Ketoprofen Lysine Salt vs. Ketoprofen Acid: Assessing the Evidence for Enhanced Safety and Efficacy
Abstract
:1. Introduction
2. Materials and Methods
3. Discussion of Findings
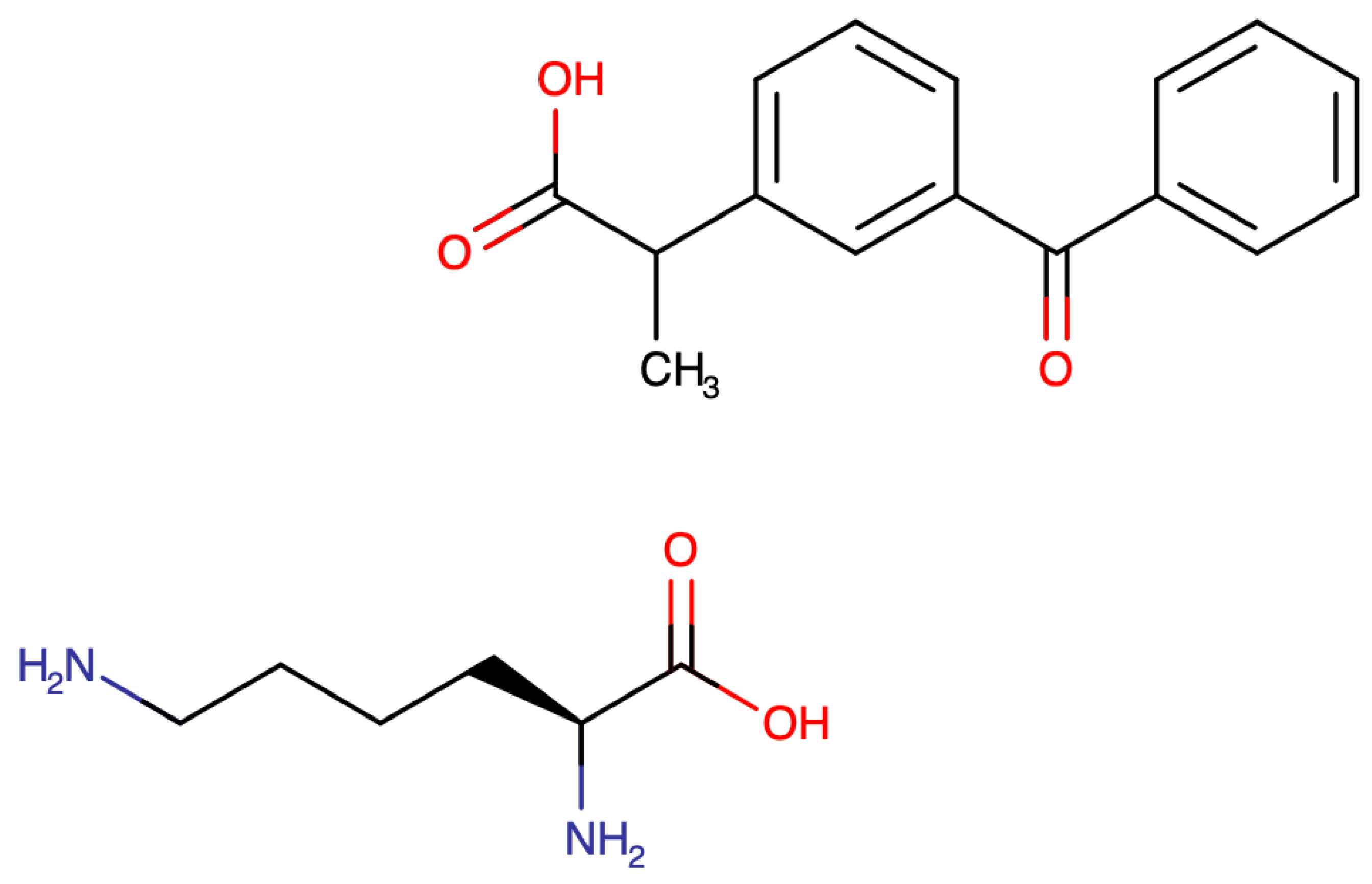
3.1. Ketoprofen
3.2. Ketoprofen Lysine Salt
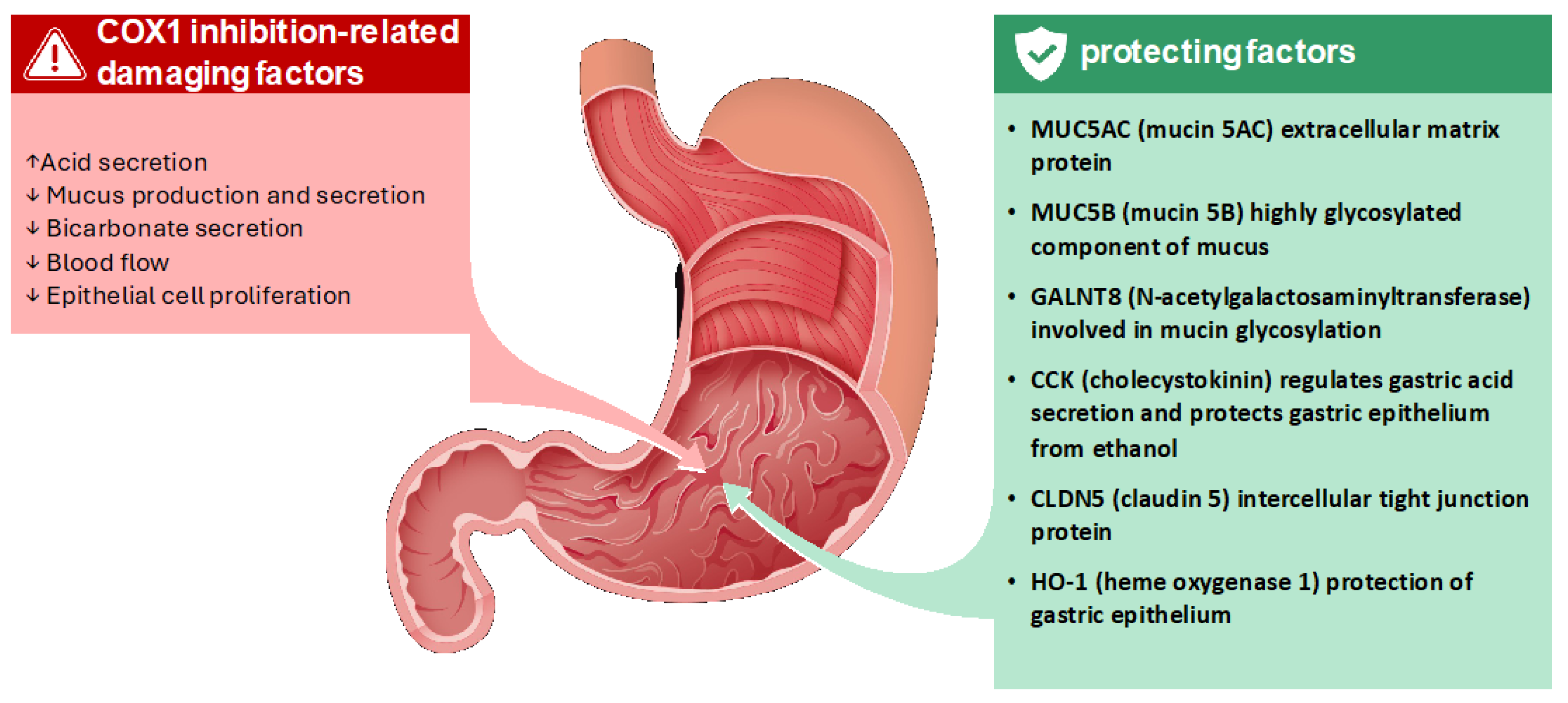
- MUC5B, a highly glycosylated component of mucus, forms a physical barrier that protects the epithelium from acid and pepsin [31];
- GALNT8, involved in mucin glycosylation, is essential for the proper formation and function of the mucus layer [32];
- CCK regulates gastric acid secretion and exerts protective effects on the gastric epithelium against damage from agents like ethanol [33];
- CLDN5, a protein of tight junctions, strengthens the intercellular connections, preventing the paracellular passage of damaging substances [34].
3.3. KLS Pharmacokinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxy-2-nonenal |
| CCK | cholecystokinin |
| Ce50 | concentration that produces one-half the maximum PID |
| CLDN5 | claudin 5 |
| Cmax | peak plasma concentrations |
| COX | cyclooxygenase |
| CSE | COX-independent cystathionine-γ-lyase |
| CV | cardiovascular |
| eNOS | nitric oxide synthase |
| GALNT8 | N-acetylgalactosaminyltransferase |
| HO-1 | heme oxygenase-1 |
| IBU | ibuprofen |
| KA | ketoprofen acid (KLS) |
| KLS | ketoprofen lysine salt |
| LD | linear dichroism |
| MDA | malondialdehyde |
| MUC5B | mucin 5B |
| NSAID | non-steroidal anti-inflammatory drugs |
| PD | pharmacodynamics |
| PID | pain intensity reduction |
| PK | pharmacokinetics |
| ROS | reactive oxygen species |
| Tmax | time to max concentration |
| UGIB | Upper Gastrointestinal Bleeding |
References
- Kantor, T.G. Ketoprofen: A Review of Its Pharmacologic and Clinical Properties. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1986, 6, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Raska, M.; Kriegova, E.; Goodman, S.B. Inflammation and its resolution and the musculoskeletal system. J. Orthop. Transl. 2017, 10, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Sci. 2019, 2019, 3418975. [Google Scholar] [CrossRef]
- Neha, K.; Wakode, S. Contemporary advances of cyclic molecules proposed for inflammation. Eur. J. Med. Chem. 2021, 221, 113493. [Google Scholar] [CrossRef]
- Meyer-Kirchrath, J.; Schror, K. Cyclooxygenase-2 inhibition and side-effects of non-steroidal antiinflammatory drugs in the gastrointestinal tract. Curr. Med. Chem. 2000, 7, 1121–1129. [Google Scholar] [CrossRef]
- D’angelo, M.; Brandolini, L.; Catanesi, M.; Castelli, V.; Giorgio, C.; Alfonsetti, M.; Tomassetti, M.; Zippoli, M.; Benedetti, E.; Cesta, M.C.; et al. Differential Effects of Nonsteroidal Anti-Inflammatory Drugs in an In Vitro Model of Human Leaky Gut. Cells 2023, 12, 728. [Google Scholar] [CrossRef]
- Carbone, C.; Rende, P.; Comberiati, P.; Carnovale, D.; Mammí, M.; De Sarro, G. The safety of ketoprofen in different ages. J. Pharmacol. Pharmacother. 2013, 4, S99–S103. [Google Scholar] [CrossRef]
- Castellsague, J.; Riera-Guardia, N.; Calingaert, B.; Varas-Lorenzo, C.; Fourrier-Reglat, A.; Nicotra, F.; Sturkenboom, M.; Perez-Gutthann, S. Individual NSAIDs and upper gastrointestinal complications: A systematic review and meta-analysis of observational studies (the SOS project). Drug Saf. 2012, 35, 1127–1146. [Google Scholar] [CrossRef]
- Varrassi, G.; Alon, E.; Bagnasco, M.; Lanata, L.; Mayoral-Rojals, V.; Paladini, A.; Pergolizzi, J.V.; Perrot, S.; Scarpignato, C.; Tölle, T. Towards an Effective and Safe Treatment of Inflammatory Pain: A Delphi-Guided Expert Consensus. Adv. Ther. 2019, 36, 2618–2637. [Google Scholar] [CrossRef] [PubMed]
- Kuczyńska, J.; Nieradko-Iwanicka, B. Future prospects of ketoprofen in improving the safety of the gastric mucosa. Biomed. Pharmacother. 2021, 139, 111608. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Shimokawa, O.; Kaneko, T.; Nagano, Y.; Rai, K.; Hyodo, I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J. Clin. Biochem. Nutr. 2011, 48, 107–111. [Google Scholar] [CrossRef]
- Bjarnason, I.; Scarpignato, C.; Holmgren, E.; Olszewski, M.; Rainsford, K.D.; Lanas, A. Mechanisms of Damage to the Gastrointestinal Tract From Nonsteroidal Anti-Inflammatory Drugs. Gastroenterology 2018, 154, 500–514. [Google Scholar] [CrossRef]
- Brandolini, L.; D’Angelo, M.; Antonosante, A.; Villa, S.; Cristiano, L.; Castelli, V.; Benedetti, E.; Catanesi, M.; Aramini, A.; Luini, A.; et al. Differential protein modulation by ketoprofen and ibuprofen underlines different cellular response by gastric epithelium. J. Cell. Physiol. 2017, 233, 2304–2312. [Google Scholar] [CrossRef]
- Cimini, A.; Brandolini, L.; Gentile, R.; Cristiano, L.; Menghini, P.; Fidoamore, A.; Antonosante, A.; Benedetti, E.; Giordano, A.; Allegretti, M. Gastroprotective effects of L-lysine salification of ketoprofen in ethanol-injured gastric mucosa. J. Cell. Physiol. 2014, 230, 813–820. [Google Scholar] [CrossRef]
- Puttini, P.S.; Atzeni, F.; Lanata, L.; Bagnasco, M.; Colombo, M.; Fischer, F.; D’Imporzano, M. Pain and ketoprofen: What is its role in clinical practice? Reumatismo 2010, 62, 172–188. [Google Scholar] [CrossRef]
- Registration Dossier—ECHA. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/12427/7/13 (accessed on 2 April 2025).
- Jamali, F.; Brocks, D.R. Clinical Pharmacokinetics of ketoprofen and its enantiomers. Clin. Pharmacokinet. 1990, 19, 197–217. [Google Scholar] [CrossRef]
- Stiller, C.; Hjemdahl, P. Lessons from 20 years with COX-2 inhibitors: Importance of dose–response considerations and fair play in comparative trials. J. Intern. Med. 2022, 292, 557–574. [Google Scholar] [CrossRef]
- Bauer, J.; Ripperger, A.; Frantz, S.; Ergün, S.; Schwedhelm, E.; Benndorf, R.A. Pathophysiology of isoprostanes in the cardiovascular system: Implications of isoprostane-mediated thromboxane A2 receptor activation. Br. J. Pharmacol. 2014, 171, 3115–3131. [Google Scholar] [CrossRef]
- Schjerning, A.-M.; McGettigan, P.; Gislason, G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat. Rev. Cardiol. 2020, 17, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Baigent, C.; Godwin, J.; Halls, H.; Emberson, J.R.; Patrono, C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006, 332, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Trelle, S.; Reichenbach, S.; Wandel, S.; Hildebrand, P.; Tschannen, B.; Villiger, P.M.; Egger, M.; Jüni, P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ 2011, 342, c7086. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, L.; Antonosante, A.; Giorgio, C.; Bagnasco, M.; D’angelo, M.; Castelli, V.; Benedetti, E.; Cimini, A.; Allegretti, M. NSAIDs-dependent adaption of the mitochondria-proteasome system in immortalized human cardiomyocytes. Sci. Rep. 2020, 10, 18337. [Google Scholar] [CrossRef]
- Masclee, G.M.C.; Straatman, H.; Arfè, A.; Castellsague, J.; Garbe, E.; Herings, R.; Kollhorst, B.; Lucchi, S.; Perez-Gutthann, S.; Romio, S.; et al. Risk of acute myocardial infarction during use of individual NSAIDs: A nested case-control study from the SOS project. PLoS ONE 2018, 13, e0204746. [Google Scholar] [CrossRef]
- Marmo, E.; Ottavo, R.; Giordano, L.; Paone, G.; Falcone, O.; Spaziante, G.; Visone, C.; Campidonico, U. Experimental assessment of some pharmacodynamic features of ketoprofen lysine. Pain relief activity, antipyretic effects, anti-inflammatory activity, anti-platelet aggregation activity and interference with the biosynthesis of prostaglandins. Arch. Sci. Med. 1980, 137, 387–404. [Google Scholar]
- Hayamizu, K.; Oshima, I.; Nakano, M. Comprehensive Safety Assessment of l-Lysine Supplementation from Clinical Studies: A Systematic Review. J. Nutr. 2020, 150, 2561S–2569S. [Google Scholar] [CrossRef]
- Lutnicki, K.; Wróbel, J.; Ledwozyw, A.; Trebas-Pietraś, E. The effect of ethyl alcohol on peroxidation processes and activity of antioxidant enzymes in rat’s gastric mucosa. Arch. Vet. Pol. 1992, 32, 117–123. [Google Scholar]
- Aburaya, M.; Tanaka, K.-I.; Hoshino, T.; Tsutsumi, S.; Suzuki, K.; Makise, M.; Akagi, R.; Mizushima, T. Heme oxygenase-1 protects gastric mucosal cells against non-steroidal anti-inflammatory Drugs. J. Biol. Chem. 2006, 281, 33422–33432. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Bergstrom, K.S.B.; Xia, L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology 2013, 23, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.E.; Schenk, S.; Nustede, R.; Holst, J.J.; Fölsch, U.R.; Creutzfeldt, W. Cholecystokinin is a negative regulator of gastric acid secretion and postprandial release of gastrin in humans. Gastroenterology 1994, 107, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight junctions: From molecules to gastrointestinal diseases. Tissue Barriers 2022, 11, 2077620. [Google Scholar] [CrossRef] [PubMed]
- Kuczyńska, J.; Nieradko-Iwanicka, B. The effect of ketoprofen lysine salt on mucosa of rat stomach after ethyl alcohol intoxication. Biomed. Pharmacother. 2021, 141, 111938. [Google Scholar] [CrossRef]
- Stevens, C.E.; Hume, I.D. Comparative Physiology of the Vertebrate Digestive System; Cambridge University Press: Cambridge, UK, 1998; 400p. [Google Scholar]
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef]
- Ziegler, A.; Gonzalez, L.; Blikslager, A. Large Animal Models: The Key to Translational Discovery in Digestive Disease Research. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 716–724. [Google Scholar] [CrossRef]
- Novelli, R.; Aramini, A.; Boccella, S.; Bagnasco, M.; Cattani, F.; Ferrari, M.P.; Goisis, G.; Minnella, E.M.; Allegretti, M.; Pace, V. Ketoprofen lysine salt has a better gastrointestinal and renal tolerability than ketoprofen acid: A comparative tolerability study in the Beagle dog. Biomed. Pharmacother. 2022, 153, 113336. [Google Scholar] [CrossRef]
- Cailleteau, J.G. Ketoprofen in dentistry: A pharmacologic review. Oral Surg. Oral Med. Oral Pathol. 1988, 66, 620–624. [Google Scholar] [CrossRef]
- Murakami, T. Absorption sites of orally administered drugs in the small intestine. Expert Opin. Drug Discov. 2017, 12, 1219–1232. [Google Scholar] [CrossRef]
- Marseglia, G.L.; Ciprandi, G. Clinical use of ketoprofen lysine salt: A reappraisal in adolescents with acute respiratory infections. Allergol. Immunopathol. 2023, 51, 76–82. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Mannila, A.; Kokki, H.; Heikkinen, M.; Laisalmi, M.; Lehtonen, M.; Louhisto, H.L.; Järvinen, T.; Savolainen, J. Cerebrospinal fluid distribution of ketoprofen after intravenous administration in young children. Clin. Pharmacokinet. 2006, 45, 737–743. [Google Scholar] [CrossRef] [PubMed]
- McCormack, K.; Brune, K. Dissociation between the antinociceptive and anti-inflammatory effects of the nonsteroidal anti-inflammatory drugs. A survey of their analgesic efficacy. Drugs 1991, 41, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I.; Scarpignato, C.; Takeuchi, K.; Rainsford, K.D. Determinants of the short-term gastric damage caused by NSAIDs in man. Aliment. Pharmacol. Ther. 2007, 26, 95–106. [Google Scholar] [CrossRef]
- Lapicque, F.; Jankowski, R.; Netter, P.; Bannwarth, B.; Guillemin, C.; Bene, M.-C.; Monot, C.; Wayoff, M. Drug assay in ground tissues: Example of ketoprofen diffusion into tonsillar tissue. J. Pharm. Sci. 1990, 79, 791–795. [Google Scholar] [CrossRef]
- Roda, A.; Sabatini, L.; Mirasoli, M.; Baraldini, M.; Roda, E. Bioavailability of a new ketoprofen formulation for once-daily oral administration. Int. J. Pharm. 2002, 241, 165–172. [Google Scholar] [CrossRef]
- Panerai, A.E.; Lanata, L.; Ferrari, M.; Bagnasco, M. A new ketoprofen lysine salt formulation: 40 mg orodispersible granules. Trends Med. 2012, 12, 159–167. [Google Scholar]

| NSAIDs | ORpooled | 95% Cls |
|---|---|---|
| Ketorolac | 1.8 | 1.49 to 2.18 |
| Indometacin | 1.51 | 1.28 to1.80 |
| Etoricoxib | 1.39 | 1.24 to 1.57 |
| Diclofenac | 1.28 | 1.22 to 1.34 |
| Ibuprofen | 1.25 | 1.18 to 1.33 |
| Celecoxib | 1.1 | 1.05 to 1.25 |
| Ketoprofen | 1.00 | 0.86 to 1.16 |
| KLS Granules | KLS Sachet | |
|---|---|---|
| dose mg | 40 | 80 |
| Tmax min | 15 | 15 |
| Ce50 mg/mL | 0.3 (Cl 0.1–0.5) | 0.3 (Cl 0.1–0.5) |
| T to Ce50 min | 6 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graziosi, A.; Senatore, M.; Gazzaniga, G.; Agliardi, S.; Pani, A.; Scaglione, F. Ketoprofen Lysine Salt vs. Ketoprofen Acid: Assessing the Evidence for Enhanced Safety and Efficacy. Life 2025, 15, 659. https://doi.org/10.3390/life15040659
Graziosi A, Senatore M, Gazzaniga G, Agliardi S, Pani A, Scaglione F. Ketoprofen Lysine Salt vs. Ketoprofen Acid: Assessing the Evidence for Enhanced Safety and Efficacy. Life. 2025; 15(4):659. https://doi.org/10.3390/life15040659
Chicago/Turabian StyleGraziosi, Agnese, Michele Senatore, Gianluca Gazzaniga, Stefano Agliardi, Arianna Pani, and Francesco Scaglione. 2025. "Ketoprofen Lysine Salt vs. Ketoprofen Acid: Assessing the Evidence for Enhanced Safety and Efficacy" Life 15, no. 4: 659. https://doi.org/10.3390/life15040659
APA StyleGraziosi, A., Senatore, M., Gazzaniga, G., Agliardi, S., Pani, A., & Scaglione, F. (2025). Ketoprofen Lysine Salt vs. Ketoprofen Acid: Assessing the Evidence for Enhanced Safety and Efficacy. Life, 15(4), 659. https://doi.org/10.3390/life15040659








