Chemical Constituents and α-Glucosidase Inhibitory Activities of the Leaves of Embelia parviflora—In Vitro and In Silico Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. General Experimental Procedures
2.3. Extraction and Isolation
2.4. Assay of α-Glucosidase Enzyme Inhibition
2.5. Molecular Docking
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Constituents and Chemotaxonomy Significance
3.2. α-Glucosidase Inhibitory Activity
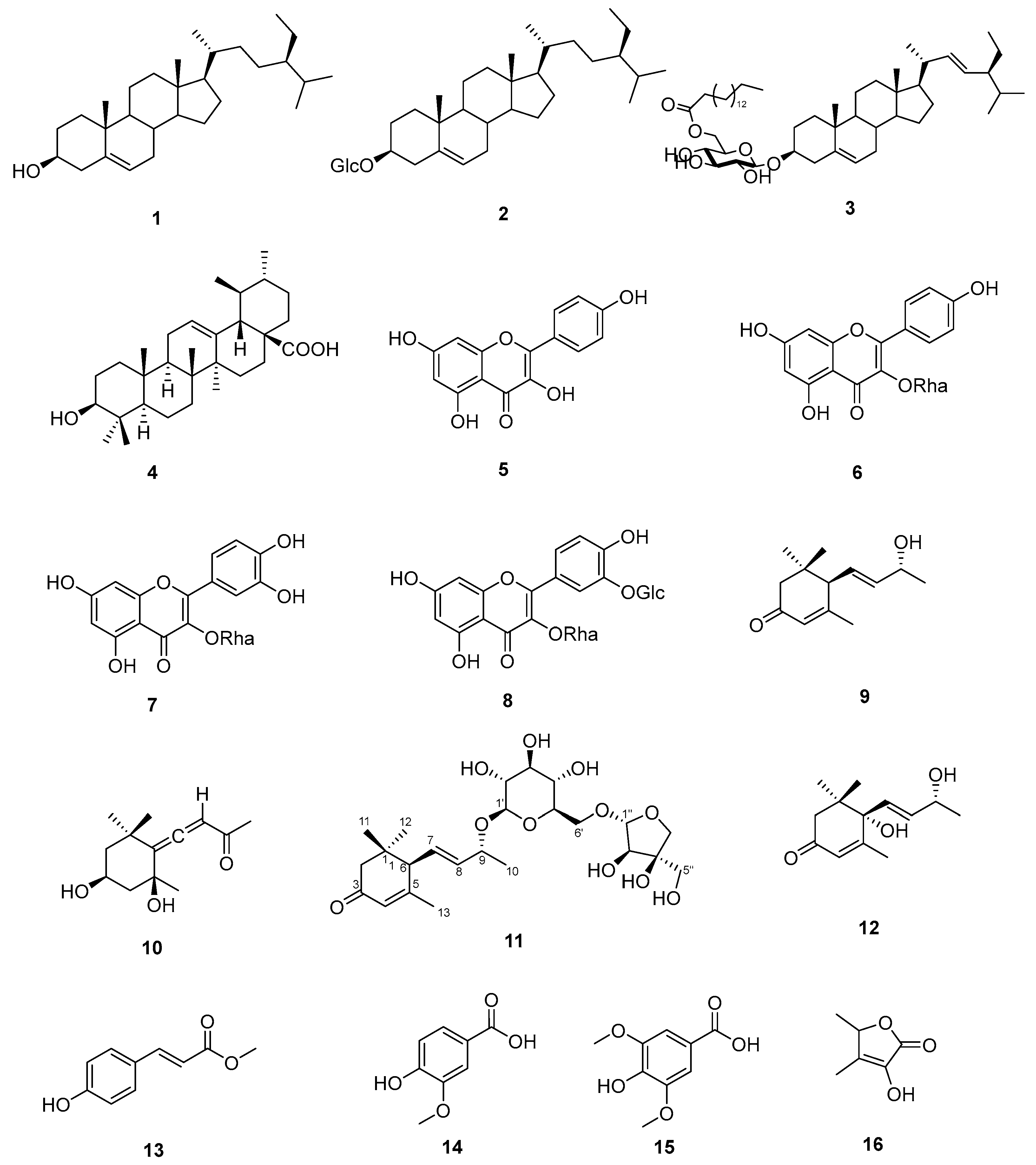
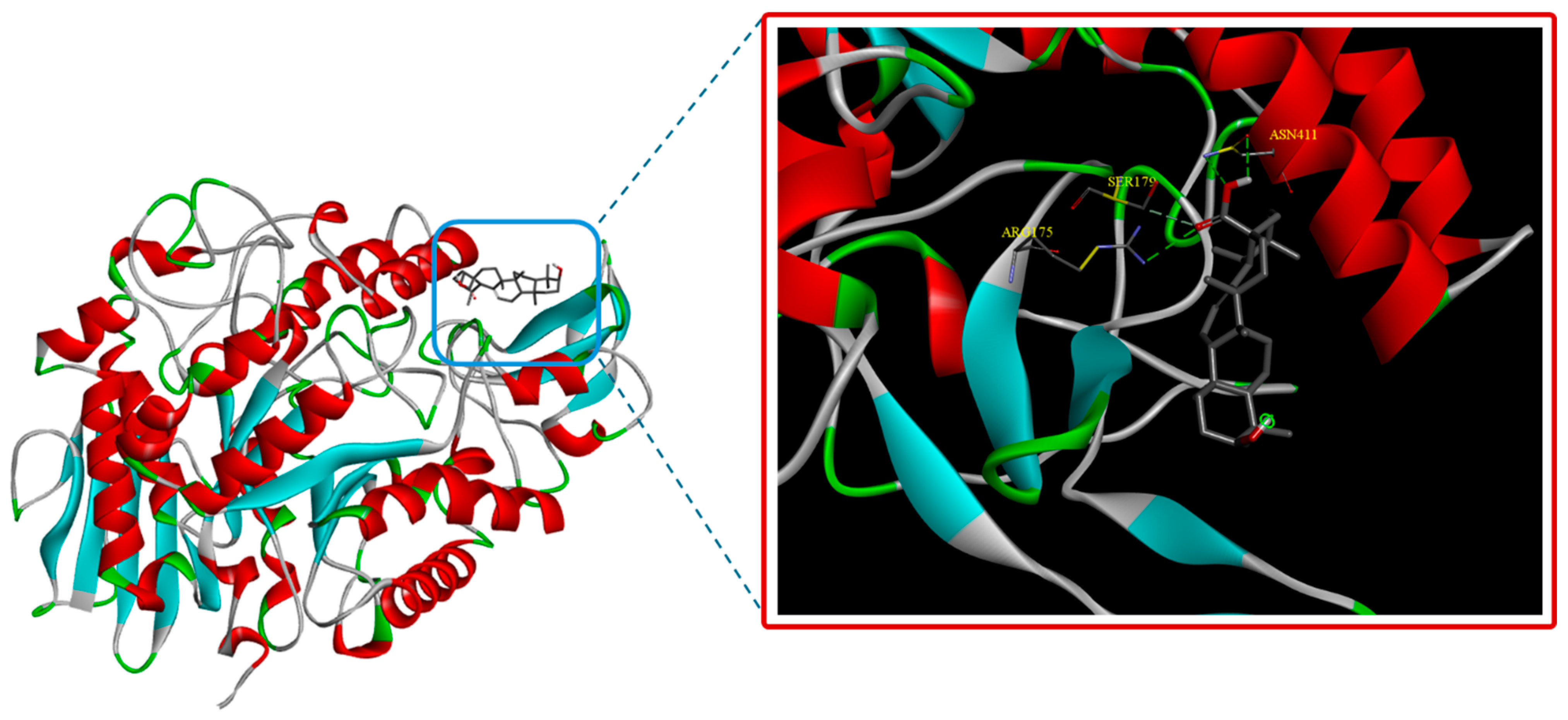
3.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubéarnès, A.; Julius, A.; Utteridge, T.M.A. A synopsis of the genus Embelia in peninsular Malaysia and Singapore. Studies in Malaysian Myrsinaceae III. Kew Bull. 2015, 70, 25. [Google Scholar] [CrossRef]
- Vijayan, K.R.; Raghu, A.V. Embelin: An HPTLC method for quantitative estimation in five species of genus Embelia Burm. f. Future J. Pharm. Sci. 2021, 7, 55. [Google Scholar] [CrossRef]
- Sharma, V.; Gautam, D.N.S.; Radu, A.F.; Behl, T.; Bungau, S.G.; Vesa, C.M. Reviewing the traditional/modern uses, phytochemistry, essential oils/extracts and pharmacology of Embelia ribes Burm. Antioxidants 2022, 11, 1359. [Google Scholar] [CrossRef] [PubMed]
- Manguro, L.O.A.; Okwiri, S.O.; Lemmen, P. Oleanane-type triterpenes of Embelia schimperi leaves. Phytochemistry 2006, 67, 2641–2650. [Google Scholar] [CrossRef]
- Bouzeko, I.L.T.; Ndontsa, B.L.; Nguekeu, Y.M.M.; Awouafack, M.D.; Wong, C.P.; Mpetga, J.D.S.; Mbouangouere, R.; Tane, P.; Morita, H. A new alkylbenzoquinone from Embelia rowlandii Gilg. (Myrsinaceae). Nat. Prod. Res. 2019, 33, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Manguro, L.O.A.; Ugi, I.; Lemmen, P. Flavonol glycosides from the leaves of Embelia keniensis. J. Chin. Chem. Soc. 2005, 52, 201–208. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, J.P.; Li, C.Y.; Zhu, L.J.; Zhang, X.; Wang, J.H.; Yao, X.S. Flavonoid glycosides from the fruits of Embelia ribes and their anti-oxidant and α-glucosidase inhibitory activities. J. Asian Nat. Prod. Res. 2021, 23, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.H.; Nguyen, H.X.; Nguyen, N.T.; Le, H.N.T.; Nguyen, M.T.T. α-Glucosidase inhibitors from the stems of Embelia ribes. Phytother. Res. 2014, 28, 1632–1636. [Google Scholar] [CrossRef]
- Dang, P.H.; Nguyen, N.T.; Nguyen, H.X.; Nguyen, L.B.; Le, T.H.; Van Do, T.N.; Can, M.V.; Nguyen, M.T.T. α-Glucosidase inhibitors from the leaves of Embelia ribes. Fitoterapia 2015, 100, 201–207. [Google Scholar] [CrossRef]
- Yang, L.J. Chemical constituents from the fruits of Embelia laeta. Chin. Pharm. J. 2016, 24, 1179–1182. [Google Scholar]
- Feng, X.; Li, Y.H.; Liang, C.Y.; Wang, H.; Zhang, X.W. Chemical constituents from Embelia laeta. Zhong Yao Cai (J. Chin. Med. Mater.) 2013, 36, 1947–1949. [Google Scholar]
- Chen, J.P.; Zhu, L.J.; Su, X.X.; Zhang, K.X.; Zhang, X.; Wang, J.H.; Yao, X.S. New alkylresorcinols from the fruits of Embelia ribes. Fitoterapia 2018, 128, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Machocho, A.K.; Kiprono, P.C.; Grinberg, S.; Bittner, S. Pentacyclic triterpenoids from Embelia schimperi. Phytochemistry 2003, 62, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Manguro, L.O.A.; Ugi, I.; Lemen, P. Further flavonol glycosides of Embelia schimperi leaves. Bull. Chem. Soc. Ethiop. 2004, 18, 51–57. [Google Scholar] [CrossRef]
- Mahendran, S.; Badami, S.; Maithili, V. Evaluation of antidiabetic effect of embelin from Embelia ribes in alloxan induced diabetes in rats. Biomed. Prev. Nutr. 2011, 1, 25–31. [Google Scholar] [CrossRef]
- Bhandari, U.; Kanojia, R.; Pillai, K.K. Effect of ethanolic extract of Embelia ribes on dyslipidemia in diabetic rats. J. Diabetes Res. 2002, 3, 159–162. [Google Scholar] [CrossRef]
- Bhandari, U.; Jain, N.; Pillai, K.K. Further studies on antioxidant potential and protection of pancreatic β-cells by Embelia ribes in experimental diabetes. J. Diabetes Res. 2007, 2007, 015803. [Google Scholar] [CrossRef] [PubMed]
- Durg, S.; Veerapur, V.P.; Neelima, S.; Dhadde, S.B. Antidiabetic activity of Embelia ribes, embelin and its derivatives: A systematic review and meta-analysis. Biomed. Pharmacother. 2017, 86, 195–204. [Google Scholar] [CrossRef]
- Bhandari, U.; Chaudhari, H.S.; Khanna, G.; Najmi, A.K. Antidiabetic effects of Embelia ribes extract in high fat diet and low dose streptozotocin-induced type 2 diabetic rats. Front. Life Sci. 2013, 7, 186–196. [Google Scholar] [CrossRef]
- Bhandari, U.; Jain, N.; Ansari, M.N.; Pillai, K.K. Beneficial effect of Embelia ribes ethanolic extract on blood pressure and glycosylated hemoglobin in streptozotocin-induced diabetes in rats. Fitoterapia 2008, 79, 351–355. [Google Scholar] [CrossRef]
- Chaudhari, H.S.; Bhandari, U.; Khanna, G. Preventive effect of embelin from Embelia ribes on lipid metabolism and oxidative stress in high-fat diet-induced obesity in rats. Planta Med. 2012, 78, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Nazish, I.; Ansari, S.H.; Arora, P. Antiobesity actions of Embelia ribes. Pharmacogn. J. 2012, 4, 73–80. [Google Scholar] [CrossRef]
- Gupta, G.; Kazmi, I.; Afzal, M.; Upadhyay, G.; Singh, R.; Habtemariam, S. Antidepressant-like activity of embelin isolated from Embelia ribes. Phytopharmacology 2013, 4, 87–95. [Google Scholar]
- Thippeswamy, B.S.; Nagakannan, P.; Shivasharan, B.D.; Mahendran, S.; Veerapur, V.P.; Badami, S. Protective effect of embelin from Embelia ribes Burm. against transient global ischemia-induced brain damage in rats. Neurotox. Res. 2011, 20, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Durg, S.; Kumar, N.; Vandal, R.; Dhadde, S.B.; Thippeswamy, B.S.; Veerapur, V.P.; Badami, S. Antipsychotic activity of embelin isolated from Embelia ribes: A preliminary study. Biomed. Pharmacother. 2017, 90, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Dhayalan, M.; Denison, M.I.J.; L, J.A.; Krishnan, K.; N, G.N. In vitro antioxidant, antimicrobial, cytotoxic potential of gold and silver nanoparticles prepared using Embelia ribes. Nat. Prod. Res. 2017, 31, 465–468. [Google Scholar] [CrossRef]
- Afzal, M.; Gupta, G.; Kazmi, I.; Rahman, M.; Upadhyay, G.; Ahmad, K.; Imam, F.; Pravez, M.; Anwar, F. Evaluation of anxiolytic activity of embelin isolated from Embelia ribes. Biomed. Aging Pathol. 2012, 2, 45–47. [Google Scholar] [CrossRef]
- Ansari, M.N.; Bhandari, U.; Islam, F.; Tripathi, C.D. Evaluation of antioxidant and neuroprotective effect of ethanolic extract of Embelia ribes Burm in focal cerebral ischemia/reperfusion-induced oxidative stress in rats. Fundam. Clin. Pharmacol. 2008, 22, 305–314. [Google Scholar] [CrossRef]
- Swamy, H.K.; Krishna, V.; Shankarmurthy, K.; Rahiman, B.A.; Mankani, K.L.; Mahadevan, K.M.; Harish, B.G.; Naika, H.R. Wound healing activity of embelin isolated from the ethanol extract of leaves of Embelia ribes Burm. J. Ethnopharmacol. 2007, 109, 529–534. [Google Scholar] [CrossRef]
- Hossan, M.S.; Fatima, A.; Rahmatullah, M.; Khoo, T.J.; Nissapatorn, V.; Galochkina, A.V.; Slita, A.V.; Shtro, A.A.; Nikolaeva, Y.; Zarubaev, V.V.; et al. Antiviral activity of Embelia ribes Burm. f. against influenza virus in vitro. Arch. Virol. 2018, 163, 2121–2131. [Google Scholar] [CrossRef]
- Rambaran, N.; Naidoo, Y.; Dwarka, D.; Mellem, J.; Thimmegowda, S.C.; Baijnath, H. In vitro antioxidant and anticancer potential of the vegetative and reproductive organs of Embelia ruminata. J. Herbs Spices Med. Plants 2024, 30, 429–441. [Google Scholar] [CrossRef]
- Lemmich, J. Anthelmintic usage of extracts of Embelia schimperi from Tanzania. J. Ethnopharmacol. 1996, 50, 35–42. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Liang, X.L.; Chen, Y.; Pang, D.; Huang, Z.; Han, Q.; Liu, W.; Chen, L. Optimization of extraction process and antioxidant activity of polysaccharide in Embelia parviflora Wall. by RSM. Med. Plant 2020, 11, 34–42. [Google Scholar] [CrossRef]
- Anh, M.T.H.; Mau, C.H.; Thuong, S.D. Morphological characteristics and sequence of trnE-trnT of Embelia parviflora Wall. ex A. DC.). TNU J. Sci. Technol. 2025, 230, 259–266. [Google Scholar] [CrossRef]
- Lu, S.H.; Li, Y.H.; Chen, Y.; Zeng, H.S.; Zhang, L.; Zheng, X.R. Study on the chemical constituents of volatile oil from different parts of Embelia parviflora Wall. ex A. DC by GC-MS. Med. Plant 2012, 3, 58–61. [Google Scholar]
- Wei, J.; Chen, Y.; Ma, X.; Qiu, M.; Qing, B.; Jiang, L.; Wen, Z.; Qin, Z. Anti-inflammatory effect of polysaccharide from Embelia parviflora Wall, on rheumatoid arthritis in rats. Med. Plant 2024, 15, 41. [Google Scholar] [CrossRef]
- Liu, W.; Que, Z.; Li, J.; Chen, Z.; Huang, Z.; Pang, D.; Chen, L.; Chen, Y. Study on sreening of effective components of Embelia parviflora for tonifying blood and its mechanism. China Pharm. 2020, 12, 293–297. [Google Scholar]
- Oanh, V.T.K.; Ha, N.T.T.; Duc, H.V.; Thuc, D.N.; Hang, N.T.M.; Thanh, L.N. New triterpene and nor-diterpene derivatives from the leaves of Adinandra poilanei. Phytochem. Lett. 2021, 46, 110–113. [Google Scholar] [CrossRef]
- Phuong, D.T.L.; Van Phuong, N.; Le Tuan, N.; Cong, N.T.; Hang, N.T.; Thanh, L.N.; Bankova, V. Antimicrobial, cytotoxic, and α-glucosidase inhibitory activities of ethanol extract and chemical constituents isolated from Homotrigona apicalis propolis—In vitro and molecular docking studies. Life 2023, 13, 1682. [Google Scholar] [CrossRef]
- Sosińska, E.; Przybylski, R.; Hazendonk, P.; Zhao, Y.Y.; Curtis, J.M. Characterisation of non-polar dimers formed during thermo-oxidative degradation of β-sitosterol. Food Chem. 2013, 139, 464–474. [Google Scholar] [CrossRef]
- Faizi, S.; Ali, M.; Saleem, R.; Irfanullah, B.S. Complete 1H and 13C NMR assignments of stigma-5-en-3-O-β-glucoside and its acetyl derivative. Magn. Reson. Chem. 2001, 39, 399–405. [Google Scholar] [CrossRef]
- Lavaud, C.; Massiot, G.; Moretti, C.; Le Men-Olivier, L. Triterpene saponins from Myrsine pellucida. Phytochemistry 1994, 37, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Acebey-Castellon, I.L.; Voutquenne-Nazabadioko, L.; Doan Thi Mai, H.; Roseau, N.; Bouthagane, N.; Muhammad, D.; Lavaud, C. Triterpenoid saponins from Symplocos lancifolia. J. Nat. Prod. 2011, 74, 163–168. [Google Scholar] [CrossRef]
- Li, Y.L.; Li, J.; Wang, N.L.; Yao, X.S. Flavonoids and a new polyacetylene from Bidens parviflora Willd. Molecules 2008, 13, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.T.; Mai, N.T. Chemical constituents of Saraca dives. Vietnam J. Sci. Technol. 2014, 123, 107–111. [Google Scholar]
- Thanh, N.T.V.; Hien, D.T.T.; Minh, T.T.; Cuong, H.D.; Nhiem, N.X.; Yen, P.H.; Van Kiem, P. Quercetin glycosides and sesquiterpenes from Phoebe poilanei Kosterm. Vietnam J. Chem. 2019, 57, 401–405. [Google Scholar] [CrossRef]
- Zhong, X.N.; Otsuka, H.; Ide, T.; Hirata, E.; Takushi, A.; Takeda, Y. Three flavonol glycosides from leaves of Myrsine seguinii. Phytochemistry 1997, 46, 943–946. [Google Scholar] [CrossRef]
- Niu, C.; Yang, L.P.; Zhang, Z.Z.; Zhou, D.J.; Kong, J.C.; Liu, Z.Q.; Wang, Z.H. Chemical constituents of Ampelopsis japonica. Chem. Nat. Comp. 2022, 58, 545–547. [Google Scholar] [CrossRef]
- Wang, H.; Cong, W.L.; Fu, Z.L.; Chen, D.F.; Wang, Q. Anti-complementary constituents of Viola kunawarensis. Nat. Prod. Res. 2017, 31, 2312–2315. [Google Scholar] [CrossRef]
- Wu, Q.L.; Wang, M.; Simon, J.E.; Yu, S.C.; Xiao, P.G.; Ho, C.T. Studies on the chemical constituents of loquat leaves (Eriobotrya japonica). Orient. Food Herbs 2003, 22, 292–306. [Google Scholar]
- Ren, Y.; Anaya-Eugenio, G.D.; Czarnecki, A.A.; Ninh, T.N.; Yuan, C.; Chai, H.B.; Soejarto, D.D.; Burdette, J.E.; de Blanco, E.J.C.; Kinghorn, A.D. Cytotoxic and NF-κB and mitochondrial transmembrane potential inhibitory pentacyclic triterpenoids from Syzygium corticosum and their semi-synthetic derivatives. Bioorg. Med. Chem. 2018, 26, 4452–4460. [Google Scholar] [CrossRef] [PubMed]
- Huong, P.T.T.; Nguyen, V.T.; Diep, C.N.; Thao, N.P.; Cuong, N.X.; Nam, N.H.; Minh, C.V. Antimicrobial compounds from Rhizophora stylosa. Vietnam J. Sci. Technol. 2015, 53, 205–210. [Google Scholar] [CrossRef]
- Khallouki, F.; Hull, W.E.; Würtele, G.; Haubner, R.; Erben, G.; Owen, R.W. Isolation of the major phenolic compounds in the pits of brined green olive drupes: Structure elucidation by comprehensive 1H/13C-NMR spectroscopy. Nat. Prod. Comm. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Watari, A.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective effect of syringic acid and vanillic acid on CCl4-induced liver injury. Biol. Pharm. Bull. 2010, 33, 983–987. [Google Scholar] [CrossRef]
- Raffauf, R.F.; Zennie, T.M.; Onan, K.D.; Le Quesne, P.W. Funebrine, a structurally novel pyrrole alkaloid, and other. gamma.-hydroxyisoleucine-related metabolites of Quararibea funebris (Llave) Vischer (Bombacaceae). J. Org. Chem. 1984, 49, 2714–2718. [Google Scholar] [CrossRef]
- Trang, B.T.; Thuong, C.H.; Thao, P.X.; Mac, D.H.; Gree, R. A new approach for the synthesis of sotolon in racemic and enantioenriched forms. Vietnam J. Chem. 2021, 59, 42–46. [Google Scholar] [CrossRef]
- Ali, Z.; Khan, I.A. Alkyl phenols and saponins from the roots of Labisia pumila (Kacip Fatimah). Phytochemistry 2011, 72, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Row, L.C.; Chen, C.M.; Ho, J.C. Two cerebrosides and one acylglycosyl sterol from Monochoria vaginalis. J. Chin. Chem. Soc. 2003, 50, 1103–1107. [Google Scholar] [CrossRef]
- Zhang, G.L.; Li, N.; Wang, Y.H.; Zheng, Y.T.; Zhang, Z.; Wang, M.W. Bioactive lignans from Peperomia heyneana. J. Nat. Prod. 2007, 70, 662–664. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, C.; Wang, G.; Xu, Q.; Bian, Y.; Li, J.; Li, J.; Ding, R.; Lin, H.; Tian, W.; et al. C-13 Norisoprenoids and Eudesmanoids from Nelumbo nucifera Gaertn. Regulate the Lipid Metabolism via the AMPK/ACC/SREBP-1c Signaling Pathway. Chem. Biodiver. 2025, 22, e202401778. [Google Scholar] [CrossRef]
- Liu, J.J.; Hao, J.J.; Tan, M.; Liao, C.C.; Liu, D.; Li, H.M.; Li, R.T. Iridoids and other constituents from the leaves and stems of Valeriana officinalis var. latifolia. Phytochemistry 2024, 218, 113934. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Xiong, J.; Zou, Y.; Mei, Q.B.; Wang, W.X.; Hu, J.F. Sesquiterpenoids from the Chinese endangered plant Manglietia aromatica. Phytochem. Lett. 2016, 18, 202–207. [Google Scholar] [CrossRef]
- Tu, P.C.; Tseng, H.C.; Liang, Y.C.; Huang, G.J.; Lu, T.L.; Kuo, T.F.; Kuo, Y.H. Phytochemical investigation of Tradescantia albiflora and anti-inflammatory butenolide derivatives. Molecules 2019, 24, 3336. [Google Scholar] [CrossRef]
- Pan, Y.B.; Song, X.Q.; Sun, P.; Zhang, H. Chemical constituents from the stems of Marsdenia tenacissima. Phytochem. Lett. 2023, 58, 24–28. [Google Scholar] [CrossRef]
- Liu, Q.W.; Chen, C.H.; Wang, X.F.; Jiang, K.; Qu, S.J.; Dai, Y.R.; Tan, C.H. Triterpenoids, megastigmanes and hydroxycinnamic acid derivatives from Anisomeles indica. Nat. Prod. Res. 2019, 33, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; Oh, K.E.; Jo, Y.H.; Ahn, J.H.; Liu, Q.; Turk, A.; Jang, J.Y.; Hwang, B.Y.; Lee, M.K. Characterization of tyrosinase inhibitory constituents from the aerial parts of Humulus japonicus using LC-MS/MS coupled online assay. Bioorg. Med. Chem. 2018, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- DellaGreca, M.; Di Marino, C.; Zarrelli, A.; D’Abrosca, B. Isolation and phytotoxicity of apocarotenoids from Chenopodium album. J. Nat. Prod. 2004, 67, 1492–1495. [Google Scholar] [CrossRef]
- Wang, H.L.; Ding, J. Extraction Method of Neoflavonoid Compound. Patent CN111732617, 1 October 2020. [Google Scholar]
- Otsuka, H.; Yao, M.; Kamada, K.; Takeda, Y. Alangionosides G-M: Glycosides of megastigmane derivatives from the leaves of Alangium premnifolium. Chem. Pharm. Bull. 1995, 43, 754–759. [Google Scholar] [CrossRef]
- De Tommasi, N.; Piacente, S.; De Simone, F.; Pizza, C. Constituents of Cydonia vulgaris: Isolation and structure elucidation of four new flavonol glycosides and nine new α-ionol-derived glycosides. J. Agric. Food Chem. 1996, 44, 1676–1681. [Google Scholar] [CrossRef]
- Srinroch, C.; Sahakitpichan, P.; Techasakul, S.; Chimnoi, N.; Ruchirawat, S.; Kanchanapoom, T. 2-Aminobenzoyl and megastigmane glycosides from Wrightia antidysenterica. Phytochem. Lett. 2019, 29, 61–64. [Google Scholar] [CrossRef]
- Michalska, K.; Galanty, A.; Le, T.N.; Malarz, J.; Vuong, N.Q.; Pham, V.C.; Stojakowska, A. New polyesterified ursane derivatives from leaves of Maesa membranacea and their cytotoxic activity. Molecules 2021, 26, 7013. [Google Scholar] [CrossRef] [PubMed]
- Manome, T.; Hara, Y.; Ishibashi, M. A new 1, 2-diketone physalin isolated from Physalis minima and TRAIL-resistance overcoming activity of physalins. J. Nat. Med. 2023, 77, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Ninh, B.H.; Dung, D.T.; Tai, B.H.; Yen, P.H.; Nhiem, N.X.; Hien, T.T.T.; Kiem, P.V. New isopropyl chromone and flavanone glucoside compounds from the leaves of Syzygium cerasiforme (Blume) Merr. & LM Perry and their inhibition of nitric oxide production. Chem. Biodiver. 2023, 20, e202201048. [Google Scholar] [CrossRef]
- Thao, N.P.; Linh, K.T.P.; Quan, N.H.; Trung, V.T.; Binh, P.T.; Cuong, N.T.; Nam, N.H.; Thanh, N.V. Cytotoxic metabolites from the leaves of the mangrove Rhizophora apiculata. Phytochem. Lett. 2022, 47, 51–55. [Google Scholar] [CrossRef]
- Kornpointner, C.; Hochenegger, N.J.; Shi, B.B.; Berger, A.; Theiner, J.; Brecker, L.; Schinnerl, J. Phytochemistry meets geochemistry—Blumenol C sulfate: A new megastigmane sulfate from Palicourea luxurians (Rubiaceae: Palicoureeae). Molecules 2022, 27, 7284. [Google Scholar] [CrossRef]
- Thapsut, M.; Singha, S.; Seeka, C.; Sutthivaiyakit, S. Megastigmanes from the aerial part of Euphorbia heterophylla. Phytochem. Lett. 2021, 44, 42–47. [Google Scholar] [CrossRef]
- Uyen, N.H.; Widyowati, R.; Sulistyowaty, M.I.; Sugimoto, S.; Yamano, Y.; Kawakami, S.; Otsuka, H.; Matsunami, K. Firmosides A and B: Two new sucrose ferulates from the aerial parts of Silene firma and evaluation of radical scavenging activities. J. Nat. Med. 2020, 74, 796–803. [Google Scholar] [CrossRef]
- Deng, L.; Zong, W.; Tao, X.; Liu, S.; Feng, Z.; Lin, Y.; Liao, Z.; Chen, M. Evaluation of the therapeutic effect against benign prostatic hyperplasia and the active constituents from Epilobium angustifolium L. J. Ethnopharmacol. 2019, 232, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.M.; Wang, Y.; Yu, J.H.; Liu, Y.L.; Wu, X.; He, X.R.; Zhou, Z.W. Tyrosinase inhibitors from the leaves of Eucalyptus globulus. Fitoterapia 2019, 139, 104418. [Google Scholar] [CrossRef]
- Yan, J.; Shi, X.; Donkor, P.O.; Zhu, H.; Gao, X.; Ding, L.; Qiu, F. Nine pairs of megastigmane enantiomers from the leaves of Eucommia ulmoides Oliver. J. Nat. Med. 2017, 71, 780–790. [Google Scholar] [CrossRef]
- Phetkul, U.; Hayiawae, N.; Khunthong, S.; Daus, M.; Voravuthikunchai, S.P.; Tamvapee, P.; Watanapokasin, R.; Chakthong, S. Zanthoisobutylamides A−C: Rare dimeric C-6 substituent dihydrobenzophenanthridine alkaloids from the roots of Zanthoxylum nitidum. Nat. Prod. Res. 2023, 37, 1249–1257. [Google Scholar] [CrossRef]
- Rahim, A.; Saito, Y.; Miyake, K.; Goto, M.; Nakagawa-Goto, K. Novel seco-phenanthroquinolizidine alkaloids from Indonesian Boehmeria virgata. Phytochem. Lett. 2021, 45, 132–136. [Google Scholar] [CrossRef]
- Shen, D.Y.; Kuo, P.C.; Huang, S.C.; Hwang, T.L.; Chan, Y.Y.; Shieh, P.C.; Ngan, N.T.; Thang, T.D.; Wu, T.S. Constituents from the leaves of Clausena lansium and their anti-inflammatory activity. J. Nat. Med. 2017, 71, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Vitek, R.; de Novais, L.M.; Torquato, H.F.; Paredes-Gamero, E.J.; de Carvalho, M.G.; de Sousa, P.T., Jr.; Jacinto, M.J.; da Silva, V.C. Chemical constituents and antileukemic activity of Eugenia dysenterica. Nat. Prod. Res. 2017, 31, 1930–1934. [Google Scholar] [CrossRef]
- Lee, M.; Lee, H.H.; Lee, J.K.; Ye, S.K.; Kim, S.H.; Sung, S.H. Anti-adipogenic activity of compounds isolated from Idesia polycarpa on 3T3-L1 cells. Bioorg. Med. Chem. Lett. 2013, 23, 3170–3174. [Google Scholar] [CrossRef] [PubMed]
- Sob, S.V.T.; Wabo, H.K.; Tang, C.P.; Tane, P.; Ngadjui, B.T.; Ye, Y. Phenol esters and other constituents from the stem barks of Stereospermum acuminatissimum. J. Asian Nat. Prod. Res. 2011, 13, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Tip-pyang, S.; Limpipatwattana, Y.; Khumkratok, S.; Siripong, P.; Sichaem, J. A new cytotoxic 1-azaanthraquinone from the stems of Goniothalamus laoticus. Fitoterapia 2010, 81, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Liu, H.K.; Lo, J.M.; Chien, S.C.; Chan, Y.F.; Lee, T.H.; Su, J.K.; Kuo, Y.H. Cytotoxic constituents of the leaves of Calocedrus formosana. J. Chin. Chem. Soc. 2003, 50, 161–166. [Google Scholar] [CrossRef]
- Pan, W.B.; Chang, F.R.; Wei, L.M.; Wu, Y.C. New flavans, spirostanol sapogenins, and a pregnane genin from Tupistra chinensis and their cytotoxicity. J. Nat. Prod. 2003, 66, 161–168. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Cheritamine, A new N-fatty acyl tryptamine and other constituents from the stems of Annona cherimola. J. Chin. Chem. Soc. 1999, 46, 77–86. [Google Scholar] [CrossRef]
- Van Nguyen Thien, T.; Do, L.T.M.; Dang, P.H.; Huynh, N.V.; Dang, H.P.; Nguyen, T.T.; Tran, K.T.; Huu, N.D.M.; That, Q.T. A new lignan from the flowers of Hibiscus sabdariffa L. (Malvaceae). Nat. Prod. Res. 2021, 35, 2218–2223. [Google Scholar] [CrossRef]
- Sheng, Z.; Dai, H.; Pan, S.; Wang, H.; Hu, Y.; Ma, W. Isolation and characterization of an α-glucosidase inhibitor from Musa spp.(Baxijiao) flowers. Molecules 2014, 19, 10563–10573. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Hu, X.; Xu, X.; Zhang, G.; Gong, D. Inhibitory mechanism of two allosteric inhibitors, oleanolic acid and ursolic acid on α-glucosidase. Inter. J. Biol. Macromol. 2018, 107, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016, 190, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Van Do, T.N.; Nguyen, H.X.; Truong, T.Q.; Ly, D.T.; Dang, N.M.T.; Le, T.H.; Nguyen, M.T.T. Some terpenoid compounds from the ethyl acetate extract of Annona muricata L. leaves and their α-glucosidase inhibitory activity. Sci. Technol. Dev. J. Nat. Sci. 2024, 8, 1–7. (In Vietnamese) [Google Scholar] [CrossRef]
- Tabussum, A.; Riaz, N.; Saleem, M.; Ashraf, M.; Ahmad, M.; Alam, U.; Jabeen, B.; Malik, A.; Jabbar, A. α-Glucosidase inhibitory constituents from Chrozophora plicata. Phytochem. Lett. 2013, 6, 614–619. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.; Xu, C.; Jiang, H.; Zhu, C.; Sun, L.; Sun, C.; Li, X. α-Glucosidase inhibitors from Chinese bayberry (Morella rubra Sieb. et Zucc.) fruit: Molecular docking and interaction mechanism of flavonols with different B-ring hydroxylations. RSC Adv. 2020, 10, 29347–29361. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Yan, Y.; Liu, D.; Wang, C.; Wang, H. Inhibition of glycosidase by ursolic acid: In vitro, in vivo and in silico study. J. Sci. Food Agric. 2020, 100, 986–994. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Thu, H.N.T.; Tu, A.L.T.; Thanh, T.N.; Thi, C.B.; Bavkov, D.; Kazakova, O.B. Structural modifications of 2,3-indolobetulinic acid: Design and synthesis of highly potent α-glucosidase inhibitors. Bioorg. Chem. 2019, 88, 102957. [Google Scholar] [CrossRef]
- Li, N.; Yang, J.; Wang, C.; Wu, L.; Liu, Y. Screening bifunctional flavonoids of anti-cholinesterase and anti-glucosidase by in vitro and in silico studies: Quercetin, kaempferol and myricetin. Food Biosci. 2023, 51, 102312. [Google Scholar] [CrossRef]
| Compounds | Name | Plants | Parts | References |
|---|---|---|---|---|
| 1 | β-Sistosterol | E. ribes | leaves | [3] |
| 2 | Daucosterol | E. rowlandii | leaves | [5] |
| E. ribes | leaves | [3] | ||
| 4 | Ursolic acid | E. ribes | leaves | [9] |
| 5 | Kaempferol | E. ribes | leaves | [9] |
| 6 | Kaempferin | E. ribes | leaves | [9] |
| 7 | Quercitrin | E. ribes | leaves | [9] |
| 14 | Vanillic acid | E. laeta | leaves | [11] |
| 15 | Syringic acid | E. laeta | leaves | [11] |
| Compounds | Species | Family | References |
|---|---|---|---|
| 3-O-(6′-O-Palmitoyl)-β-D-glucopyranosyl stigmasterol (3) | Myrsine pellucida | Primulaceae | [42] |
| Labisia pumila | Primulaceae | [57] | |
| Monochoria vaginalis | Pontederiaceae | [58] | |
| Quercetin-3-rhamnoside-3′-glucoside (8) | Myrsine seguinii | Primulaceae | [47] |
| (6R,9R)-9-Hydroxy-4,7-megastigmadien-3-one (9) | Peperomia heyneana | Piperaceae | [59] |
| Nelumbo nucifera | Nelumbonaceae | [60] | |
| Valeriana officinalis var. latifolia | Caprifoliaceae | [61] | |
| Manglietia aromatica | Magnoliaceae | [62] | |
| Tradescantia albiflora | Commelinaceae | [63] | |
| Grasshopper ketone (10) | Nelumbo nucifera | Nelumbonaceae | [60] |
| Marsdenia tenacissima | Apocynaceae | [64] | |
| Anisomeles indica | Lamiaceae | [65] | |
| Humulus japonicus | Cannabaceae | [66] | |
| Chenopodium album | Chenopodiaceae | [67] | |
| (6R,7E,9R)-9-Hydroxy-4,7-megastigmadien-3-one, 9-O-β-D-Apiofuranosyl(1->6)-β-D-glucopyranoside (11) | Eriobotrya japonica | Rosaceae | [68] |
| Alangium premnifolium | Cornaceae | [69] | |
| Cydonia vulgaris | Rosaceae | [70] | |
| Wrightia antidysenterica | Apocynaceae | [71] | |
| Vomifoliol (12) | Maesa membranacea | Primulaceae | [72] |
| Physalis minima | Solanaceae | [73] | |
| Syzygium cerasiforme | Myrtaceae | [74] | |
| Rhizophora apiculata | Rhizophoraceae | [75] | |
| Palicourea adusta | Rubiaceae | [76] | |
| Euphorbia heterophylla | Euphorbiaceae | [77] | |
| Silene firma | Caryophyllaceae | [78] | |
| Epilobium angustifolium | Onagraceae | [79] | |
| Eucalyptus globulus | Myrtaceae | [80] | |
| Eucommia ulmoides | Eucommiaceae | [81] | |
| Methyl trans-p-coumarate (13) | Zanthoxylum nitidum | Rutaceae | [82] |
| Boehmeria virgata | Urticaceae | [83] | |
| Clausena lansium | Rutaceae | [84] | |
| Eugenia dysenterica | Myrtaceae | [85] | |
| Idesia polycarpa | Salicaceae | [86] | |
| Stereospermum acuminatissimum | Bignoniaceae | [87] | |
| Goniothalamus laoticus | Annonaceae | [88] | |
| Calocedrus formosana | Cupressaceae | [89] | |
| Tupistra chinensis | Liliaceae | [90] | |
| Annona cherimola | Annonaceae | [91] | |
| Hibiscus sabdariffa | Malvaceae | [92] | |
| Sotolone (16) | Quararibea funebris | Bombacaceae | [55] |
| No. | Compounds | IC50 (µg/mL) | No. | Compounds | IC50 (µg/mL) |
|---|---|---|---|---|---|
| 1 | 1 | >256 | 9 | 9 | >256 |
| 2 | 2 | >256 | 10 | 10 | >256 |
| 3 | 3 | >256 | 11 | 11 | >256 |
| 4 | 4 | 1.40 ± 0.06 | 12 | 12 | >256 |
| 5 | 5 | 1.75 ± 0.08 | 13 | 13 | >256 |
| 6 | 6 | 162.13 ± 3.28 | 14 | 16 | >256 |
| 7 | 7 | 168.01 ± 4.15 | 15 | MeOH extract | 12.80 ± 0.62 |
| 8 | 8 | >256 | 16 | Acarbose | 198.5 ± 6.25 |
| Compound | Binding Energy (kcal/mol) | Interacted Residues | |
|---|---|---|---|
| Active Site | Allosteric Site | ||
| 4 | N.D * | −9.2 | Arg175, Ser179, Asn411 |
| 5 | −7.9 | N.D * | Glu304, Arg312, Arg439, Asp408, Phe157 |
| 6 | −9.3 | N.D * | Lys155, Asp349, Phe157, Arg312, His239 |
| 7 | −9.1 | N.D * | Arg312, Glu304, |
| Acarbose | −6.7 | N.D * | Glu304, His279, Pro309, Phe300, Arg312, Glu276, Gln350, Asp349, Tyr313, Asp408, Phe157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thuong, S.D.; Anh, M.T.H.; Phuong, N.V.; Mau, C.H.; Quan, N.H.; Cong, N.T.; Thanh, L.N. Chemical Constituents and α-Glucosidase Inhibitory Activities of the Leaves of Embelia parviflora—In Vitro and In Silico Studies. Life 2025, 15, 680. https://doi.org/10.3390/life15050680
Thuong SD, Anh MTH, Phuong NV, Mau CH, Quan NH, Cong NT, Thanh LN. Chemical Constituents and α-Glucosidase Inhibitory Activities of the Leaves of Embelia parviflora—In Vitro and In Silico Studies. Life. 2025; 15(5):680. https://doi.org/10.3390/life15050680
Chicago/Turabian StyleThuong, Sy Danh, Mai Thi Hoang Anh, Nguyen Van Phuong, Chu Hoang Mau, Nguyen Huu Quan, Nguyen Thanh Cong, and Le Nguyen Thanh. 2025. "Chemical Constituents and α-Glucosidase Inhibitory Activities of the Leaves of Embelia parviflora—In Vitro and In Silico Studies" Life 15, no. 5: 680. https://doi.org/10.3390/life15050680
APA StyleThuong, S. D., Anh, M. T. H., Phuong, N. V., Mau, C. H., Quan, N. H., Cong, N. T., & Thanh, L. N. (2025). Chemical Constituents and α-Glucosidase Inhibitory Activities of the Leaves of Embelia parviflora—In Vitro and In Silico Studies. Life, 15(5), 680. https://doi.org/10.3390/life15050680






