (accessed on 18 March 2025).
- Herpes Simplex Virus: Adult and Adolescent OIs | NIH. 26 May 2020. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/herpes-simplex (accessed on 18 March 2025).
- Delic, N.C.; Lyons, J.G.; Di Girolamo, N.; Halliday, G.M. Damaging Effects of Ultraviolet Radiation on the Cornea. Photochem. Photobiol. 2017, 93, 920–929. [Google Scholar] [CrossRef]
- Mao, X.W.; Boerma, M.; Rodriguez, D.; Campbell-Beachler, M.; Jones, T.; Stanbouly, S.; Sridharan, V.; Wroe, A.; Nelson, G.A. Acute effect of low-dose space radiation on mouse retina and retinal endothelial cells. Radiat. Res. 2018, 190, 45–52. [Google Scholar] [CrossRef]
- Özelbaykal, B.; Öğretmenoğlu, G.; Gedik, Ş. The Effects of Space Radiation and Microgravity on Ocular Structures. Turk. J. Ophthalmol. 2022, 52, 57–63. [Google Scholar] [CrossRef]
- Asaithamby, A.; Chen, D.J. Mechanism of cluster DNA damage repair in response to high-atomic number and energy particles radiation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2011, 711, 87–99. [Google Scholar] [CrossRef]
- Waisberg, E.; Ong, J.; Masalkhi, M.; Shimada, K.; Lee, A.G. Artificial gravity as a potential countermeasure for Spaceflight Associated Neuro-Ocular Syndrome. Eye 2024, 38, 2847–2848. [Google Scholar] [CrossRef]
- Pohlen, M.; Carroll, D.; Prisk, G.K.; Sawyer, A.J. Overview of lunar dust toxicity risk. Npj Microgravity 2022, 8, 55. [Google Scholar] [CrossRef]
- James, J.T.; Kahn-Mayberry, N. Risk of Adverse Health Effects from Lunar Dust Exposure. In Human Health and Performance Risks of Space Exploration Missions; NASA: Washington, DC, USA, 2009; pp. 317–330. [Google Scholar]
- Meyers, V.E.; Garcìa, H.D.; Monds, K.; Cooper, B.L.; James, J.T. Ocular toxicity of authentic lunar dust. BMC Ophthalmol. 2012, 12, 26. [Google Scholar]
- Mader, T.H.; Gibson, C.R.; Pass, A.F.; Kramer, L.A.; Lee, A.G.; Fogarty, J.; Tarver, W.J.; Dervay, J.P.; Hamilton, D.R.; Sargsyan, A.; et al. Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight. Ophthalmology 2011, 118, 2058–2069. [Google Scholar] [CrossRef]
- Hull, S.M.; Lindsay, C.D.; Brunel, L.G.; Shiwarski, D.J.; Tashman, J.W.; Roth, J.G.; Myung, D.; Feinberg, A.W.; Heilshorn, S.C. 3D Bioprinting using UNIversal Orthogonal Network (UNION) Bioinks. Adv. Funct. Mater. 2021, 31, 2007983. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef]
- Xie, Z.; Yuan, B.; Chi, M.; Hong, J. Focus on seed cells: Stem cells in 3D bioprinting of corneal grafts. Front. Bioeng. Biotechnol. 2024, 12, 1423864. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharashi, S.A.; Al-Obailan, M.M.; Almohaimeed, M.; Al-Torbak, A.A. Deep anterior lamellar Keratoplasty. Saudi J. Ophthalmol 2009, 23, 203–209. [Google Scholar] [CrossRef]
- Chandran, C.; Santra, M.; Rubin, E.; Geary, M.L.; Yam, G.H.-F. Regenerative Therapy for Corneal Scarring Disorders. Biomedicines 2024, 12, 649. [Google Scholar] [CrossRef]
- Altay, Y.; Tamer, S.; Kaya, A.S.; Balta, O.; Burcu, A.; Ornek, F. The outcome of penetrating keratoplasty for corneal scarring due to herpes simplex keratitis. Arq. Bras. Oftalmol. 2017, 80, 41–45. [Google Scholar] [CrossRef]
- Yang, G.N.; Roberts, P.K.; Gardner-Russell, J.; Shah, M.H.; Couper, T.A.; Zhu, Z.; Pollock, G.A.; Dusting, G.J.; Daniell, M. From bench to clinic: Emerging therapies for corneal scarring. Pharmacol. Ther. 2023, 242, 108349. [Google Scholar] [CrossRef]
- Petsoglou, C.; Weinel, L. Biomaterials and their impact on corneal transplantation and eye banking. Clin. Exper. Ophthalmol. 2023, 51, 7–8. [Google Scholar] [CrossRef]
- Stuart, A.J.; Romano, V.; Virgili, G.; Shortt, A.J. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst. Rev. 2018, 2018, CD012097. [Google Scholar] [CrossRef]
- Ghosh, A.; Singh, V.K.; Singh, V.; Basu, S.; Pati, F. Recent Advancements in Molecular Therapeutics for Corneal Scar Treatment. Cells 2022, 11, 3310. [Google Scholar] [CrossRef]
- Vanathi, M. Changing inclinations of eye banking. Indian J. Ophthalmol. 2023, 71, 3121–3122. [Google Scholar] [CrossRef] [PubMed]
- Chameettachal, S.; Venuganti, A.; Parekh, Y.; Prasad, D.; Joshi, V.P.; Vashishtha, A.; Basu, S.; Singh, V.; Bokara, K.K.; Pati, F. Human cornea-derived extracellular matrix hydrogel for prevention of post-traumatic corneal scarring: A translational approach. Acta Biomater. 2023, 171, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Van Ombergen, A.; Chalupa-Gantner, F.; Chansoria, P.; Colosimo, B.M.; Costantini, M.; Domingos, M.; Dufour, A.; De Maria, C.; Groll, J.; Jungst, T.; et al. 3D Bioprinting in Microgravity: Opportunities, Challenges, and Possible Applications in Space. Adv. Healthc. Mater. 2023, 12, 2300443. [Google Scholar] [CrossRef]
- Gingras, A.A.; Jansen, P.A.; Smith, C.; Zhang, X.; Niu, Y.; Zhao, Y.; Roberts, C.J.; Herderick, E.D.; Swindle-Reilly, K.E. 3D Bioprinting of Acellular Corneal Stromal Scaffolds with a Low Cost Modified 3D Printer: A Feasibility Study. Curr. Eye Res. 2023, 48, 1112–1121. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, Q.; Li, J.; Ma, L.; Yao, Y.; Ye, H.; Cui, Z.; Yang, H. 3D bioprinting for artificial cornea: Challenges and perspectives. Med. Eng. Phys. 2019, 71, 68–78. [Google Scholar] [CrossRef]
- Jamieson, C.; Keenan, P.; Kirkwood, D.; Oji, S.; Webster, C.; Russell, K.A.; Koch, T.G. A Review of Recent Advances in 3D Bioprinting With an Eye on Future Regenerative Therapies in Veterinary Medicine. Front. Vet. Sci. 2021, 7, 584193. [Google Scholar] [CrossRef]
- Al-Atawi, S. Three-dimensional bioprinting in ophthalmic care. Int. J. Ophthalmol. 2023, 16, 1702–1711. [Google Scholar] [CrossRef]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Kumar, H.; Mashayekhan, S.; Baradaran-Rafii, A.; Kim, K. Stereolithography 3D Bioprinting Method for Fabrication of Human Corneal Stroma Equivalent. Ann. Biomed. Eng. 2020, 48, 1955–1970. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, Y.; Liu, T.; Xu, R.; Mao, S.; Mo, X.; Zhang, T.; Ouyang, L.; Xiong, Z.; Sun, W. Advances in 3D Bioprinting. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100011. [Google Scholar] [CrossRef]
- Mirshafiei, M.; Rashedi, H.; Yazdian, F.; Rahdar, A.; Baino, F. Advancements in tissue and organ 3D bioprinting: Current techniques, applications, and future perspectives. Mater. Des. 2024, 240, 112853. [Google Scholar] [CrossRef]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, A.L. Corneal stem cells and tissue engineering: Current advances and future perspectives. World J. Stem Cells 2015, 7, 806. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 2018, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, Z.; Bagheri, M.; Rostaminasab, G.; Mikaeili, A.; Djalilian, A.R.; Rezakhani, L. Tissue engineering strategies for ocular regeneration; from bench to the bedside. Heliyon 2024, 10, e39398. [Google Scholar] [CrossRef]
- Utine, C.A.; Güven, S. Tissue Engineering and Ophthalmology. Turk. J. Ophthalmol. 2024, 54, 159–169. [Google Scholar] [CrossRef]
- Montesinos, C.A.; Khalid, R.; Cristea, O.; Greenberger, J.S.; Epperly, M.W.; Lemon, J.A.; Boreham, D.R.; Popov, D.; Gorthi, G.; Ramkumar, N.; et al. Space Radiation Protection Countermeasures in Microgravity and Planetary Exploration. Life 2021, 11, 829. [Google Scholar] [CrossRef]
- Williams, D.R.; Bashshur, R.L.; Pool, S.L.; Doarn, C.R.; Merrell, R.C.; Logan, J.S. A Strategic Vision for Telemedicine and Medical Informatics in Space Flight. Telemed. J. E-Health 2000, 6, 441–448. [Google Scholar] [CrossRef]
- Nanodropper’s Eyedrop Adaptor Is Heading to Space. Eyes on Eyecare. Available online: https://glance.eyesoneyecare.com/stories/2024-02-27/nanodropper-s-eyedrop-adaptor-is-heading-to-space/ (accessed on 17 March 2025).
- Rippy, L.O. NASA Human Systems Integration Handbook. Published online 1 November 2021. Available online: https://ntrs.nasa.gov/citations/20210010952 (accessed on 17 March 2025).
- Semp, D.A.; Beeson, D.; Sheppard, A.L.; Dutta, D.; Wolffsohn, J.S. Artificial Tears: A Systematic Review. OPTO 2023, 15, 9–27. [Google Scholar] [CrossRef]
- Wei, Z.; Su, Y.; Su, G.; Baudouin, C.; Labbé, A.; Liang, Q. Effect of artificial tears on dynamic optical quality in patients with dry eye disease. BMC Ophthalmol. 2022, 22, 64. [Google Scholar] [CrossRef]
- Suh, A.; Ong, J.; Waisberg, E.; Lee, A.G. Corneal thermal burn injuries during long-duration spaceflight: Mechanisms, evaluation, and management. Eye 2024, 38, 2488–2490. [Google Scholar] [CrossRef]
- Meshkin, R.S.; Armstrong, G.W.; Hall, N.E.; Rossin, E.J.; Hymowitz, M.B.; Lorch, A.C. Effectiveness of a telemedicine program for triage and diagnosis of emergent ophthalmic conditions. Eye 2023, 37, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Thirsk, R.B. Health care for deep space explorers. Ann. ICRP 2020, 49 (Suppl. S1), 182–184. [Google Scholar] [CrossRef] [PubMed]
- Asrar, F.M.; Saint-Jacques, D.; Williams, D. Outer space assets offer benefits to health care: Family doctors play a key role in supporting innovative work. Can. Fam. Physician 2022, 68, 797–798, 800. [Google Scholar] [CrossRef]
- Doarn, C.R.; Nicogossian, A.E.; Merrell, R.C. Applications of Telemedicine in the United States Space Program. Telemed. J. 1998, 4, 19–30. [Google Scholar] [CrossRef]
- Urlings, C.; Baselet, B.; Tabury, K.; Baatout, S. Virtual medical astronaut avatars for future deep space missions. Front. Space Technol. 2024, 5, 1423138. [Google Scholar] [CrossRef]
- Ganapathy, K. Space Medicine: The Ultimate in Remote Healthcare. Telehealth Med. Today 2020, 5, 1. [Google Scholar] [CrossRef]
- Haney, N.M.; Urman, A.; Waseem, T.; Cagle, Y.; Morey, J.M. AI’s role in deep space. J. Med. Artif. Intell. 2020, 3, 11. [Google Scholar] [CrossRef]
- Greatbatch, C. The Role of Artificial Intelligence in Space Medicine. J. Australas. Soc. Aerosp. Med. 2024, 13, 1–5. [Google Scholar] [CrossRef]
- Mackintosh, G. AI Applications for Astronaut Health. 2020. Available online: https://www.nasa.gov/wp-content/uploads/2019/10/space_portal_graham_mackintosh.pdf (accessed on 3 April 2025).
- Waisberg, E.; Ong, J.; Paladugu, P.; Kamran, S.A.; Zaman, N.; Lee, A.G.; Tavakkoli, A. Challenges of Artificial Intelligence in Space Medicine. Space Sci. Technol. 2022, 2022, 9852872. [Google Scholar] [CrossRef]
- Da Silva, R.G.L. The advancement of artificial intelligence in biomedical research and health innovation: Challenges and opportunities in emerging economies. Glob. Health 2024, 20, 44. [Google Scholar] [CrossRef]
- Shimizu, E.; Ishikawa, T.; Tanji, M.; Agata, N.; Nakayama, S.; Nakahara, Y.; Yokoiwa, R.; Sato, S.; Hanyuda, A.; Ogawa, Y.; et al. Artificial intelligence to estimate the tear film breakup time and diagnose dry eye disease. Sci. Rep. 2023, 13, 5822. [Google Scholar] [CrossRef]
- Choo, S.; Yoo, S.; Endo, K.; Truong, B.; Son, M.H. Advancing Clinical Chatbot Validation Using AI-Powered Evaluation With a New 3-Bot Evaluation System: Instrument Validation Study. JMIR Nurs. 2025, 8, e63058. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Han, B.; Ma, Z.-C. Femtosecond Laser-Assisted Ophthalmic Surgery: From Laser Fundamentals to Clinical Applications. Micromachines 2022, 13, 1653. [Google Scholar] [CrossRef]
- Yule, S.; Robertson, J.M.; Mormann, B.; Smink, D.S.; Lipsitz, S.; Abahuje, E.; Kennedy-Metz, L.; Park, S.; Miccile, C.; Pozner, C.N.; et al. Crew Autonomy During Simulated Medical Event Management on Long Duration Space Exploration Missions. Hum. Factors 2023, 65, 1221–1234. [Google Scholar] [CrossRef]
- Minard, C.G.; Carvalho, M.F.D.; Iyengar, M.S. Optimizing Medical Resources for Spaceflight Using the Integrated Medical Model. Aviat. Space Environ. Med. 2011, 82, 890–894. [Google Scholar] [CrossRef]
- Keller, N.; Whittle, R.S.; McHenry, N.; Johnston, A.; Duncan, C.; Ploutz-Snyder, L.; Torre, G.G.D.L.; Sheffield-Moore, M.; Chamitoff, G.; Diaz-Artiles, A. Virtual Reality “exergames”: A promising countermeasure to improve motivation and restorative effects during long duration spaceflight missions. Front. Physiol. 2022, 13, 932425. [Google Scholar] [CrossRef]
- Houston, S.C. How NASA Uses Virtual Reality to Train Astronauts. Space Center Houston. 25 September 2018. Available online: https://spacecenter.org/how-nasa-uses-virtual-reality-to-train-astronauts/ (accessed on 17 March 2025).
- Kamran, S.A.; Hossain, K.F.; Ong, J.; Zaman, N.; Waisberg, E.; Paladugu, P.; Lee, A.G.; Tavakkoli, A. SANS-CNN: An automated machine learning technique for spaceflight associated neuro-ocular syndrome with astronaut imaging data. Npj Microgravity 2024, 10, 40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, J.; Ong, J.; Lee, R.; Suh, A.; Waisberg, E.; Gibson, C.R.; Berdahl, J.; Mader, T.H. Risk of Permanent Corneal Injury in Microgravity: Spaceflight-Associated Hazards, Challenges to Vision Restoration, and Role of Biotechnology in Long-Term Planetary Missions. Life 2025, 15, 602. https://doi.org/10.3390/life15040602
Shah J, Ong J, Lee R, Suh A, Waisberg E, Gibson CR, Berdahl J, Mader TH. Risk of Permanent Corneal Injury in Microgravity: Spaceflight-Associated Hazards, Challenges to Vision Restoration, and Role of Biotechnology in Long-Term Planetary Missions. Life. 2025; 15(4):602. https://doi.org/10.3390/life15040602
Chicago/Turabian StyleShah, Jainam, Joshua Ong, Ryung Lee, Alex Suh, Ethan Waisberg, C. Robert Gibson, John Berdahl, and Thomas H. Mader. 2025. "Risk of Permanent Corneal Injury in Microgravity: Spaceflight-Associated Hazards, Challenges to Vision Restoration, and Role of Biotechnology in Long-Term Planetary Missions" Life 15, no. 4: 602. https://doi.org/10.3390/life15040602
APA StyleShah, J., Ong, J., Lee, R., Suh, A., Waisberg, E., Gibson, C. R., Berdahl, J., & Mader, T. H. (2025). Risk of Permanent Corneal Injury in Microgravity: Spaceflight-Associated Hazards, Challenges to Vision Restoration, and Role of Biotechnology in Long-Term Planetary Missions. Life, 15(4), 602. https://doi.org/10.3390/life15040602







