Abstract
Background: Varicella zoster virus (VZV) infection can be life-threatening for fragile and immunosuppressed patients. Recombinant VZ vaccination (RVZV) has been recommended for vulnerable patients to reduce the risk of reactivation. Hemodialysis (HD) patients often have weakened immune systems and a high prevalence of comorbidities, which may justify the use of RVZV. This study examines the difference in VZ antibody levels following RVZV and its significance in HD patients. Methods: We measured the levels of immunoglobulin G antibodies against VZ (VZ-IgG) in the HD population. We also collected demographic and clinical data for each patient, including their age, length of time on dialysis, Charlson Comorbidity Index (CCI), and markers of nutritional and inflammatory status. Results: A total of 160 patients were evaluated, with 111 (69.4%) male and 143 (89.3%) Caucasian. The mean VZ-IgG levels after one year were significantly higher in patients who received RVZV than those who did not (2177 ± 834 versus 1494 ± 882, p < 0.001). Additionally, among all other risk factors, only CCI harmed the VZ-IgG levels in non-vaccinated HD patients (B −403 with 95%CI −778 −27.9, p = 0.039). Overall, 98.8% of patients were found to be seropositive for VZ, with only one patient in each group (RVZV and non-RVZV) testing negative. Conclusions: Patients who received RVZV showed higher VZ IgG levels after one year compared to those who did not. Moreover, unvaccinated patients with more comorbidities had lower anti-VZ IgG titers.
1. Introduction
Shingles is caused by the reactivation of latent VZV following a primary infection with VZV, and it presents as a unilateral, vesicular rash in a single dermatome accompanied by pain, which can be severe and persistent. The rate of shingles in the general population increases with age; the incidence is estimated to be between 6 and 8/1000 person-years over 60 and between 8 and 12/1000 person-years over 80 years of age [1]. It occurs due to a decline in VZ-specific immunity. A typical long-term issue is post-herpetic neuralgia [2,3], which is intense burning pain in one area that lasts for at least three months [4]. This pain is often resistant to treatment, affecting patients’ quality of life [5].
Hemodialysis (HD) patients have chronic low-grade inflammation, which compromises the immune response [6,7,8,9], significantly increasing their vulnerability to infections and the reactivation of dormant viruses [7]. Previous studies indicate that these patients face a twofold risk of viral reactivation compared to the general population [10,11] and a heightened likelihood of encountering severe complications associated with such events. Additionally, these individuals have higher resistance to antiviral treatment, necessitating longer treatment durations [12]. Furthermore, they are at a greater risk for neurotoxicity related to antiviral treatment [13]. Given these peculiarities, implementing a prophylactic strategy to prevent the reactivation of the varicella zoster virus (VZ) in this at-risk population seems a prudent action.
Since 2017 in the United States and 2018 in Europe, a recombinant vaccine for VZ (RVZV) has been available for adult patients over 50 and for those over 18 with immunological impairment to reduce the risk of VZ reactivation. According to its FDA and EMA approval [14,15], RVZV can prevent shingles [16,17] and post-herpetic neuralgia for at least four years and can reduce the rate of serious complications such as viral dissemination, stroke, encephalitis, and visual impairment without the risks of a live attenuated vaccine. The indications for fragile patients were determined in two studies on hematological patients [15]. One study showed a 68% reduction in VZ reactivation in people who had received an autologous stem cell transplant, and the other research observed an 87% reduction in patients with blood cancer. Afterward, other trials reported the potential usefulness in other immunological impairment conditions, such as in human immunodeficiency virus infection [18], in autologous stem cell transplantation [19], in kidney transplantation [20], in patients with solid tumors before or during chemotherapy [21], and in patients with immune-mediated diseases [22]. Conversely, only one recent report has described surrogate biomarkers for the efficacy of RVZV with one year of follow-up in hemodialysis patients [23]. No other study has explored the actual role of RVZV in shingle prevention in HD patients. Additionally, no studies have examined the baseline conditions and fragility to assess the risk of shingles among HD patients.
Both humoral and cell-mediated immunity are involved in primary VZ infection and vaccination [24], but, while cell-mediated immunity is predominant in VZ reactivation [25,26], the role of humoral immunity is minor. Specifically, the only study published about the immune response to VZ in dialysis patients suggested that cellular and humoral immunity showed no impairment and seemed similar to that of matched healthy controls [27], revealing that the underlying mechanism is not entirely understood and needs further investigation. Conversely, some studies have shown a significant correlation between the VZ antibody titer and cell-mediated immunity tests [28,29,30,31,32] and a significant VZ antibody titer increase after vaccination [33,34,35]. Cell-mediated immunity assessment requires the analysis of qualitative/quantitative lymphocyte subpopulations and antigen-specific T-cells, a methodology that is currently not always feasible in all clinical contexts. In contrast, the VZ antibody titer is a widely used measure in evaluating VZ contact, as it is more straightforward, quicker, and cheaper to apply. In this clinical context, evaluating VZ IgG titers seems a reasonable strategy to understand the immune status versus VZ reactivation [36].
The VZ vaccination campaign for patients undergoing HD impacts public and private health system expenditures, which could represent a barrier to wide vaccine diffusion. For example, the RVZV vaccine costs approximately EUR 180 per person for a single dose in Italy. Given the estimated 45,000 dialysis patients in the country [37], the total financial burden related to this vaccination initiative would amount to around EUR 16 million. Despite the cost-effectiveness of VZ vaccination, which seems to favor its widespread diffusion, especially in vulnerable patients, definitive conclusions about the real benefit of RVZV are still lacking [38]. Further investigations into the role of the patient’s age, the vaccine’s price and efficacy, and the incidence of shingles complications are necessary. Considering the lack of cost-effectiveness evidence in the HD population, the uncertainties surrounding the vaccine’s efficacy for post-herpetic neuralgia [17], and the different rates of major complications between patients and treatment modalities [39], we aim to share our clinical experiences with RVZV vaccination to enhance the current understanding of its effects on HD patients. This retrospective report seeks to thoroughly discuss antibody titers against varicella zoster virus (VZ) as a potential indicator of the risk of developing shingles in patients undergoing hemodialysis (HD). Additionally, the study explores various clinical conditions that may influence the VZ-IgG levels in these patients to discover higher-risk patients. By identifying these factors, we aim to enhance the understanding of the shingles risk in HD patients and suggest the prioritization of RVZV administration among individuals on renal replacement therapy.
2. Materials and Methods
We conducted a retrospective study at Padua University Hospital in Italy to assess the antibody titer in HD patients and the impact of the clinical basal conditions on its value. The study was approved by the local ethics committee and conducted according to the principles expressed in the Declaration of Helsinki.
All HD patients >19 years old were considered eligible for the study.
For each patient, we evaluated the following features.
- -
- Demographic characteristics such as age, gender, dialytic vintage, and history of shingles in the year before the blood examination were documented, According to the reports in clinical notes.
- -
- Previous RVZV administration. Specifically, vaccinated patients received a two-dose series from August 2022 to January 2023, with a period between the first and second dose of about two months.
- -
- The Charlson Comorbidity Index (CCI) was recorded to assess the patient’s fragility [40]. Specifically, the CCI adjusted by the patient’s age (+1 in patients between 50 and 59 years old, +2 in patients between 60 and 69 years old, +3 in patients between 70 and 79 years old, +4 in patients over 80 years old) was determined, evaluating the following conditions: diabetes, congestive heart failure, peripheral vascular disease, chronic pulmonary disease, liver disease, hemiplegia, renal disease, hematological or metastatic cancer, and acquired immunodeficiency syndrome.
- -
- Blood examinations related to the patient’s well-being, such as hemoglobin, albumin, C-reactive protein (CRP), white cell count, platelet count, and urea, were evaluated. Anti-VZ IgG was detected. A serum sample was collected from each participant and tested by chemiluminescence using LIAISON® VZV IgG, a semi-quantitative method performed with a standardized commercial kit (Diasorin Italia S.p.A, Saluggia, Italy). According to the manufacturer’s instructions, an anti-VZ IgG titer > 150 mIU/mL was scored positively.
We collected all blood samples during the regular monthly HD controls. Specifically, in RVZV patients, the test for VZ titers was performed one year after vaccination, while, in non-RVZV patients, we collected the sample in the first two months of 2024.
Sample size: As a retrospective study, we determined the sample size as 131 cases by applying the following equation for sample size calculation [41]:
where n = desired number of samples, Z1−β = desired power (1.28 for 90% power), Z1−α/2 = standardized value for the corresponding confidence level (at a 99% CI or 1% type I error, it is 2.58), d = margin of error or rate of precision, and σ = standard deviation estimated according to the results published by Hielscher F on the hemodialysis population [18]. Furthermore, considering the size of our cohort, we decided to enroll all hemodialysis patients who consented to participate in the study to avoid selection bias.
n = [(r + 1)/r] × [σ2 × (Z1−β + Z 1−α/2)]/d2
Statistical analysis: Continuous variables were reported as the mean ± standard deviation (SD) or median with interquartile range (IQR) according to their distribution, while categorical variables were reported as numbers or percentages. The Shapiro–Wilk test evaluated the normality of distribution for continuous variables. A paired t-test and the Mann–Whitney U-test were used to compare the levels of anti-VZ IgG between the groups according to the variable distribution.
Univariate and multivariate linear regression were performed to identify the predictors of the anti-VZ IgG titer. All continuous variables that were non-normally distributed were normalized by natural log transformation. All reported p-values were two-sided, and statistical significance was set at p < 0.05. Statistical analysis was performed with IBM SPSS Statistics (Version 28.0).
3. Results
3.1. HD Cohort Description
A total of 160 HD patients were enrolled, of which 111 (69.3%) were male. Among them, 72 patients (45.3%) received two subsequent doses of RVZV within two months. In our patient cohort, the median age was 67.5 years, with a median Charlson Comorbidity Index (CCI) of 8. Notably, the mean anti-VZ IgG level was 1799 mIU/mL. The characteristics of the HD cohort are detailed in Table 1.

Table 1.
Characteristics of HD patients.
3.2. Anti-VZ IgG Titer
We did not find any significant differences in the anti-VZ IgG levels among the explored variables based on gender, race, or the presence of diabetes and hypertension. In contrast, patients who received RVZV twelve months prior to blood sampling exhibited higher levels of anti-VZ IgG compared to non-vaccinated patients, with means of 2177 (±834) and 1494 (±882), respectively. Table 2 indicates that the differences in the anti-VZ IgG levels correlate with the categorical risk factors.

Table 2.
Anti-VZ IgG titers according to basal conditions.
Additionally, the CCI showed a significant negative correlation with the anti-VZ IgG titer in non-vaccinated patients, while it exhibited no significant correlation in vaccinated patients. No significant correlation was found between anti-VZ IgG and the other continuous variables. Specifically, Table 3 presents the relationship between humoral immunity and the risk associated with the continuous variable factors.

Table 3.
Pearson’s rho indices between continuous variables and anti-VZ titer.
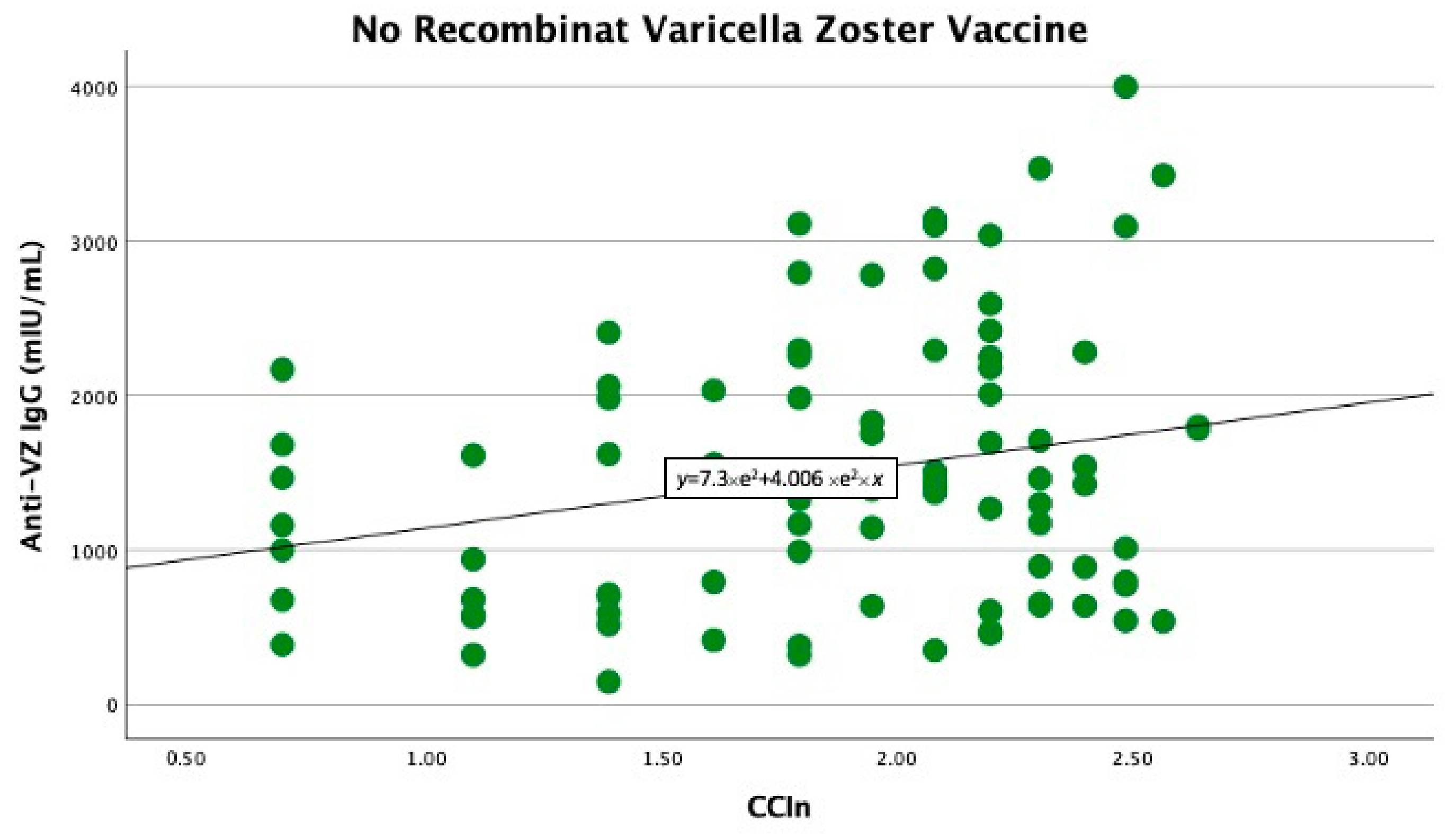
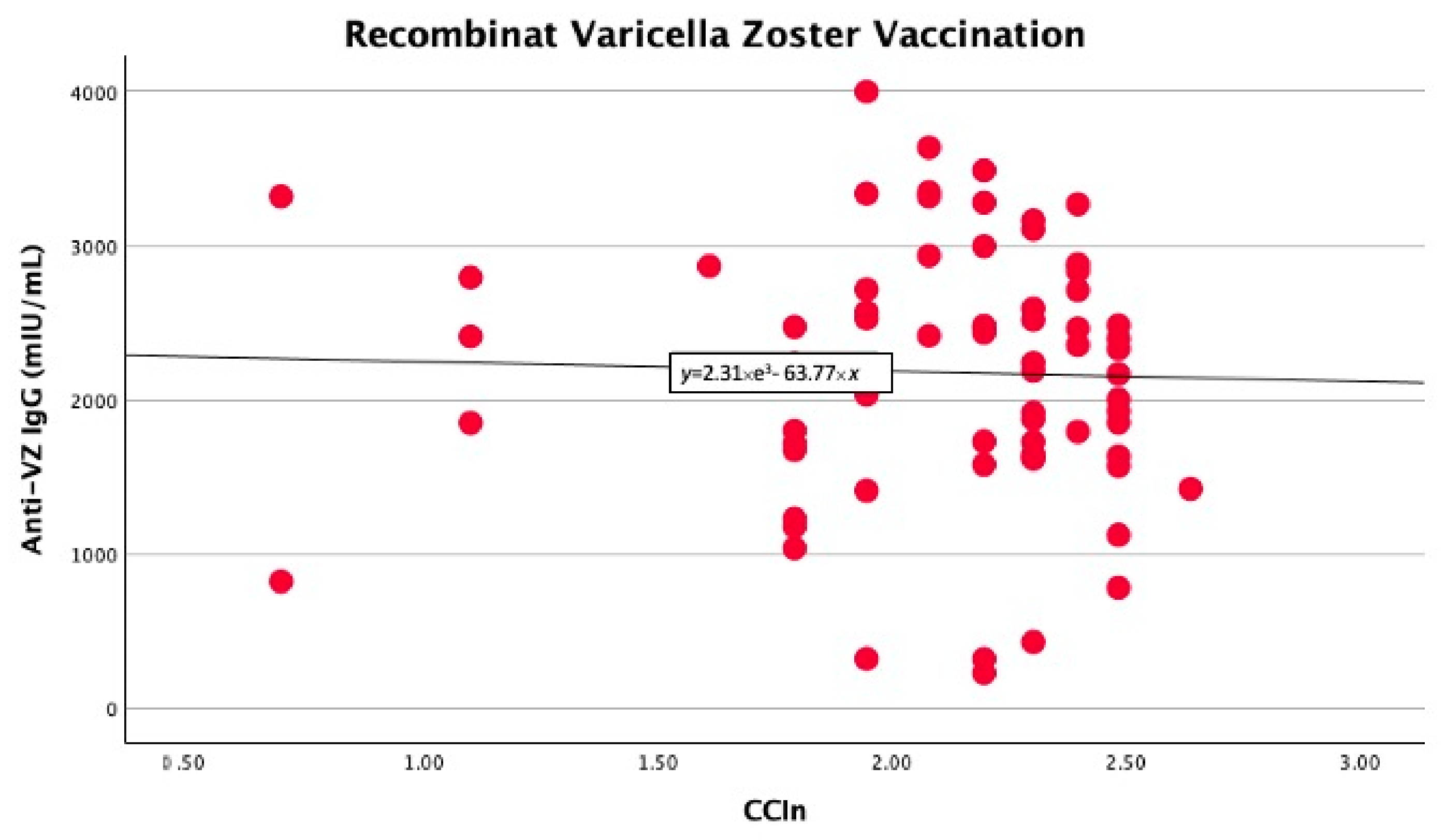
In the univariate linear regression analysis, we found that the CCIn predicted significantly higher anti-VZ IgG titers only in non-vaccinated patients (B −403 with 95% CI −778 −27.9, p = 0.039). In contrast, in vaccinated patients, the comorbidity index did not significantly predict anti-VZ IgG titers (B −74.7 with 95% CI −571.9 and 422.4, p = 0.76), as shown in Figure 1 and Figure 2.
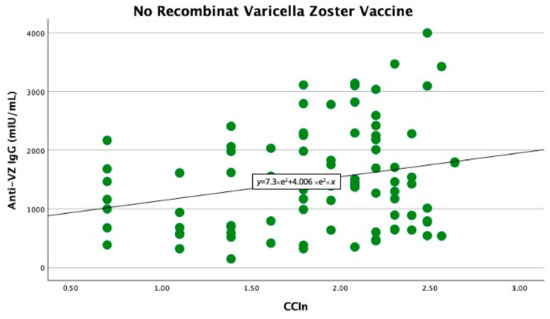
Figure 1.
Significant linear relationship between anti-VZ IgG titer and CCIn in non-vaccinated patients.
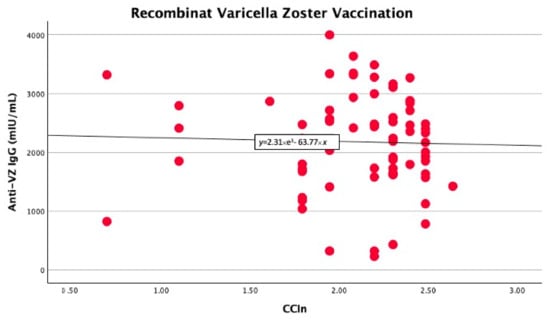
Figure 2.
No significant linear correlation between anti-VZ IgG titer and CCIn in vaccinated patients.
Finally, in our cohort, only two patients (1.2%) had anti-VZ IgG levels below 150 mIU/mL; of these, one had received two doses of RVZV, while the other had no vaccination. Notably, among the HD patients who had received two previous doses of RVZV, one patient experienced a shingles episode in the year following vaccination.
4. Discussion
Our retrospective study revealed a remarkably high prevalence of HD patients with anti-VZ IgG titers exceeding 150 mUI/mL. Specifically, only two patients were VZ seronegative, one of whom had received RVZV in the year prior. Furthermore, we showed that patients who were vaccinated with RVZV in the previous year had significantly higher anti-VZ IgG titers compared to those who were not vaccinated.
Finally, our report demonstrated a significant negative correlation between lower anti-VZ IgG titers and a higher comorbidity index in unvaccinated patients. Meanwhile, no other conditions significantly impacted the anti-VZ IgG levels.
4.1. Prevalence of Seropositive VZV
The large number of HD patients testing positive for VZ antibodies, along with the patients’ ages, indicates that chickenpox was widespread in Italy during the twentieth century. The Italian population born before 1998 did not receive the chickenpox vaccine in childhood, according to the Italian National Immunization Plan prior to 2003. Therefore, the presence of anti-VZ antibodies in non-vaccinated individuals suggests a history of infection. After a long period following the primary infection, the well-preserved anti-VZ IgG titer is likely related to a more robust immunity response to VZ infection than chickenpox vaccination [33]. Notably, one non-vaccinated patient and one RVZV HD patient had undetectable anti-VZ IgG titers. The non-vaccinated patient may have never had a primary VZ infection, or their titer could have declined to undetectable levels. Conversely, the vaccinated patient demonstrated an inadequate response to RVZV, as did the patient who experienced shingles after two doses of RVZV. These findings in HD patients highlight the need to prioritize VZ vaccination for seronegative patients and those with lower antibody titers based on their childhood chickenpox history.
4.2. VZ Anti-IgG Titer After RVZV
The higher levels of anti-VZ IgG titers in vaccinated patients underscore an immune response at one year after RVZV. Our findings are aligned with this and support the findings of previous studies, which showed a significant increase after vaccination [42,43,44] due to the effect of the immune response to the vaccination. Specifically, our report is consistent with a study by Hielscher F. et al. [23], which found a 2.2-fold increase in VZV-specific IgG levels and a significant correlation with neutralizing activity over one year.
It is challenging to assess the effectiveness of RVZV in HD patients without studies focused on this specific population. Currently, no reports exist about the rate of VZ reactivation in HD patients who have received RVZV; the only reports on RVZV have evaluated the safety of the vaccination and its side effects [45], as well as humoral and cell-mediated immunity [23] during the first year after vaccination. However, given the aging of HD patients and their comorbidities, along with the lack of significant differences in the humoral and cellular immune responses to VZ [27] compared to the general population, we can speculate that HD patients may experience vaccine efficacy comparable to the lower end of the confidence interval for individuals over 70 years old. Therefore, HD patients might have vaccine efficacy of around 85% in preventing shingles, based on the findings of Cunningham et al., where the vaccine efficacy ranged from 84.2% to 93.7% in older patients [46]. Simultaneously, HD patients could have vaccine efficacy of about 70% for post-herpetic neuralgia, which was estimated to be between 68.7% and 97.1% in older adults [46]. Additional reports on the incidence of shingles and neuralgia following RVZV are necessary to determine the actual benefits of vaccination in dialysis patients, particularly in light of the questionable advantages of RVZV concerning post-herpetic neuralgia even within the general population [17]. Consequently, we plan to extend our study’s observation period to four years, emphasizing the ongoing nature of our research and the necessity for further investigation in this area.
Given the uncertain evidence regarding vaccination’s benefits in all hemodialysis patients, assessing antibody titers prior to vaccination may be an appropriate procedure in clinical practice. This initial step could help to identify higher-risk patients, which is a significant aspect of personalized medicine. Due to their unique circumstances, dialysis patients present challenges in utilizing biomarkers to determine pathophysiological processes or prognosis [47,48,49,50,51]. This peculiarity arises not only from end-stage kidney disease but also from the potential effects of the hemodialysis procedure on biomarker levels [52,53,54,55,56]. In this context, the results of anti-VZ IgG assessment over time in dialysis patients could enhance our understanding of the kinetics of VZ antibodies, regardless of their immune system characteristics [6,7,8,57], thereby further emphasizing the importance of personalized medicine. Future studies are required to support these intriguing hypotheses.
4.3. Comorbidity Score and VZ Anti-IgG Titer
Interestingly, our series revealed a negative correlation between the CCI score and VZ anti-IgG titer in non-vaccinated patients, whereas vaccinated patients showed no significant correlation. Our findings align with other studies, indicating a decline in anti-VZ antibody titers among frail and older patients [58,59] and a heightened risk of VZ reactivation in high-comorbidity HD patients. The influence of comorbidities on the risk of shingles is well documented; other reports identify a high CCI as a risk factor for the development of shingles in the general population [60,61] and in specific groups, such as stroke patients [62]. Meanwhile, the lack of a significant correlation between VZ anti-IgG titers and the CCI in vaccinated patients suggests a robust humoral immune response to RVZV, highlighting its effectiveness even among frail patients. Based on these findings, stratifying the shingles risk by the CCI seems to be a reasonable strategy for the RVZV campaign in HD patients to prioritize vaccine administration.
Our study has some limitations. The first are those associated with the retrospective study design and analysis. Secondly, the number of patients could limit the reliability of our analysis, reducing the actual effects of other covariates. Nevertheless, we enrolled all our HD patients and calculated the sample size according to the only previous report on hemodialysis patients. Thirdly, we did not consider the possible differences in antibody titers between the dialysis prescriptions. Still, the retrospective nature of our report and the high prevalence of bicarbonate dialysis in our cohort limited the reliability of this type of sub-analysis.
5. Conclusions
Our study cohort showed a high prevalence of seropositivity for VZV among vaccinated and unvaccinated patients. Vaccinated patients exhibited significantly higher levels of anti-VZ IgG than unvaccinated patients, suggesting an effective immune response to vaccination regardless of the patient’s overall health status. Conversely, among unvaccinated patients, those with more comorbidities had lower anti-VZ IgG titers. This finding suggests that greater patient fragility may increase the risk of VZ reactivation. Consequently, a higher CCI and lower anti-VZ IgG titer could help the nephrologist to assess the RVZV priority in HD patients.
Author Contributions
Conceptualization, F.K.M.; methodology, F.K.M.; software, M.C. and E.B.; validation, L.A.C. and F.N.; formal analysis, F.K.M.; investigation, all authors; resources, all authors; data curation, M.C. and E.B.; writing—original draft preparation, F.K.M.; writing—review and editing, F.K.M.; visualization, all authors; supervision, L.A.C. and F.N.; project administration, F.K.M.; funding acquisition, F.N. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the grant DOR n. 2307847/2023 from the University of Padova, awarded to F.N.
Institutional Review Board Statement
This study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of Padua University Hospital (CET code: 453n/AO/24, date of approval: 22 February 2024).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VZV | Varicella Zoster Virus |
| RVZV | Recombinant Varicella Zoster Vaccination |
| HD | Hemodialysis |
| VZ-IgG | Immunoglobulin G Antibodies against Varicella Zoster |
| CCI | Charlson Comorbidity Index |
| SD | Standard Deviation |
| IQR | Interquartile Range |
References
- Kawai, K.; Gebremeskel, B.G.; Acosta, C.J. Systematic review of incidence and complications of herpes zoster: Towards a global perspective. BMJ Open 2014, 10, e004833. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Goldust, M.; Wollina, U. Herpes zoster: A Review of Clinical Manifestations and Management. Viruses 2022, 14, 192. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.Z.J.; Tey, H.L.; Salada, B.M.A.; Oon, J.E.L.; Seah, E.D.; Chandran, N.S.; Pan, J.Y. Herpes Zoster and Post-Herpetic Neuralgia-Diagnosis, Treatment, and Vaccination Strategies. Pathogens 2024, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Adriaansen, E.J.M.; Jacobs, J.G.; Vernooij, L.M.; van Wijck, A.J.M.; Cohen, S.P.; Huygen, F.J.P.M.; Rijsdijk, M. 8. Herpes zoster and post herpetic neuralgia. Pain Pract. 2024, 25, e13423. [Google Scholar] [CrossRef]
- Gobbi, L.; Martino, F.K.; Sgrò, E.; Nalesso, F.; Calo’, L.A. Varicella Zoster vaccination in hemodialysis patients: The state of the art. Hum. Vaccines Immunother. 2023, 19, 2286689. [Google Scholar] [CrossRef]
- Caprara, C.; Kinsey, G.R.; Corradi, V.; Xin, W.; Ma, J.Z.; Scalzotto, E.; Martino, F.K.; Okusa, M.D.; Nalesso, F.; Ferrari, F.; et al. The Influence of Hemodialysis on T Regulatory Cells: A Meta-Analysis and Systematic Review. Blood Purif. 2016, 42, 307–313. [Google Scholar] [CrossRef]
- Sharif, M.R.; Chitsazian, Z.; Moosavian, M.; Raygan, F.; Nikoueinejad, H.; Sharif, A.R.; Einollahi, B. Immune disorders in hemodialysis patients. Iran. J. Kidney Dis. 2015, 9, 84–96. [Google Scholar]
- Hauser, A.B.; Stinghen, A.E.; Kato, S.; Bucharles, S.; Aita, C.; Yuzawa, Y.; Pecoits-Filho, R. Characteristics and causes of immune dysfunction related to uremia and dialysis. Perit. Dial. Int. 2008, 28 (Suppl. S3), S183–S187. [Google Scholar] [CrossRef]
- Babel, N.; Hugo, C.; Westhoff, T.H. Vaccination in patients with kidney failure: Lessons from COVID-19. Nat. Rev. Nephrol. 2022, 18, 708–723. [Google Scholar] [CrossRef]
- Kuo, C.C.; Lee, C.T.; Lee, I.M.; Ho, S.C.; Yang, C.Y. Risk of herpes zoster in patients treated with long-term hemodialysis: A matched cohort study. Am. J. Kidney Dis. 2012, 59, 428–433. [Google Scholar] [CrossRef]
- Wu, M.Y.; Hsu, Y.H.; Su, C.L.; Lin, Y.F.; Lin, H.W. Risk of herpes zoster in CKD: A matched-cohort study based on administrative data. Am. J. Kidney Dis. 2012, 60, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Mesar, I.; Basić-Jukić, N.; Hudolin, T.; Katalinić, L.; Kes, P. Varicella zoster virus reactivation in hemodialysis patients: Manifestations, treatment, complications and outcome. Acta Clin. Croat. 2011, 50, 549–552. [Google Scholar] [PubMed]
- Wang, Y.C.; Juan, S.H.; Li, C.H.; Chou, C.L.; Chen, L.Y.; Chien, L.N.; Fang, T.C. Valacyclovir-associated neurotoxicity among patients on hemodialysis and peritoneal dialysis: A nationwide population-based study. Front. Med. 2022, 9, 997379. [Google Scholar] [CrossRef]
- US Food & Drugs Administration. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/shingrix (accessed on 31 August 2024).
- European Medicine Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/shingrix (accessed on 31 August 2024).
- Amirthalingam, G.; Andrews, N.; Keel, P.; Mullett, D.; Correa, A.; de Lusignan, S.; Ramsay, M. Evaluation of the effect of the herpes zoster vaccination programme 3 years after its introduction in England: A population-based study. Lancet Public Health 2018, 3, e82–e90. [Google Scholar] [CrossRef]
- Chen, N.; Li, Q.; Zhang, Y.; Zhou, M.; Zhou, D.; He, L. Vaccination for preventing postherpetic neuralgia. Cochrane Database Syst. Rev. 2011, CD007795. [Google Scholar] [CrossRef]
- Berkowitz, E.M.; Moyle, G.; Stellbrink, H.J.; Schürmann, D.; Kegg, S.; Stoll, M.; El Idrissi, M.; Oostvogels, L.; Heineman, T.C.; Zoster-015 HZ/su Study Group. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: A phase 1/2a randomized, placebo-controlled study. J. Infect. Dis. 2015, 211, 1279–1287. [Google Scholar] [CrossRef]
- Bastidas, A.; de la Serna, J.; El Idrissi, M.; Oostvogels, L.; Quittet, P.; López-Jiménez, J.; Vural, F.; Pohlreich, D.; Zuckerman, T.; Issa, N.C.; et al. Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster After Autologous Stem Cell Transplantation: A Randomized Clinical Trial. JAMA 2019, 322, 123–133. [Google Scholar] [CrossRef]
- Vink, P.; Ramon Torrell, J.M.; Sanchez Fructuoso, A.; Kim, S.J.; Kim, S.I.; Zaltzman, J.; Ortiz, F.; Campistol Plana, J.M.; Fernandez Rodriguez, A.M.; Rebollo Rodrigo, H.; et al. Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Chronically Immunosuppressed Adults Following Renal Transplant: A Phase 3, Randomized Clinical Trial. Clin. Infect. Dis. 2020, 70, 181–190. [Google Scholar] [CrossRef]
- Vink, P.; Delgado Mingorance, I.; Maximiano Alonso, C.; Rubio-Viqueira, B.; Jung, K.H.; Rodriguez Moreno, J.F.; Grande, E.; Marrupe Gonzalez, D.; Lowndes, S.; Puente, J.; et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: A randomized trial. Cancer 2019, 125, 1301–1312, Erratum in Cancer 2020, 126, 2941. [Google Scholar] [CrossRef]
- Dagnew, A.F.; Rausch, D.; Hervé, C.; Zahaf, T.; Levin, M.J.; Schuind, A.; ZOE-50/70 Study Group. Efficacy and serious adverse events profile of the adjuvanted recombinant zoster vaccine in adults with pre-existing potential immune-mediated diseases: A pooled post hoc analysis on two parallel randomized trials. Rheumatology 2021, 60, 1226–1233. [Google Scholar] [CrossRef]
- Hielscher, F.; Schmidt, T.; Enders, M.; Leyking, S.; Gerhart, M.; van Bentum, K.; Mihm, J.; Schub, D.; Sester, U.; Sester, M. The inactivated herpes zoster vaccine HZ/su induces a varicella zoster virus specific cellular and humoral immune response in patients on dialysis. EBioMedicine 2024, 108, 105335. [Google Scholar] [CrossRef]
- Otani, N.; Shima, M.; Yamamoto, T.; Okuno, T. Effect of Routine Varicella Immunization on the Epidemiology and Immunogenicity of Varicella and Shingles. Viruses 2022, 14, 588. [Google Scholar] [CrossRef] [PubMed]
- Asada, H. VZV-specific cell-mediated immunity, but not humoral immunity, correlates inversely with the incidence of herpes zoster and the severity of skin symptoms and zoster-associated pain: The SHEZ study. Vaccine 2019, 37, 6776–6781. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Weinberg, A. Immune responses to zoster vaccines. Hum. Vaccines Immunother. 2019, 15, 772–777. [Google Scholar] [CrossRef]
- Rondaan, C.; de Joode, A.A.E.; van Assen, S.; Bos, N.A.; Westerhuis, R.; Westra, J. Increased incidence of herpes zoster in patients on renal replacement therapy cannot be explained by intrinsic defects of cellular or humoral immunity to varicella-zoster virus. Antivir. Res. 2018, 158, 206–212. [Google Scholar] [CrossRef]
- Besbassi, H.; Garcia-Fogeda, I.; Quinlivan, M.; Breuer, J.; Abrams, S.; Hens, N.; Ogunjimi, B.; Beutels, P. Modeling antibody dynamics following herpes zoster indicates that higher varicella-zoster virus viremia generates more VZV-specific antibodies. Front. Immunol. 2023, 14, 1104605. [Google Scholar] [CrossRef]
- Kho, M.M.L.; Weimar, W.; Malahe, S.R.K.; Zuijderwijk, J.M.; de Kuiper, R.; Boer-Verschragen, M.J.; van der Eijk, A.A.; Hesselink, D.A.; Reinders, M.E.J.; van Besouw, N.M. Boosting the VZV-Specific Memory B and T Cell Response to Prevent Herpes Zoster After Kidney Transplantation. Front. Immunol. 2022, 13, 927734. [Google Scholar] [CrossRef]
- Sun, X.; Zang, Y.S.; Xu, Y.; Wang, W. Assessment of the humoral immune status of varicella-zoster virus in patients with diffuse connective tissue diseases. Front. Med. 2024, 11, 1470068. [Google Scholar] [CrossRef]
- Levin, M.J.; Schmader, K.E.; Pang, L.; Williams-Diaz, A.; Zerbe, G.; Canniff, J.; Johnson, M.J.; Caldas, Y.; Cho, A.; Lang, N.; et al. Cellular and Humoral Responses to a Second Dose of Herpes Zoster Vaccine Administered 10 Years After the First Dose Among Older Adults. J. Infect. Dis. 2016, 213, 14–22. [Google Scholar] [CrossRef]
- Weinberg, A.; Zhang, J.H.; Oxman, M.N.; Johnson, G.R.; Hayward, A.R.; Caulfield, M.J.; Irwin, M.R.; Clair, J.; Smith, J.G.; Stanley, H.; et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J. Infect. Dis. 2009, 200, 1068–1077. [Google Scholar] [CrossRef]
- Jenke, A.C.; Klein, S.; Baiker, A.; Wirth, S.; Sander, M.; Noelting, C.; Boecher, O.; Vizoso-Pinto, M.G. Serologic analysis of the IgG anti-body response in children with varicella zoster virus wild-type infection and vaccination. Pediatr. Infect. Dis. J. 2012, 31, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Sieiro-Santos, C.; Herrero, J.G.; Ordas Martínez, J.; Álvarez Castro, C.; López Robles, A.; Colindres, R.; Martín, E.R.; Sahagun, A.M.; Ruiz de Morales, J.G. Immunogenicity to Herpes Zoster recombinant subunit vaccine in immune-mediated rheumatic patients under treatment with JAK inhibitors. Rheumatology 2024, keae584. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.S.; Miao, C.; Leung, J.; Johnson, M.; Weinberg, A.; Levin, M.J. Comparative Antibody Responses to the Live-Attenuated and Recombinant Herpes Zoster Vaccines. J. Virol. 2021, 95, e00240-21. [Google Scholar] [CrossRef]
- Pan, D.; Wang, W.; Cheng, T. Current Methods for the Detection of Antibodies of Varicella-Zoster Virus: A Review. Microorganisms 2023, 11, 519. [Google Scholar] [CrossRef]
- Società Italiana di Nefrologia. Available online: https://ridt.sinitaly.org/2023/11/22/report-2021-2/ (accessed on 31 August 2024).
- Szucs, T.D.; Pfeil, A.M. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics 2013, 31, 125–136. [Google Scholar] [CrossRef]
- Lin, S.Y.; Liu, J.H.; Lin, C.L.; Tsai, I.J.; Chen, P.C.; Chung, C.J.; Liu, Y.L.; Wang, I.K.; Lin, H.H.; Huang, C.C. A comparison of herpes zoster incidence across the spectrum of chronic kidney disease, dialysis and transplantation. Am. J. Nephrol. 2012, 36, 27–33. [Google Scholar] [CrossRef]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mudgal, S.K.; Thakur, K.; Gaur, R. How to calculate sample size for observational and experimental nursing research studies? Natl. J. Physiol. Pharm. Pharmacol. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Krasselt, M.; Baerwald, C.; Liebert, U.G.; Seifert, O. Humoral immunity to varicella zoster virus is altered in patients with rheumatoid arthritis. Clin. Rheumatol. 2019, 38, 2493–2500. [Google Scholar] [CrossRef]
- Koh, J.H.; Lee, J.; Kim, S.H.; Kwok, S.K.; Ju, J.H.; Park, S.H. Safety, and Humoral and Cell-mediated Immune Responses to Herpes Zoster Vaccine in Patients with Rheumatoid Arthritis. J. Rheumatol. 2018, 45, 465–469. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kang, M.G.; Park, K.U.; Park, W.B.; Kim, K.I.; Kim, E.S.; Kim, H.B.; Song, K.H.; Kim, C.H. Immunogenicity of the Varicella-Zoster Vaccine in Community-Dwelling Non-robust Elderly Individuals Compared to Robust Elderly Individuals: A Prospective Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.K.; Pini, S.; Scaparrotta, G.; Schirinzi, M.; Gnappi, M.; Fragasso, A.; Zanella, R.; Naso, E.; De Giorgi, M.L.; Carraro, G.; et al. Recombinant Varicella Zoster vaccine in haemodialysis facilities: Adherence and safety. J. Nephrol. 2023, 36, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.J.; Díez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barberà, J.; et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Silva de Almeida, C.C.; Guerra, D.C.; Vannucchi, M.T.; Geleilete, T.J.; Vannucchi, H.; Chiarello, P.G. What is the meaning of homocysteine in patients on dialysis? J. Ren. Nutr. 2011, 21, 394–400. [Google Scholar] [CrossRef]
- Martino, F.K.; Filippi, I.; Giavarina, D.; Kaushik, M.; Rodighiero, M.P.; Crepaldi, C.; Teixeira, C.; Nadal, A.F.; Rosner, M.H.; Ronco, C. Neutrophil gelatinase-associated lipocalin in the early diagnosis of peritonitis: The case of neutrophil gelatinase-associated lipocalin. Contrib. Nephrol. 2012, 178, 258–263. [Google Scholar] [CrossRef]
- Okuno, S.; Inaba, M.; Kitatani, K.; Ishimura, E.; Yamakawa, T.; Nishizawa, Y. Serum levels of C-terminal telopeptide of type I collagen: A useful new marker of cortical bone loss in hemodialysis patients. Osteoporos. Int. 2005, 16, 501–509. [Google Scholar] [CrossRef]
- Chen, X.; Guo, W.; Diao, Z.; Huang, H.; Liu, W. Lymphocyte-to-C reactive protein ratio as novel inflammatory marker for predicting outcomes in hemodialysis patients: A multicenter observational study. Front. Immunol. 2023, 14, 1101222. [Google Scholar] [CrossRef]
- Crepaldi, C.; Rosner, M.; Teixeira, C.; Martos, L.B.; Martino, F.K.; Rodighiero, M.P.; Ronco, C. Is brain natriuretic peptide a reliable biomarker of hydration status in all peritoneal dialysis patients? Blood Purif. 2014, 37, 238–242. [Google Scholar] [CrossRef]
- Belmouaz, M.; Bauwens, M.; Hauet, T.; Bossard, V.; Jamet, P.; Joly, F.; Chikhi, E.; Joffrion, S.; Gand, E.; Bridoux, F. Comparison of the removal of uraemic toxins with medium cut-off and high-flux dialysers: A randomized clinical trial. Nephrol. Dial. Transplant. 2020, 35, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Wolley, M.; Jardine, M.; Hutchison, C.A. Exploring the Clinical Relevance of Providing Increased Removal of Large Middle Molecules. Clin. J. Am. Soc. Nephrol. 2018, 13, 805–814. [Google Scholar] [CrossRef]
- Kandi, M.; Brignardello-Petersen, R.; Couban, R.; Wu, C.; Nesrallah, G. Effects of Medium Cut-Off Versus High-Flux Hemodialysis Membranes on Biomarkers: A Systematic Review and Meta-Analysis. Can. J. Kidney Health Dis. 2022, 9, 20543581211067090. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Virzì, G.M.; Brocca, A.; Garzotto, F.; Kim, J.C.; Ramponi, F.; de Cal, M.; Lorenzin, A.; Brendolan, A.; Nalesso, F.; et al. In vitro Cytotoxicity of Bisphenol A in Monocytes Cell Line. Blood Purif. 2015, 40, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A.; Daugirdas, J.T. Kinetics of β -2-Microglobulin with Hemodiafiltration and High-Flux Hemodialysis. Clin. J. Am. Soc. Nephrol. 2024, 19, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Kartsios, C.; Yiannaki, E.; Antoniadi, G.; Kazila, P.; Pliakos, K.; Liakopoulos, V.; Markala, D. Decreased CD3+CD16+ natural killer-like T-cell percentage and zeta-chain expression accompany chronic inflammation in haemodialysis patients. Nephrology 2009, 14, 471–475. [Google Scholar] [CrossRef]
- Zorzoli, E.; Pica, F.; Masetti, G.; Franco, E.; Volpi, A.; Gabutti, G. Herpes zoster in frail elderly patients: Prevalence, impact, management, and preventive strategies. Aging Clin. Exp. Res. 2018, 30, 693–702. [Google Scholar] [CrossRef]
- Del Giudice, G.; Goronzy, J.J.; Grubeck-Loebenstein, B.; Lambert, P.H.; Mrkvan, T.; Stoddard, J.J.; Doherty, T.M. Fighting against a protean enemy: Immunosenescence, vaccines, and healthy aging. NPJ Aging Mech. Dis. 2017, 4, 1. [Google Scholar] [CrossRef]
- Hashizume, H.; Nakatani, E.; Sato, Y.; Goto, H.; Yagi, H.; Miyachi, Y. A new susceptibility index to predict the risk of severe herpes zoster-associated pain: A Japanese regional population-based cohort study, the Shizuoka study. J. Dermatol. Sci. 2022, 105, 170–175, Erratum in J. Dermatol. Sci. 2023, 112, 117. [Google Scholar] [CrossRef]
- Cho, S.I.; Lee, D.H.; Park, Y.M. Identification of herpes zoster high-risk group using Charlson comorbidity index: A nationwide retrospective cohort study. J. Dermatol. 2020, 47, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Tu, H.P.; Wu, M.K.; Kuo, K.L.; Su, Y.F.; Lu, Y.Y.; Lin, C.L.; Wu, C.H. Higher risk of herpes zoster in stroke patients. PLoS ONE 2020, 15, e0228409. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).