Extracellular Traps in Inflammation: Pathways and Therapeutic Targets
Abstract
1. Introduction
1.1. Context and Objectives
1.2. From Immune Cells to ET
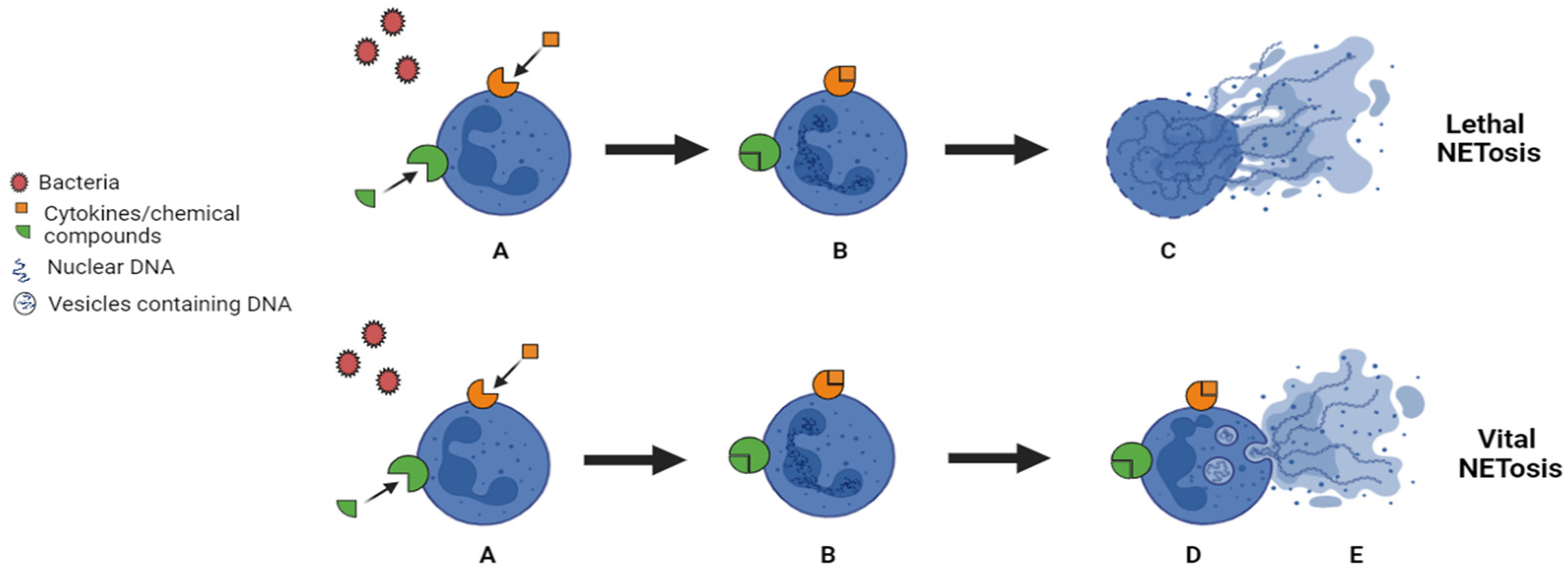
1.3. ETs Production
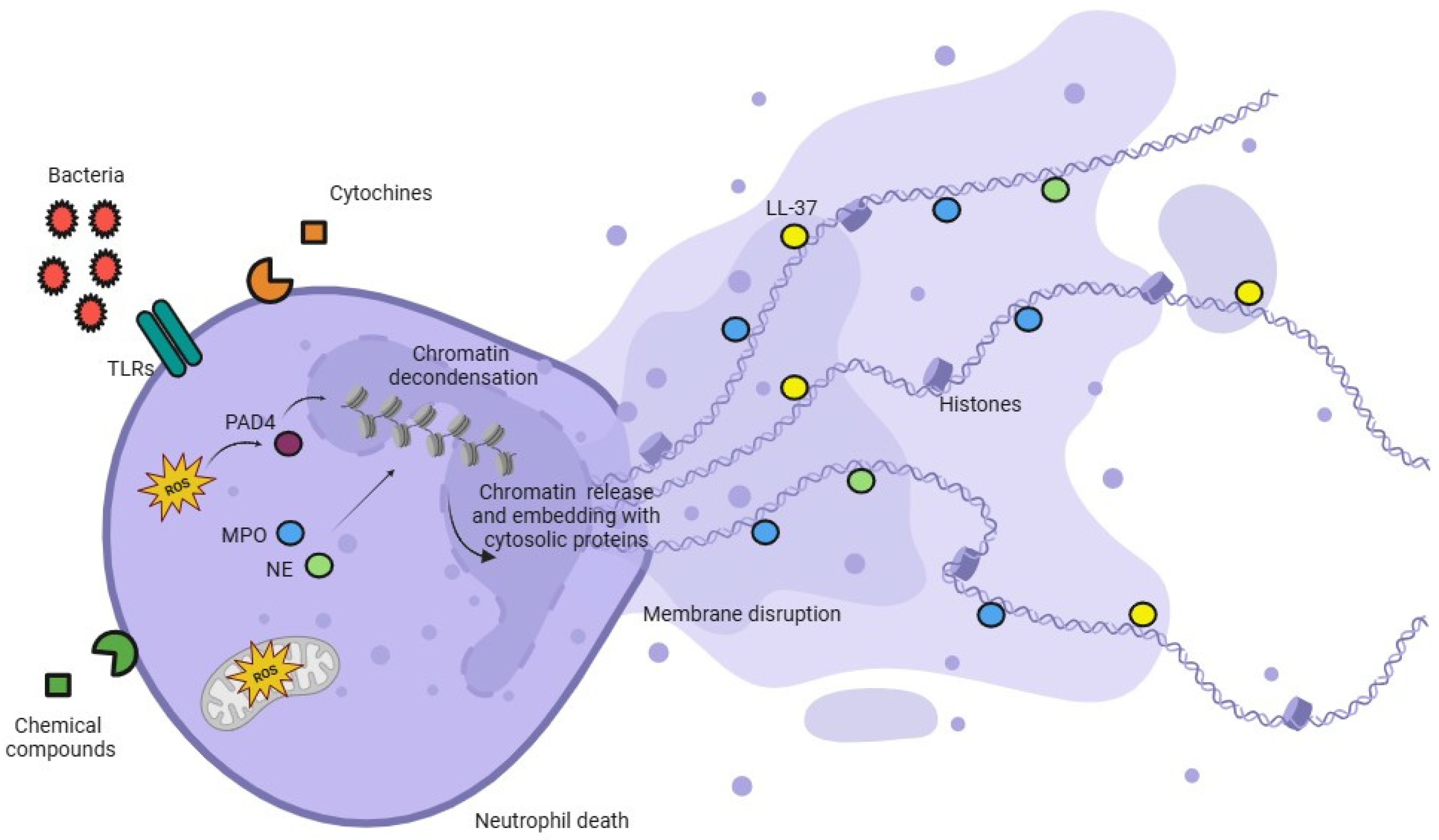
1.4. The Molecular Mechanism Involved in the ETosis Process
1.5. ETs and Pathology: A General Overview
1.6. ETs and Skin Affections
1.7. ETs and Chronic Inflammatory States in Smokers
1.8. ETs and Lung Pathologies
1.9. ETs and Kidney Disease
1.10. ETs and Neurodegenerative Disease
1.11. ETs and Liver Disease
2. Therapeutic Strategies
3. Conclusions and Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| NETs | Neutrophil extracellular traps |
| ET s | Extracellular traps |
| MPO | Myeloperoxidase |
| PMNs | Polymorphonuclear neutrophils |
| ROS | Reactive oxygen species |
| ALI | Acute lung injury |
| ARDS | Acute respiratory distress syndrome |
| METs | Monocyte/Macrophage extracellular traps |
| PMA | Phorbol Myristic Acid |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| TLRs | Toll-like receptors |
| COPS | Chronic Obstructive Pulmonary Disease |
| Ds-DNA | Double-stranded DNA |
| MPO-DNA | Myeloperoxidase-DNA |
| Cit-H3 | Citrullinated histone |
| EETs | Eosinophil extracellular traps |
| SA | Severe asthma |
| ICSs | Inhaled corticosteroids |
| ECP | Eosinophil cationic protein |
| MBP | Major basic protein |
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane regulator |
| NE | Neutrophils elastase |
| HMGB-1 | High-Mobility Group Box 1 |
| ANCAs | Antineutrophil Cytoplasmatic Antibodies |
| DKD | Diabetic kidney disease |
| A.D. | Alzheimer’s disease |
| NASH | Non-Alcoholic Steatohepatitis |
| HCC | Hepatocellular carcinoma |
| TME | Tumor microenvironment |
| S100A9 | S100 Calcium-Binding Protein A9 |
| HFD | High-fat diet |
| ND | Normal diet |
| CSE | Cigarette smoke extract |
| RNO | Roflumilast N-Oxide |
| DAPT | Dual antiplatelet therapy |
| iCCA | Intrahepatic cholangiocarcinoma |
References
- Rizzi, M.; Carniato, F.; Tonello, S.; Migliario, M.; Invernizzi, M.; Rocchetti, V.; Marchese, L.; Renò, F. Charged molecular silica trigger in vitro NETosis in human granulocytes via both oxidative and autophagic pathways. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7058–7068. [Google Scholar] [CrossRef] [PubMed]
- Migliario, M.; Tonello, S.; Rocchetti, V.; Rizzi, M.; Renò, F. Near infrared laser irradiation induces NETosis via oxidative stress and autophagy. Lasers Med. Sci. 2018, 33, 1919–1924. [Google Scholar] [CrossRef]
- Tonello, S.; Carniato, F.; Rizzi, M.; Migliario, M.; Rocchetti, V.; Marchese, L.; Renò, F. Charged polyhedral oligomeric silsesquioxanes trigger in vitro METosis via both oxidative stress and autophagy. Life Sci. 2017, 190, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Malech, H.L.; Deleo, F.R.; Quinn, M.T. The role of neutrophils in the immune system: An overview. Methods Mol. Biol. 2019, 1124, 3–10. [Google Scholar] [CrossRef]
- Wang, J.; Arase, H. Regulation of immune responses by neutrophils. Ann. N. Y. Acad. Sci. 2014, 1319, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Scapini, P.; Cassatella, M.A. Social networking of human neutrophils within the immune system. Blood 2014, 124, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Ocana, A.; Nieto-Jiménez, C.; Pandiella, A.; Templeton, A.J. Neutrophils in cancer: Prognostic role and therapeutic strategies. Mol. Cancer 2017, 16, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Koenderman, L.; Tesselaar, K.; Vrisekoop, N. Human neutrophil kinetics: A call to revisit old evidence. Trends Immunol. 2022, 43, 868–876. [Google Scholar] [CrossRef]
- Tseng, C.W.; Liu, G.Y. Expanding roles of neutrophils in aging hosts. Curr. Opin. Immunol. 2014, 29, 43–48. [Google Scholar] [CrossRef]
- Tonello, S.; Rizzi, M.; Migliario, M.; Rocchetti, V.; Renò, F. Low concentrations of neutrophil extracellular traps induce proliferation in human keratinocytes via NF-kB activation. J. Dermatol. Sci. 2017, 88, 110–116. [Google Scholar] [CrossRef]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Zhong, L.; Ye, D.; Zhang, J.; Tu, Y.; Bornstein, S.R.; Zhou, Z.; Lam, K.S.; Xu, A. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes. Diabetes 2014, 63, 4239–4248. [Google Scholar] [CrossRef]
- Navegantes, K.C.; de Souza Gomes, R.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 36–57. [Google Scholar] [CrossRef]
- Neves, J.S.; Weller, P.F. Functional extracellular eosinophil granules: Novel implications in eosinophil immunobiology. Curr. Opin. Immunol. 2009, 21, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Tokunaga, T.; Fujieda, S.; Honda, K.; Hirokawa, M.; Spencer, L.A.; Weller, P.F. Eosinophil ETosis and DNA Traps: A New Look at Eosinophilic Inflammation. Curr. Allergy Asthma Rep. 2016, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Tilley, D.O.; Zychlinsky, A. Neutrophil Extracellular Traps: The Biology of Chromatin Externalization. Dev. Cell 2018, 44, 542–553. [Google Scholar] [CrossRef] [PubMed]
- von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Chuammitri, P.; Ostojić, J.; Andreasen, C.B.; Redmond, S.B.; Lamont, S.J.; Palić, D. Chicken heterophil extracellular traps (HETs): Novel defense mechanism of chicken heterophils. Vet. Immunol. Immunopathol. 2009, 129, 126–131. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, F.; Fortier, M.; Portes, M.; Demattei, C.; Mousty, E.; Nouvellon, E.; Mercier, E.; Chea, M.; Letouzey, V.; Gris, J.C.; et al. Vital NETosis vs. suicidal NETosis during normal pregnancy and preeclampsia. Front. Cell Dev. Biol. 2023, 10, 1099038. [Google Scholar] [CrossRef]
- Chow, O.A.; von Köckritz-Blickwede, M.; Bright, A.T.; Hensler, M.E.; Zinkernagel, A.S.; Cogen, A.L.; Gallo, R.L.; Monestier, M.; Wang, Y.; Glass, C.K.; et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 2010, 8, 445–454. [Google Scholar] [CrossRef]
- Altincicek, B.; Stötzel, S.; Wygrecka, M.; Preissner, K.T.; Vilcinskas, A. Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J. Immunol. 2008, 181, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Kenno, S.; Perito, S.; Mosci, P.; Vecchiarelli, A.; Monari, C. Autophagy and Reactive Oxygen Species Are Involved in Neutrophil Extracellular Traps Release Induced by C. albicans Morphotypes. Front. Microbiol. 2016, 7, 879. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V.; Pinegin, B.V. Neutrophil extracellular traps: Mechanisms of formation and role in health and disease. Biochemistry 2014, 79, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef]
- Phillipson, M.; Kubes, P. The neutrophil in vascular inflammation. Nat. Med. 2011, 17, 1381–1390. [Google Scholar] [CrossRef]
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Leshner, M.; Wang, S.; Lewis, C.; Zheng, H.; Chen, X.A.; Santy, L.; Wang, Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012, 3, 307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, E.; Zhang, N.; Krysko, O.; Lan, F.; Holtappels, G.; De Ruyck, N.; Nauwynck, H.; Yousefi, S.; Simon, H.U.; Bachert, C. Extracellular eosinophilic traps in association with Staphylococcus aureus at the site of epithelial barrier defects in patients with severe airway inflammation. J. Allergy Clin. Immunol. 2017, 139, 1849–1860.e6. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, O.E.; Borregaard, N. Neutrophil extracellular traps—The dark side of neutrophils. J. Clin. Investig. 2016, 126, 1612–1620. [Google Scholar] [CrossRef]
- Itakura, A.; McCarty, O.J. Pivotal role for the mTOR pathway in the formation of neutrophil extracellular traps via regulation of autophagy. Am. J. Physiol. Cell Physiol. 2013, 305, C348–C354. [Google Scholar] [CrossRef] [PubMed]
- Remijsen, Q.; Vanden Berghe, T.; Wirawan, E.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011, 21, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.; Kumar, S.V.; Darisipudi, M.N.; Anders, H.J. Extracellular histones in tissue injury and inflammation. J. Mol. Med. 2014, 92, 465–472. [Google Scholar] [CrossRef]
- Stephan, A.; Fabri, M. The NET, the trap and the pathogen: Neutrophil extracellular traps in cutaneous immunity. Exp. Dermatol. 2015, 24, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, C.; Zou, Z.; Fan, E.K.Y.; Chen, L.; Li, Y.; Billiar, T.R.; Wilson, M.A.; Shi, X.; Fan, J. Aging-related Atg5 defect impairs neutrophil extracellular traps formation. Immunology 2017, 151, 417–432. [Google Scholar] [CrossRef]
- Teimourian, S.; Moghanloo, E. Role of PTEN in neutrophil extracellular trap formation. Mol. Immunol. 2015, 66, 319–324. [Google Scholar] [CrossRef]
- Germic, N.; Hosseini, A.; Yousefi, S.; Karaulov, A.; Simon, H.U. Regulation of eosinophil functions by autophagy. Semin. Immunopathol. 2021, 43, 347–362. [Google Scholar] [CrossRef]
- Pieterse, E.; Rother, N.; Yanginlar, C.; Hilbrands, L.B.; van der Vlag, J. Neutrophils Discriminate between Lipopolysaccharides of Different Bacterial Sources and Selectively Release Neutrophil Extracellular Traps. Front. Immunol. 2016, 7, 484. [Google Scholar] [CrossRef]
- Tsai, S.R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Germic, N.; Stojkov, D.; Oberson, K.; Yousefi, S.; Simon, H.U. Neither eosinophils nor neutrophils require ATG5-dependent autophagy for extracellular DNA trap formation. Immunology 2017, 152, 517–525. [Google Scholar] [CrossRef]
- Apostolidou, E.; Kambas, K.; Chrysanthopoulou, A.; Kourtzelis, I.; Speletas, M.; Ritis, K.; Mitroulis, I. Genetic analysis of C5a receptors in neutrophils from patients with familial Mediterranean fever. Mol. Biol. Rep. 2012, 39, 5503–5510. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, E.; Skendros, P.; Kambas, K.; Mitroulis, I.; Konstantinidis, T.; Chrysanthopoulou, A.; Nakos, K.; Tsironidou, V.; Koffa, M.; Boumpas, D.T.; et al. Neutrophil extracellular traps regulate IL-1β-mediated inflammation in familial Mediterranean fever. Ann. Rheum. Dis. 2016, 75, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Mitroulis, I.; Ritis, K. Autophagy in Neutrophils: From Granulopoiesis to Neutrophil Extracellular Traps. Front. Cell Dev. Biol. 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef]
- Cristinziano, L.; Modestino, L.; Antonelli, A.; Marone, G.; Simon, H.U.; Varricchi, G.; Galdiero, M.R. Neutrophil extracellular traps in cancer. Semin. Cancer Biol. 2022, 79, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Herre, M.; Cedervall, J.; Mackman, N.; Olsson, A.K. Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases. Physiol. Rev. 2023, 103, 277–312. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, H.; Lin, W.; Lu, L.; Su, J.; Chen, X. Signaling pathways and targeted therapies for psoriasis. Signal Transduct. Target. Ther. 2023, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, Y.S.; Lee, B.S.; Han, C.H.; Kim, S.G.; Kim, C.H. NLRP3 inflammasome activation and NETosis positively regulate each other and exacerbate proinflammatory responses: Implications of NETosis inhibition for acne skin inflammation treatment. Cell Mol. Immunol. 2024, 21, 466–478. [Google Scholar] [CrossRef]
- Damiani, G.; Bragazzi, N.L.; Karimkhani Aksut, C.; Wu, D.; Alicandro, G.; McGonagle, D.; Guo, C.; Dellavalle, R.; Grada, A.; Wong, P.; et al. The Global, Regional, and National Burden of Psoriasis: Results and Insights From the Global Burden of Disease 2019 Study. Front. Med. 2021, 8, 743180. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.B.; Jerome, D.; Yeung, J. Diagnosis and management of psoriasis. Can. Fam. Physician 2017, 63, 278–285. [Google Scholar]
- Shen, C.; Han, Y.; Wang, B.; Tang, J.; Chen, H.; Lin, Q. Correction: Ocular biocompatibility evaluation of POSS nanomaterials for biomedical material applications. RSC Adv. 2024, 14, 10262. [Google Scholar] [CrossRef] [PubMed]
- D’Onghia, D.; Colangelo, D.; Bellan, M.; Tonello, S.; Puricelli, C.; Virgilio, E.; Apostolo, D.; Minisini, R.; Ferreira, L.L.; Sozzi, L.; et al. Gas6/TAM system as potential biomarker for multiple sclerosis prognosis. Front Immunol. 2024, 15, 1362960. [Google Scholar] [CrossRef]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Lai, D.; Zhang, P.; Yang, Y.; Li, Y.; Fei, K.; Jiang, G.; Fan, J. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018, 9, 597. [Google Scholar] [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Manuela, R.; Mario, M.; Vincenzo, R.; Filippo, R. Nicotine stimulation increases proliferation and matrix metalloproteinases-2 and -28 expression in human dental pulp cells. Life Sci. 2015, 135, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Thompson, P.R.; Segal, B.H.; Urban, C.F. Nicotine induces neutrophil extracellular traps. J. Leukoc. Biol. 2016, 100, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, H.; Bajrami, B.; Kwak, H.; Cao, S.; Liu, P.; Zhou, J.; Zhou, Y.; Zhu, H.; Ye, K.; et al. Cigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphate. Proc. Natl. Acad. Sci. USA 2013, 110, 7726–7731. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.L.; Zhang, H.; Tang, Q.Y.; Bai, J.; He, Z.Y.; Zhang, J.Q.; Li, M.H.; Deng, J.M.; Liu, G.N.; Zhong, X.N. Neutrophil extracellular traps induced by cigarette smoke activate plasmacytoid dendritic cells. Thorax 2017, 72, 1084–1093. [Google Scholar] [CrossRef]
- Renò, F.; Rocchetti, V.; Migliario, M.; Rizzi, M.; Cannas, M. Chronic exposure to cigarette smoke increases matrix metalloproteinases and Filaggrin mRNA expression in oral keratinocytes: Role of nicotine stimulation. Oral Oncol. 2011, 47, 827–830. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Chen, P.; Liu, X.M. The role of cigarette smoke-induced pulmonary vascular endothelial cell apoptosis in COPD. Respir. Res. 2021, 22, 39. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Y.; Li, X.; Wang, R.; Zeng, Z.; Cheng, M.; Gao, L.; Xu, D.; Wen, F.; Wang, T.; et al. Inhibition of neutrophil elastase prevents cigarette smoke exposure-induced formation of neutrophil extracellular traps and improves lung function in a mouse model of chronic obstructive pulmonary disease. Int. Immunopharmacol. 2023, 114, 109537. [Google Scholar] [CrossRef]
- Dicker, A.J.; Crichton, M.L.; Pumphrey, E.G.; Cassidy, A.J.; Suarez-Cuartin, G.; Sibila, O.; Furrie, E.; Fong, C.J.; Ibrahim, W.; Brady, G.; et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 117–127. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef]
- Wright, T.K.; Gibson, P.G.; Simpson, J.L.; McDonald, V.M.; Wood, L.G.; Baines, K.J. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology 2016, 21, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.; Tosi, I.; Hego, A.; Maréchal, P.; Marichal, T.; Radermecker, C. Neutrophil Extracellular Traps Are Found in Bronchoalveolar Lavage Fluids of Horses With Severe Asthma and Correlate With Asthma Severity. Front. Immunol. 2022, 13, 921077. [Google Scholar] [CrossRef] [PubMed]
- Porto, B.N.; Stein, R.T. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front. Immunol. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Shamji, M.; Marone, G.; Durham, S.R.; Scadding, G.W.; Varricchi, G. Neutrophil Extracellular Traps in Asthma: Friends or Foes? Cells 2022, 11, 3521. [Google Scholar] [CrossRef] [PubMed]
- Granger, V.; Taillé, C.; Roach, D.; Letuvé, S.; Dupin, C.; Hamidi, F.; Noël, B.; Neukirch, C.; Aubier, M.; Pretolani, M.; et al. Circulating neutrophil and eosinophil extracellular traps are markers of severe asthma. Allergy 2020, 75, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, Y.M.; Lee, H.R.; Mun, J.; Sim, S.; Lee, D.H.; Pham, D.L.; Kim, S.H.; Shin, Y.S.; Lee, S.W.; et al. Eosinophil extracellular traps activate type 2 innate lymphoid cells through stimulating airway epithelium in severe asthma. Allergy 2020, 75, 95–103. [Google Scholar] [CrossRef]
- Trian, T.; Benard, G.; Begueret, H.; Rossignol, R.; Girodet, P.O.; Ghosh, D.; Ousova, O.; Vernejoux, J.M.; Marthan, R.; Tunon-de-Lara, J.M.; et al. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J. Exp. Med. 2007, 204, 3173–3181. [Google Scholar] [CrossRef]
- Xu, W.; Ghosh, S.; Comhair, S.A.; Asosingh, K.; Janocha, A.J.; Mavrakis, D.A.; Bennett, C.D.; Gruca, L.L.; Graham, B.B.; Queisser, K.A.; et al. Increased mitochondrial arginine metabolism supports bioenergetics in asthma. J. Clin. Investig. 2016, 126, 2465–2481. [Google Scholar] [CrossRef]
- Esteves, P.; Blanc, L.; Celle, A.; Dupin, I.; Maurat, E.; Amoedo, N.; Cardouat, G.; Ousova, O.; Gales, L.; Bellvert, F.; et al. Crucial role of fatty acid oxidation in asthmatic bronchial smooth muscle remodelling. Eur. Respir. J. 2021, 58, 2004252. [Google Scholar] [CrossRef]
- Koranteng, J.; Chung, K.F.; Michaeloudes, C.; Bhavsar, P. The role of mitochondria in eosinophil function: Implications for severe asthma pathogenesis. Front. Cell Dev. Biol. 2024, 12, 1360079. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.S.; Antunes, G.L.; Kaiber, D.B.; da Costa, M.S.; Marques, E.P.; Ferreira, F.S.; Gassen, R.B.; Breda, R.V.; Wyse, A.T.S.; Pitrez, P.; et al. Reactive oxygen species are involved in eosinophil extracellular traps release and in airway inflammation in asthma. J. Cell Physiol. 2019, 234, 23633–23646. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Boivin, R.; Cano, P.; Murcia, Y.; Bazin, I.; Lavoie, J.P. Neutrophil extracellular traps are downregulated by glucocorticosteroids in lungs in an equine model of asthma. Respir. Res. 2017, 18, 207. [Google Scholar] [CrossRef]
- Martínez-Alemán, S.R.; Campos-García, L.; Palma-Nicolas, J.P.; Hernández-Bello, R.; González, G.M.; Sánchez-González, A. Understanding the Entanglement: Neutrophil Extracellular Traps (NETs) in Cystic Fibrosis. Front. Cell Infect. Microbiol. 2017, 7, 104. [Google Scholar] [CrossRef]
- Ambardar, S.R.; Hightower, S.L.; Huprikar, N.A.; Chung, K.K.; Singhal, A.; Collen, J.F. Post-COVID-19 Pulmonary Fibrosis: Novel Sequelae of the Current Pandemic. J. Clin. Med. 2021, 10, 2452. [Google Scholar] [CrossRef] [PubMed]
- Stoian, M.; Roman, A.; Boeriu, A.; Onișor, D.; Bandila, S.R.; Babă, D.F.; Cocuz, I.; Niculescu, R.; Costan, A.; Laszlo, S.Ș.; et al. Long-Term Radiological Pulmonary Changes in Mechanically Ventilated Patients with Respiratory Failure due to SARS-CoV-2 Infection. Biomedicines 2023, 11, 2637. [Google Scholar] [CrossRef]
- Doster, R.S.; Rogers, L.M.; Gaddy, J.A.; Aronoff, D.M. Macrophage Extracellular Traps: A Scoping Review. J. Innate Immun. 2018, 10, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kummarapurugu, A.B.; Zheng, S.; Ma, J.; Ghosh, S.; Hawkridge, A.; Voynow, J.A. Neutrophil Elastase Triggers the Release of Macrophage Extracellular Traps: Relevance to Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 66, 76–85. [Google Scholar] [CrossRef]
- Peng, H.H.; Liu, Y.J.; Ojcius, D.M.; Lee, C.M.; Chen, R.H.; Huang, P.R.; Martel, J.; Young, J.D. Mineral particles stimulate innate immunity through neutrophil extracellular traps containing HMGB1. Sci. Rep. 2017, 7, 16628. [Google Scholar] [CrossRef]
- Tonello, S.; D’Onghia, D.; Di Ruscio, A.; Mora, S.M.; Vincenzi, F.; Caria, G.; Fracchia, A.; Vercellino, N.; Bussolati, B.; Tanzi, A.; et al. Extracellular Vesicles as a Potential Biomarker of Pulmonary Arterial Hypertension in Systemic Sclerosis. Pharmaceuticals 2025, 18, 259. [Google Scholar] [CrossRef]
- Rizzi, M.; Tonello, S.; Morani, F.; Rizzi, E.; Casciaro, G.F.; Matino, E.; Costanzo, M.; Zecca, E.; Croce, A.; Pedrinelli, A.; et al. CGRP Plasma Levels Correlate with the Clinical Evolution and Prognosis of Hospitalized Acute COVID-19 Patients. Viruses 2022, 14, 2123. [Google Scholar] [CrossRef] [PubMed]
- Cano Plá, S.M.; D’Urso, A.; Fernández-Sánchez, J.F.; Colangelo, D.; Choquesillo-Lazarte, D.; Ferracini, R.; Bosetti, M.; Prat, M.; Gómez-Morales, J. Biomimetic Citrate-Coated Luminescent Apatite Nanoplatforms for Diclofenac Delivery in Inflammatory Environments. Nanomaterials 2022, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kaplan, M.J. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat. Rev. Nephrol. 2016, 12, 402–413. [Google Scholar] [CrossRef]
- Sethi, S.; De Vriese, A.S.; Fervenza, F.C. Acute glomerulonephritis. Lancet 2022, 399, 1646–1663. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Bellan, M.; Lombino, F.; Alciato, F.; Carecchio, M.; Galimberti, D.; Fenoglio, C.; Scarpini, E.; Cantello, R.; Pirisi, M.; et al. Growth Arrest Specific 6 Concentration is Increased in the Cerebrospinal Fluid of Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 55, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Alaygut, D.; Ozturk, I.; Ulu, S.; Gungor, O. NETosis and kidney disease: What do we know? Int. Urol. Nephrol. 2023, 55, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, K.; Fatima, S.; Ambreen, S.; Zimmermann, S.; Younis, R.; Krishnan, S.; Rana, R.; Gadi, I.; Schwab, C.; et al. Neutrophil Extracellular Traps Promote NLRP3 Inflammasome Activation and Glomerular Endothelial Dysfunction in Diabetic Kidney Disease. Nutrients 2022, 14, 2965. [Google Scholar] [CrossRef] [PubMed]
- Reidy, K.; Kang, H.M.; Hostetter, T.; Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Investig. 2014, 124, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Thålin, C.; Hisada, Y.; Lundström, S.; Mackman, N.; Wallén, H. Neutrophil Extracellular Traps: Villains and Targets in Arterial, Venous, and Cancer-Associated Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1724–1738. [Google Scholar] [CrossRef]
- Döring, Y.; Soehnlein, O.; Weber, C. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ. Res. 2017, 120, 736–743. [Google Scholar] [CrossRef]
- D’Abbondanza, M.; Martorelli, E.E.; Ricci, M.A.; De Vuono, S.; Migliola, E.N.; Godino, C.; Corradetti, S.; Siepi, D.; Paganelli, M.T.; Maugeri, N.; et al. Increased plasmatic NETs by-products in patients in severe obesity. Sci. Rep. 2019, 9, 14678. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Díez-Delhoyo, F.; Gutiérrez-Ibañes, E.; Sanz-Ruiz, R.; Vázquez-Álvarez, M.E.; González Saldívar, H.; Rivera Juárez, A.; Sarnago, F.; Martínez-Sellés, M.; Bermejo, J.; Soriano, J.; et al. Prevalence of Microvascular and Endothelial Dysfunction in the Nonculprit Territory in Patients with Acute Myocardial Infarction. Circ. Cardiovasc. Interv. 2019, 12, e007257. [Google Scholar] [CrossRef]
- Pietronigro, E.C.; Della Bianca, V.; Zenaro, E.; Constantin, G. NETosis in Alzheimer’s Disease. Front. Immunol. 2017, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Angelillo-Scherrer, A.; Burnier, L.; Flores, N.; Savi, P.; DeMol, M.; Schaeffer, P.; Herbert, J.M.; Lemke, G.; Goff, S.P.; Matsushima, G.K.; et al. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J. Clin. Investig. 2005, 115, 237–246. [Google Scholar] [CrossRef]
- Kretzschmar, G.C.; Bumiller-Bini, V.; Gasparetto Filho, M.A.; Zonta, Y.R.; Yu, K.S.T.; de Souza, R.L.R.; Dias-Melicio, L.A.; Boldt, A.B.W. Neutrophil Extracellular Traps: A Perspective of Neuroinflammation and Complement Activation in Alzheimer’s Disease. Front. Mol. Biosci. 2021, 8, 630869. [Google Scholar] [CrossRef] [PubMed]
- de Bont, C.M.; Boelens, W.C.; Pruijn, G.J.M. NETosis, complement, and coagulation: A triangular relationship. Cell Mol. Immunol. 2019, 16, 19–27. [Google Scholar] [CrossRef]
- Zenaro, E.; Pietronigro, E.; Della Bianca, V.; Piacentino, G.; Marongiu, L.; Budui, S.; Turano, E.; Rossi, B.; Angiari, S.; Dusi, S.; et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015, 21, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Burlone, M.E.; Pedrinelli, A.R.; Giarda, P.; Minisini, R.; Pirisi, M. Influence of age on sex-related differences among patients with hepatitis C. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1100. [Google Scholar] [CrossRef]
- Aslam, H.; Oza, F.; Ahmed, K.; Kopel, J.; Aloysius, M.M.; Ali, A.; Dahiya, D.S.; Aziz, M.; Perisetti, A.; Goyal, H. The Role of Red Cell Distribution Width as a Prognostic Marker in Chronic Liver Disease: A Literature Review. Int. J. Mol. Sci. 2023, 24, 3487. [Google Scholar] [CrossRef] [PubMed]
- Burlone, M.E.; Budkowska, A. Hepatitis C virus cell entry: Role of lipoproteins and cellular receptors. J. Gen. Virol. 2009, 90 Pt 5, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Varley, P.R.; Goswami, J.; Yazdani, H.O.; Tohme, S.; Loughran, P.; O’Doherty, R.M.; Minervini, M.I.; et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018, 68, 1347–1360. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Wang, Y.; Brown, Z.J.; Xia, Y.; Huang, Z.; Shen, C.; Hu, Z.; Beane, J.; Ansa-Addo, E.A.; et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J. Hepatol. 2021, 75, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- De Meo, M.L.; Spicer, J.D. The role of neutrophil extracellular traps in cancer progression and metastasis. Semin. Immunol. 2021, 57, 101595. [Google Scholar] [CrossRef]
- Yang, L.Y.; Luo, Q.; Lu, L.; Zhu, W.W.; Sun, H.T.; Wei, R.; Lin, Z.F.; Wang, X.Y.; Wang, C.Q.; Lu, M.; et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J. Hematol. Oncol. 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Wu, R.; Kong, X.H.; You, Y.; He, K.; Sun, X.Y.; Huang, Y.; Chen, W.X.; Duan, L. Elevated neutrophil extracellular traps by HBV-mediated S100A9-TLR4/RAGE-ROS cascade facilitate the growth and metastasis of hepatocellular carcinoma. Cancer Commun. 2023, 43, 225–245. [Google Scholar] [CrossRef]
- Agraz-Cibrián, J.M.; Delgado-Rizo, V.; Segura-Ortega, J.E.; Maldonado-Gómez, H.A.; Zambrano-Zaragoza, J.F.; Durán-Avelar, M.J.; Vibanco-Perez, N.; Fafutis-Morris, M. Impaired neutrophil extracellular traps and inflammatory responses in the peritoneal fluid of patients with liver cirrhosis. Scand. J. Immunol. 2018, 88, e12714. [Google Scholar] [CrossRef] [PubMed]
- Tonello, S.; Rizzi, M.; Matino, E.; Costanzo, M.; Casciaro, G.F.; Croce, A.; Rizzi, E.; Zecca, E.; Pedrinelli, A.; Vassia, V.; et al. Baseline Plasma Gas6 Protein Elevation Predicts Adverse Outcomes in Hospitalized COVID-19 Patients. Dis Markers. 2022, 29, 1568352. [Google Scholar] [CrossRef] [PubMed]
- Sola, D.; Tonello, S.; Casciaro, G.F.; Rizzi, E.; D’Onghia, D.; Pirisi, M.; Caldera, F.; Rizzi, M.; Colangelo, D.; Del Duca, N.; et al. Higher Serum Monolaurin Is Associated with a Lower Risk of COVID-19: Results from a Prospective Observational Cohort Study. Int. J. Mol. Sci. 2025, 26, 2452. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Pogliani, G.; Marconi, C.; Minisini, R.; Franzosi, L.; Alciato, F.; Magri, A.; Avanzi, G.C.; Pirisi, M.; Sainaghi, P.P. Gas6 as a putative noninvasive biomarker of hepatic fibrosis. Biomark. Med. 2016, 10, 1241–1249. [Google Scholar] [CrossRef]
- Cichon, I.; Ortmann, W.; Kolaczkowska, E. Metabolic Pathways Involved in Formation of Spontaneous and Lipopolysaccharide-Induced Neutrophil Extracellular Traps (NETs) Differ in Obesity and Systemic Inflammation. Int. J. Mol. Sci. 2021, 22, 7718. [Google Scholar] [CrossRef]
- Hernández González, L.L.; Pérez-Campos Mayoral, L.; Hernández-Huerta, M.T.; Mayoral Andrade, G.; Martínez Cruz, M.; Ramos-Martínez, E.; Pérez-Campos Mayoral, E.; Cruz Hernández, V.; Antonio García, I.; Matias-Cervantes, C.A.; et al. Targeting Neutrophil Extracellular Trap Formation: Exploring Promising Pharmacological Strategies for the Treatment of Preeclampsia. Pharmaceuticals 2024, 17, 605. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Xiang, X.D.; Sun, F.; Xiao, B.W.; Yan, M.Y.; Peng, B.; Liu, D. Simvastatin Reduces NETosis to Attenuate Severe Asthma by Inhibiting PAD4 Expression. Oxid. Med. Cell Longev. 2023, 2023, 1493684. [Google Scholar] [CrossRef] [PubMed]
- Demkow, U. Molecular Mechanisms of Neutrophil Extracellular Trap (NETs) Degradation. Int. J. Mol. Sci. 2023, 24, 4896. [Google Scholar] [CrossRef]
- Shen, Y.; You, Q.; Wu, Y.; Wu, J. Inhibition of PAD4-mediated NET formation by cl-amidine prevents diabetes development in nonobese diabetic mice. Eur. J. Pharmacol. 2022, 916, 174623. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Liu, L.; Cao, Y.; Ma, G.; Zhu, Y.; Xu, J.; Zhang, X.; Li, T.; Mi, L.; Jia, H.; et al. Inhibition of neutrophil extracellular trap formation ameliorates neuroinflammation and neuronal apoptosis via STING-dependent IRE1α/ASK1/JNK signaling pathway in mice with traumatic brain injury. J. Neuroinflamm. 2023, 20, 222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qiu, S.L.; Tang, Q.Y.; Zhou, X.; Zhang, J.Q.; He, Z.Y.; Bai, J.; Li, M.H.; Deng, J.M.; Liang, Y.; et al. Erythromycin suppresses neutrophil extracellular traps in smoking-related chronic pulmonary inflammation. Cell Death Dis. 2019, 10, 678. [Google Scholar] [CrossRef]
- Farrera, C.; Fadeel, B. Macrophage clearance of neutrophil extracellular traps is a silent process. J. Immunol. 2013, 191, 2647–2656. [Google Scholar] [CrossRef]
- Sollberger, G.; Choidas, A.; Burn, G.L.; Habenberger, P.; Di Lucrezia, R.; Kordes, S.; Menninger, S.; Eickhoff, J.; Nussbaumer, P.; Klebl, B.; et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar6689. [Google Scholar] [CrossRef]
- Totani, L.; Amore, C.; Piccoli, A.; Dell’Elba, G.; Di Santo, A.; Plebani, R.; Pecce, R.; Martelli, N.; Rossi, A.; Ranucci, S.; et al. Type-4 Phosphodiesterase (PDE4) Blockade Reduces NETosis in Cystic Fibrosis. Front. Pharmacol. 2021, 12, 702677. [Google Scholar] [CrossRef]
- Le Joncour, A.; Régnier, P.; Maciejewski-Duval, A.; Charles, E.; Barete, S.; Fouret, P.; Rosenzwajg, M.; Klatzmann, D.; Cacoub, P.; Saadoun, D. Reduction of Neutrophil Activation by Phosphodiesterase 4 Blockade in Behçet’s Disease. Arthritis Rheumatol. 2023, 75, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Shishikura, K.; Horiuchi, T.; Sakata, N.; Trinh, D.A.; Shirakawa, R.; Kimura, T.; Asada, Y.; Horiuchi, H. Prostaglandin E2 inhibits neutrophil extracellular trap formation through production of cyclic AMP. Br. J. Pharmacol. 2016, 173, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Kagawa, S.; Kajioka, H.; Taniguchi, A.; Kuroda, S.; Kikuchi, S.; Kakiuchi, Y.; Yagi, T.; Nogi, S.; Teraishi, F.; et al. Dual antiplatelet therapy inhibits neutrophil extracellular traps to reduce liver micrometastases of intrahepatic cholangiocarcinoma. Cancer Lett. 2023, 567, 216260. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Reference | Anti-NETs | Main Findings |
|---|---|---|---|---|
| Chen et al. | 2023 | [123] | Simvastatin | In a mouse model of severe asthma, Simvastatin decreases Th17-mediated neutrophilic inflammation and airway hyperreactivity through the reduction in PAD4 expression, resulting in NETosis inhibition. |
| Demkow U | 2023 | [124] | DNase I and II families 3’-exonucleases (TREX1 and TREX2) | These families of DNAse and exonucleases are involved in NETs degradation. |
| Shen et al. | 2022 | [125] | Cl-amidine | It has been shown that Cl-amidine is involved in the inhibition of PAD4-dependent NETs formation, avoiding NETosis. NET inhibition with Clamidine alleviated neuroinfammation, neuronal apoptosis, and neurological deficits through STING-dependent IRE1α/ASK1/JNK signaling pathway in mice with traumatic brain injury. |
| Shi et al. | 2023 | [126] | ||
| Wang et al. | 2023 | [68] | GW31161A | GW311616A represents a selective and potent inhibitor of neutrophile elastase (NE). It prevents cigarette smoke extract (CSE)-induced NETs formation through the blocking of NE nuclear translocation and subsequent chromatin decondensation. In vivo, GW311616A reduces pulmonary generation of NETs, attenuates neutrophil numbers and percentages, and the levels of neutrophil chemotactic factors and proinflammatory cytokines in BALF. Moreover, it is able to improve lung function in the COPD mouse model. |
| Wang et al. | 2023 | [68] | CXCR2 antagonist | It blocks the trafficking of the neutrophil from the blood into the airways. Additionally, in COPD patients, the CXCR2 antagonist partially inhibits spontaneous NETosis in sputum neutrophils of COPD patients. |
| Zang et al. | 2019 | [127,128] | Erythromycin | Erythromycin inhibits CSE-induced NET formation. In mice chronically exposed to cigarette smoke, erythromycin decreases NETs in the airway and ameliorates emphysema by downregulating Th1 and Th17 cells and suppressing CD40+ and CD86+ mDCs. |
| Farrera et al. | 2013 | [128] | Cytochalasin Nystatin | The administration of cytochalasin, an inhibitor of actin polymerization, and nystatin, belonging to the endocytosis inhibitors family, are involved in NETs clearance mediated by the macrophages, allowing an active and endocytosis-dependent process. |
| Sollberger et al. | 2018 | [129] | Molecules based on the pyrazolo-oxazepine scaffold (LDC7559) | A class of molecules based on the pyrazolo-oxazepine scaffold is capable of inhibiting gasdermin D and consequently NET formation. LDC7559 is able to inhibit PMA-induced NET formation without interfering with phagocytosis and impacting the physiological activity of Nox, NE, or MPO. |
| Totani et al. | 2021 | [130] | Roflumilast | They are selective PDE4 inhibitors. Roflumilast N-oxide (RNO) blocks prothrombotic functions: it inhibits Pyk2 and Akt phosphorylation, and curbs NETs production by PMNs adherent on fibrinogen-coated surfaces, and it has been approved as an adjuvant to reduce the risk of exacerbation in patients with severe COPD. Apremilast, inhibiting PDE4, prevents the activation of neutrophil surface markers as well as NETosis, ROS production, intracellular signaling and genes, and pathways related to innate immunity, and chemotaxis. Rolipram strongly affects PMA-induced NETosis and inhibits isolated neutrophil adhesion. |
| Le Joncour et al. | 2023 | [131] | Apremilast | |
| Shishikura et al. | 2016 | [132] | Rolipram (PDE4 inhibitors) | |
| Yoshimoto et al. | 2023 | [133] | Dual antiplatelet therapy (DAPT): aspirin and ticagrelor | Suppressing NETs production and avoiding platelet activation may prevent the implantation of intrahepatic cholangiocarcinoma (iCCA) cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonello, S.; Vercellino, N.; D’Onghia, D.; Fracchia, A.; Caria, G.; Sola, D.; Tillio, P.A.; Sainaghi, P.P.; Colangelo, D. Extracellular Traps in Inflammation: Pathways and Therapeutic Targets. Life 2025, 15, 627. https://doi.org/10.3390/life15040627
Tonello S, Vercellino N, D’Onghia D, Fracchia A, Caria G, Sola D, Tillio PA, Sainaghi PP, Colangelo D. Extracellular Traps in Inflammation: Pathways and Therapeutic Targets. Life. 2025; 15(4):627. https://doi.org/10.3390/life15040627
Chicago/Turabian StyleTonello, Stelvio, Nicole Vercellino, Davide D’Onghia, Alessia Fracchia, Giulia Caria, Daniele Sola, Paolo Amedeo Tillio, Pier Paolo Sainaghi, and Donato Colangelo. 2025. "Extracellular Traps in Inflammation: Pathways and Therapeutic Targets" Life 15, no. 4: 627. https://doi.org/10.3390/life15040627
APA StyleTonello, S., Vercellino, N., D’Onghia, D., Fracchia, A., Caria, G., Sola, D., Tillio, P. A., Sainaghi, P. P., & Colangelo, D. (2025). Extracellular Traps in Inflammation: Pathways and Therapeutic Targets. Life, 15(4), 627. https://doi.org/10.3390/life15040627







