Photic Retinopathy: Diagnosis and Management of This Phototoxic Maculopathy
Abstract
:1. Introduction
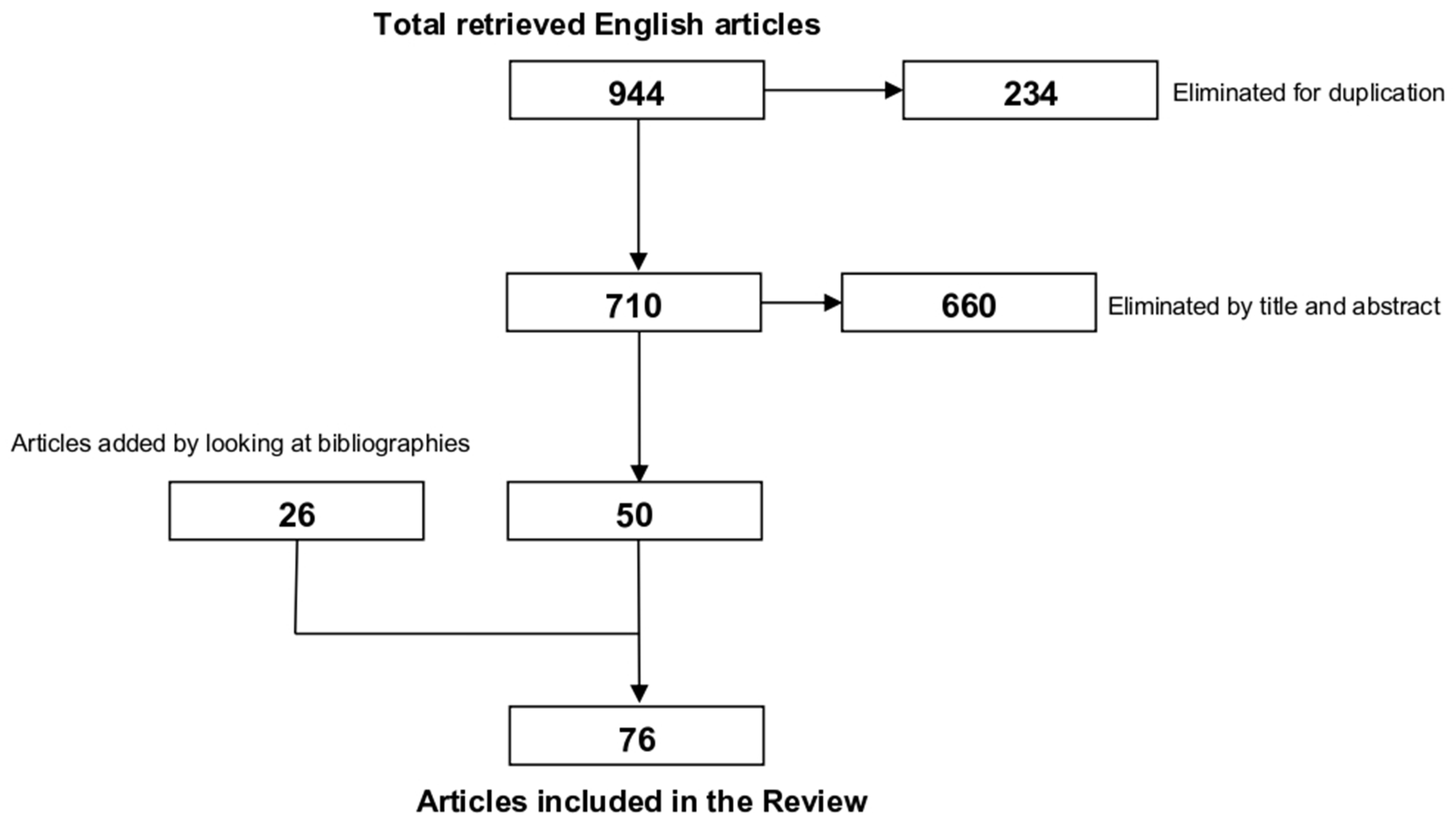
2. Materials and Methods
3. Different Clinical Entities of Photic Retinopathy
4. Diagnosis
5. Treatment
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martín-Moro, J.G.; Verdejo, J.L.H.; Gallardo, J.Z. Photic maculopathy: A review of the literature (II). Archivos De La Sociedad Española De Oftalmología (Engl. Ed.) 2018, 93, 542–550. [Google Scholar] [CrossRef]
- Mainster, M.A.; Ajlan, R. Clinical Photic Retinopathy: Mechanisms, Manifestations, and Misperceptions BT—Albert and Jakobiec’s Principles and Practice of Ophthalmology; Albert, D., Miller, J., Azar, D., Young, L.H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–30. [Google Scholar]
- Torp-Pedersen, T.; Welinder, L.; Justesen, B.; Christensen, U.C.; Bjerrum, S.S.; La Cour, M.; Saunte, J.P. Laser pointer maculopathy—On the rise? Acta Ophthalmol. 2018, 96, 749–754. [Google Scholar] [CrossRef]
- Raevis, J.; Shrier, E. Pediatric Bilateral Blue Laser Pointer-Induced Maculopathy. Case Rep. Ophthalmol. 2017, 8, 152–156. [Google Scholar] [CrossRef]
- Zamir, E.; Kaiserman, I.; Chowers, I. Laser pointer maculopathy. Am. J. Ophthalmol. 1999, 127, 728–729. [Google Scholar] [CrossRef]
- Michaelides, M.; Rajendram, R.; Marshall, J.; Keightley, S. Eclipse retinopathy. Eye 2001, 15, 148–151. [Google Scholar] [CrossRef]
- Marticorena, J.; Honrubia, A.; Ascaso, J. Solar maculopathy secondary to sunlight exposure reflected from the screen of mobile devices: Two case reports. J. Med. Case Rep. 2022, 16, 338. [Google Scholar] [CrossRef]
- Kim, M.-S.; Lee, S.-G.; Kim, J.Y.; Kang, M.-Y. Maculopathy from an accidental exposure to welding arc. BMJ Case Rep. 2019, 12, bcr-2018-227677. [Google Scholar] [CrossRef]
- Yang, X.; Shao, D.; Ding, X.; Liang, X.; Yang, J.; Li, J. Chronic phototoxic maculopathy caused by welding arc in occupational welders. Can. J. Ophthalmol. 2012, 47, 45–50. [Google Scholar] [CrossRef]
- Rohring, V.; Rehmani, A.; Smith, E.; Smith, E.; Berg, P. Drone retinopathy. J. Curr. Ophthalmol. 2019, 31, 106–108. [Google Scholar] [CrossRef]
- Postel, E.A.; Pulido, J.S.; Byrnes, G.A.; Heier, J.; Waterhouse, W.; Han, D.P.; Mieler, W.F.; Guse, C.; Wipplinger, W. Long-term Follow-up of Iatrogenic Phototoxicity. Arch. Ophthalmol. 1998, 116, 753–757. [Google Scholar] [CrossRef]
- Ruiz-Moreno, J.M.; García-Zamora, M.; Ruiz-Medrano, J. Retinal phototoxicity after macular hole surgery in a patient under paclitaxel. BMC Ophthalmol. 2023, 23, 342. [Google Scholar] [CrossRef]
- Michels, M.; Lewis, H.; Abrams, G.W.; Han, D.P.; Mieler, W.F.; Neitz, J. Macular phototoxicity caused by fiberoptic endoillumination during pars plana vitrectomy. Am. J. Ophthalmol. 1992, 114, 287–296. [Google Scholar] [CrossRef]
- Kleinmann, G.; Hoffman, P.; Schechtman, E.; Pollack, A. Microscope-induced retinal phototoxicity in cataract surgery of short duration. Ophthalmology 2002, 109, 334–338. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef]
- Boulton, M.; Różanowska, M.; Różanowski, B. Retinal photodamage. J. Photochem. Photobiol. B Biol. 2001, 64, 144–161. [Google Scholar] [CrossRef]
- Bruè, C.; Mariotti, C.; De Franco, E.; Fisher, Y.; Guidotti, J.M.; Giovannini, A. Solar Retinopathy: A Multimodal Analysis. Case Rep. Ophthalmol. Med. 2013, 2013, 906920. [Google Scholar] [CrossRef]
- Cellini, M.; Gattegna, R.; Toschi, P.G.; Strobbe, E.; Campos, E.C. Multifocal electroretinogram and Optical Coherence tomography spectral-domain in arc welding macular injury: A case report. BMC Ophthalmol. 2011, 11, 40. [Google Scholar] [CrossRef]
- Suhr, C.L.; Buffano, R.M.; Sellers, A. The use of optical coherence tomography to aid in diagnosing solar maculopathy. Optom.-J. Am. Optom. Assoc. 2011, 82, 481–484. [Google Scholar] [CrossRef]
- Green, W.R.; Robertson, D.M. Pathologic findings of photic retinopathy in the human eye. Am. J. Ophthalmol. 1991, 112, 520–527. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Fisher, Y.L.; Slakter, J.S.; Krueger, A. Solar retinopathy. A photobiologic and geophysical analysis. Retina 1989, 9, 28–43. [Google Scholar] [CrossRef]
- Taylor, H.R. The biological effects of UV-B on the eye. Photochem. Photobiol. 1989, 50, 489–492. [Google Scholar] [CrossRef]
- Clarke, A.M.; Behrendt, T. Solar Retinitis And Pupillary Reaction. Am. J. Ophthalmol. 1972, 73, 700–703. [Google Scholar] [CrossRef]
- Han, C.; Zheng, X.-X.; Zhang, W.-F. High altitude retinopathy: An overview and new insights. Travel Med. Infect. Dis. 2024, 58, 102689. [Google Scholar] [CrossRef]
- Shukla, D. Optical coherence tomography and autofluorescence findings in chronic phototoxic maculopathy secondary to snow-reflected solar radiation. Indian. J. Ophthalmol. 2015, 63, 455–457. [Google Scholar] [CrossRef]
- Bhavsar, K.V.; Michel, Z.; Greenwald, M.; Cunningham, E.T.; Freund, K.B. Retinal injury from handheld lasers: A review. Surv. Ophthalmol. 2021, 66, 231–260. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, A.; Nie, H.; Bhavsar, K.V.; Xu, Y.; Sliney, D.H.; Trokel, S.L.; Tsang, S.H. Laser-Induced Photic Injury Phenocopies Macular Dystrophy. Ophthalmic Genet. 2016, 37, 59–67. [Google Scholar] [CrossRef]
- Chakravarthy, H.; Georgyev, V.; Wagen, C.; Hosseini, A.; Matsubara, J. Blue light-induced phototoxicity in retinal cells: Implications in age-related macular degeneration. Front. Aging Neurosci. 2024, 16, 1509434. [Google Scholar] [CrossRef]
- Park, D.-W.; Alonzo, B.; Faridi, A.; Bhavsar, K.V. Multimodal Imaging of Photic Maculopathy from Arc Welding. Retin. Cases Brief Rep. 2021, 15, 468–472. [Google Scholar] [CrossRef]
- Shang, Y.-M.; Wang, G.-S.; Sliney, D.H.; Yang, C.-H.; Lee, L.-L. Light-emitting-diode induced retinal damage and its wavelength dependency in vivo. Int. J. Ophthalmol. 2017, 10, 191–202. [Google Scholar] [CrossRef]
- Krigel, A.; Berdugo, M.; Picard, E.; Levy-Boukris, R.; Jaadane, I.; Jonet, L.; Dernigoghossian, M.; Andrieu-Soler, C.; Torriglia, A.; Behar-Cohen, F. Light-induced retinal damage using different light sources, protocols and rat strains reveals LED phototoxicity. Neuroscience 2016, 339, 296–307. [Google Scholar] [CrossRef]
- Jaadane, I.; Boulenguez, P.; Chahory, S.; Carré, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Retinal damage induced by commercial light emitting diodes (LEDs). Free Radic. Biol. Med. 2015, 84, 373–384. [Google Scholar] [CrossRef]
- Aydin, B.; Dinç, E.; Yilmaz, Ş.N.; Altiparmak, U.E.; Yülek, F.; Ertekin, S.; Yilmaz, M.; Yakın, M. Retinal endoilluminator toxicity of xenon and light-emitting diode (LED) light source: Rabbit model. Cutan. Ocul. Toxicol. 2014, 33, 192–196. [Google Scholar] [CrossRef]
- Menezo, J.L.; Peris-Martínez, C.; Esteve, J.T. Macular phototrauma after cataract extraction and multifocal lens implantation: Case report. Eur. J. Ophthalmol. 2002, 12, 247–249. [Google Scholar] [CrossRef]
- Byrnes, G.A.; Antoszyk, A.N.; Mazur, D.O.; Kao, T.C.; Miller, S.A. Photic maculopathy after extracapsular cataract surgery: A prospective study. Ophthalmology 1992, 99, 731–738. [Google Scholar] [CrossRef]
- Kweon, E.Y.; Ahn, M.; Lee, D.W.; You, I.C.; Kim, M.J.; Cho, N.C. Operating microscope light-induced phototoxic maculopathy after transscleral sutured posterior chamber intraocular lens implantation. Retina 2009, 29, 1491–1495. [Google Scholar] [CrossRef]
- Manzouri, B.; Egan, C.A.; Hykin, P.G. Phototoxic maculopathy following uneventful cataract surgery in a predispose patient. Br. J. Ophthalmol. 2002, 86, 705–706. [Google Scholar] [CrossRef]
- Sauder, G.; Degenring, R.F.; Jaeger, M.; Heyer, C.; Jonas, J.B. Phototoxic maculopathy after secondary intraocular lens implantation. J. Cataract Refract. Surg. 2004, 30, 2620–2622. [Google Scholar] [CrossRef]
- Gomolin, J.E.; Koenekoop, R.K. Presumed photic retinopathy after cataract surgery: An angiographic study. Can. J. Ophthalmol. 1993, 28, 221–224. [Google Scholar]
- Kuhn, F.; Morris, R.; Massey, M. Photic Retinal InjurjrFrom Endoillumination During Vitrectomy. Am. J. Ophthalmol. 1991, 111, 42–46. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, K.S.; Lee, W.K. Outer retinal changes in endoilluminator-induced phototoxic maculopathy evident on spectral-domain optical coherence tomography. Clin. Exp. Optom. 2015, 98, 381–384. [Google Scholar] [CrossRef]
- Kingham, J.D. Photic maculopathy in young males with intraocular foreign body. Mil. Med. 1991, 156, 44–47. [Google Scholar] [CrossRef]
- Jiang, F.; Shi, Y.; Wang, Z.; Yang, X. Case series of photic maculopathy associated with exposure to a plasma flash induced by a femtosecond laser. Lasers Surg. Med. 2022, 54, 631–638. [Google Scholar] [CrossRef]
- de Juan-Marcos, L.; Cañete-Campos, C.; Cruz-González, F.; López-Corral, A.; Hernández-Galilea, E. Bilateral macular injury caused by a femtosecond laser. Arch. Soc. Esp. Oftalmol. 2014, 89, 459–462. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, F.; Song, Y.; Peng, C.; Sheng, S.; Li, X. Accidental Macular Injury from Prolonged Viewing of a Plasma Flash Produced by a Femtosecond Laser. Ophthalmology 2010, 117, 972–975. [Google Scholar] [CrossRef]
- Rai, N.; Thuladar, L.; Brandt, F.; Arden, G.B.; Berninger, T.A. Solar retinopathy: A study from Nepal and from Germany. Doc. Ophthalmol. 1998, 95, 99–108. [Google Scholar] [CrossRef]
- Aygün, F.B.; Tellioğlu, H.T.; Kadayıfcılar, S. Impact of Solar Eclipses on Vision: Insights from Optical Coherence Tomography and Optical Coherence Tomography Angiography Analysis. Curr. Eye Res. 2024, 49, 988–995. [Google Scholar] [CrossRef]
- Abdellah, M.M.; Mostafa, E.M.; Anber, M.A.; El Saman, I.S.; Eldawla, M.E. Solar maculopathy: Prognosis over one year follow up. BMC Ophthalmol. 2019, 19, 201. [Google Scholar] [CrossRef]
- Lodha, D.S.; Ramesh, B.; Takkar, B. Rise and Set of Solar Retinopathy on OCT. Ophthalmol. Retin. 2022, 6, 160. [Google Scholar] [CrossRef]
- Klemencic, S.; McMahon, J.; Upadhyay, S.; Messner, L. Spectral domain optical coherence tomography as a predictor of visual function in chronic solar maculopathy. Optom. Vis. Sci. 2011, 88, 1014–1019. [Google Scholar] [CrossRef]
- Combillet, F.; Saunier, V.; Rougier, M.B.; Delyfer, M.N.; Korobelnik, J.-F. Multimodal imaging in a case of self-inflicted laser-induced maculopathy. Eur. J. Ophthalmol. 2016, 26, e155–e157. [Google Scholar] [CrossRef]
- Rabiolo, A.; Sacconi, R.; Giuffrè, C.; Corbelli, E.; Carnevali, A.; Querques, L.; Sarraf, D.; Freund, K.B.; Sadda, S.; Bandello, F.; et al. Self-Inflicted Laser Handheld Laser-Induced Maculopathy: A Novel Ocular Manifestation of Factitious Disorder. Retin. Cases Brief Rep. 2018, 12, S46–S50. [Google Scholar] [CrossRef]
- Tomasso, L.; Benatti, L.; La Spina, C.; Lattanzio, R.; Baldin, G.; Carnevali, A.; De Vitis, L.A.; Querques, L.; Bandello, F.; Querques, G. Optical coherence tomography angiography findings in laser maculopathy. Eur. J. Ophthalmol. 2017, 27, e13–e15. [Google Scholar] [CrossRef] [PubMed]
- Comander, J.; Gardiner, M.; Loewenstein, J. High-resolution optical coherence tomography findings in solar maculopathy and the differential diagnosis of outer retinal holes. Am. J. Ophthalmol. 2011, 152, 413–419.e6. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.Y.; Mrejen, S.; Nghiem-Buffet, S.; Dubois, L.; Fajnkuchen, F.; Gaudric, A. Outer Foveal Microdefects. Ophthalmol. Retin. 2021, 5, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Bonyadi, M.H.J. Early and late spectral domain optical coherence tomography features of acute welding maculopathy. J. Ophthalmic Vis. Res. 2013, 8, 391–392. [Google Scholar]
- Wu, C.Y.; Jansen, M.E.; Andrade, J.; Chui, T.Y.P.; Do, A.T.; Rosen, R.B.; Deobhakta, A. Acute Solar Retinopathy Imaged With Adaptive Optics, Optical Coherence Tomography Angiography, and En Face Optical Coherence Tomography. JAMA Ophthalmol. 2018, 136, 82–85. [Google Scholar] [CrossRef]
- Querques, L.; Querques, G.; Cascavilla, M.L.; Triolo, G.; Lattanzio, R.; Introini, U.; Bandello, F. Natural course of photic maculopathy secondary to uncomplicated cataract surgery. Clin. Exp. Optom. 2014, 97, 175–177. [Google Scholar] [CrossRef]
- Schatz, P.; Eriksson, U.; Ponjavic, V.; Andréasson, S. Multifocal electroretinography and optical coherence tomography in two patients with solar retinopathy. Acta Ophthalmol. Scand. 2004, 82, 476–480. [Google Scholar] [CrossRef]
- Stangos, A.N.; Petropoulos, I.K.; Pournaras, J.-A.C.; Zaninetti, M.; Borruat, F.-X.; Pournaras, C.J. Optical coherence tomography and multifocal electroretinogram findings in chronic solar retinopathy. Am. J. Ophthalmol. 2007, 144, 131–134. [Google Scholar] [CrossRef]
- Dell’omo, R.; Konstantopoulou, K.; Wong, R.; Pavesio, C. Presumed idiopathic outer lamellar defects of the fovea and chronic solar retinopathy: An OCT and fundus autofluorescence study. Br. J. Ophthalmol. 2009, 93, 1483–1487. [Google Scholar] [CrossRef]
- Jourieh, M. Solar retinopathy: A literature review. Oman J. Ophthalmol. 2024, 17, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Youssef, P.N.; Sheibani, N.; Albert, D.M. Retinal light toxicity. Eye 2010, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, L.S.; Idil, A.; Can, D. Early and late visual prognosis in solar retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 1995, 233, 801–804. [Google Scholar] [CrossRef]
- Stoyanovsky, D.A.; Goldman, R.; Darrow, R.M.; Organisciak, D.T.; Kagan, V.E. Endogenous ascorbate regenerates vitamin E in the retina directly and in combination with exogenous dihydrolipoic acid. Curr. Eye Res. 1995, 14, 181–189. [Google Scholar] [CrossRef] [PubMed]
- D’angelo, A.; Vitiello, L.; Gagliardi, V.; Salerno, G.; De Pascale, I.; Coppola, A.; Abbinante, G.; Pellegrino, A.; Giannaccare, G. The Role of Oral Supplementation for the Management of Age-Related Macular Degeneration: A Narrative Review. J. Pers. Med. 2024, 14, 653. [Google Scholar] [CrossRef]
- Rosner, M.; Lam, T.T.; Fu, J.; Tso, M.O.M. Methylprednisolone ameliorates retinal photic injury in rats. Arch. Ophthalmol. 1992, 110, 857–861. [Google Scholar] [CrossRef]
- Nakamura, M.; Komatsu, K.; Katagiri, S.; Hayashi, T.; Nakano, T. Reconstruction of Photoreceptor Outer Layers after Steroid Therapy in Solar Retinopathy. Case Rep. Ophthalmol. Med. 2018, 2018, 7850467. [Google Scholar] [CrossRef]
- Marashi, A.; Baba, M.; Zazo, A. Managing solar retinopathy with suprachoroidal triamcinolone acetonide injection in a young girl: A case report. J. Med. Case Rep. 2021, 15, 577. [Google Scholar] [CrossRef]
- Mtanes, K.; Mimouni, M.; Zayit-Soudry, S. Laser Pointer-Induced Maculopathy: More Than Meets the Eye. J. Pediatr. Ophthalmol. Strabismus 2018, 55, 312–318. [Google Scholar] [CrossRef]
- Farassat, N.; Boehringer, D.; Luebke, J.; Ness, T.; Agostini, H.; Reinhard, T.; Lagrèze, W.A.; Reich, M. Incidence and long-term outcome of laser pointer maculopathy in children. Int. Ophthalmol. 2023, 43, 2397–2405. [Google Scholar] [CrossRef]
- Bouzas, E.A.; Moret, P.; Pournaras, C.J. Central serous chorioretinopathy complicating solar retinopathy treated with glucocorticoids. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 237, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Lee, L.R. Solar retinopathy and associated optical coherence tomography findings. Clin. Exp. Optom. 2004, 87, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.; Slotnick, S. Physiological causes of solar maculopathy. Letter. Optom.-J. Am. Optom. Assoc. 2012, 83, 6. [Google Scholar] [CrossRef]
- Zhang, C.; Dang, G.; Zhao, T.; Wang, D.; Su, Y.; Qu, Y. Predictive value of spectral-domain optical coherence tomography features in assessment of visual prognosis in eyes with acute welding arc maculopathy. Int. Ophthalmol. 2018, 39, 1081–1088. [Google Scholar] [CrossRef]
- Jorge, R.; Costa, R.A.; Quirino, L.S.; Paques, M.W.; Calucci, D.; Cardillo, J.A.; Scott, I.U. Optical coherence tomography findings in patients with late solar retinopathy. Am. J. Ophthalmol. 2004, 137, 1139–1142. [Google Scholar] [CrossRef]
| Etiology | Clinical and Diagnostic Features | Optical Coherence Tomography (OCT) | Treatment |
|---|---|---|---|
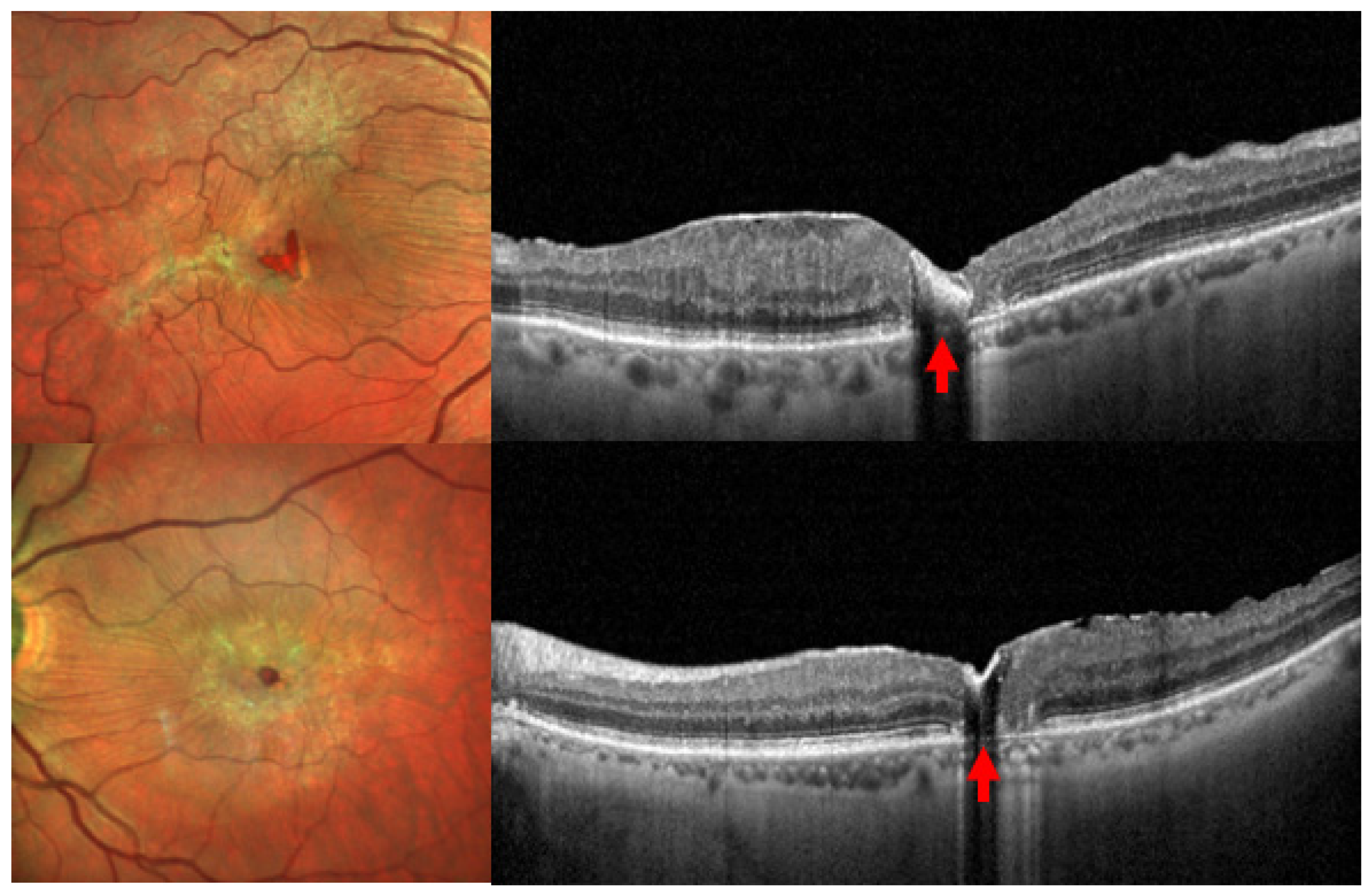
| Solar retinopathy | Bilateral but asymmetric, with greater impact on the dominant eye. Symptoms: Blurred vision → scotoma within hours. A light spot near fixation before scotoma/metamorphopsia [73]. Color vision defects: Mainly blue-yellow axis. | Acute phase [1]:
| |
| Laser pointer maculopathy | More severe forms compared with solar lesions [29]. Yellowish lesions appear in acute phase, later leading to pigment changes [1,5]. Evolution to cystic lesions in the external retina, replacing lost photoreceptors [55]. | Acute phase:
| |
| Arc welding maculopathy | Acute welding toxicity is very rare, most common due to chronic exposure. Retinal changes are frequent but have minimal impact on vision. | Acute phase:
| |
| Iatrogenic photic maculopathy secondary to ocular surgery | Clinical presentation is similar to other photic retinopathies. More extensive lesions, often above or below the fovea (due to lack of direct foveal fixation) [1,41]. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofte Zorila, M.M.; Vitiello, L.; Lixi, F.; Coppola, A.; Cukurova, F.; Pellegrino, A.; Giannaccare, G. Photic Retinopathy: Diagnosis and Management of This Phototoxic Maculopathy. Life 2025, 15, 639. https://doi.org/10.3390/life15040639
Timofte Zorila MM, Vitiello L, Lixi F, Coppola A, Cukurova F, Pellegrino A, Giannaccare G. Photic Retinopathy: Diagnosis and Management of This Phototoxic Maculopathy. Life. 2025; 15(4):639. https://doi.org/10.3390/life15040639
Chicago/Turabian StyleTimofte Zorila, Mihaela Madalina, Livio Vitiello, Filippo Lixi, Alessia Coppola, Feyza Cukurova, Alfonso Pellegrino, and Giuseppe Giannaccare. 2025. "Photic Retinopathy: Diagnosis and Management of This Phototoxic Maculopathy" Life 15, no. 4: 639. https://doi.org/10.3390/life15040639
APA StyleTimofte Zorila, M. M., Vitiello, L., Lixi, F., Coppola, A., Cukurova, F., Pellegrino, A., & Giannaccare, G. (2025). Photic Retinopathy: Diagnosis and Management of This Phototoxic Maculopathy. Life, 15(4), 639. https://doi.org/10.3390/life15040639








