Potential Role of Inorganic Confined Environments in Prebiotic Phosphorylation
Abstract
:1. Introduction
2. General Context of Phosphate Condensation
ΔrG° = 10.6 kcal/mol
3. Anomalous Water Properties in Confined Environments
4. Effect of Confinement on Energetics
5. Geological Environment: Organic Versus Inorganic
6. Conclusions
Conflicts of Interest
References
- Trevors, J.T.; Pollack, G.H. Hypothesis: The origin of life in a hydrogel environment. Prog. Biophys. Mol. Biol. 2005, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J. Macromolecular crowding: An important but neglected aspect of the intracellular environment. Curr. Opin. Struct. Biol. 2001, 11, 114–119. [Google Scholar] [CrossRef]
- Grover, M.; He, C.; Hsieh, M.-C.; Yu, S.-S. A Chemical Engineering Perspective on the Origins of Life. Processes 2015, 3, 309–338. [Google Scholar] [CrossRef]
- Ricci, M.A.; Bruni, F.; Gallo, P.; Rovere, M.; Soper, A.K. Water in confined geometries: Experiments and simulations. J. Phys. Condens. Matter 2000, 12, A345. [Google Scholar] [CrossRef]
- Venables, D.S.; Huang, K.; Schmuttenmaer, C.A. Effect of reverse micelle size on the librational band of confined water and methanol. J. Phys. Chem. B 2001, 105, 9132–9138. [Google Scholar] [CrossRef]
- Minton, A.P. The Influence of Macromolecular Crowding and Macromolecular Confinement on Biochemical Reactions in Physiological Media. J. Biol. Chem. 2001, 276, 10577–10580. [Google Scholar] [CrossRef] [PubMed]
- Dauty, E.; Verkman, A.S. Molecular crowding reduces to a similar extent the diffusion of small solutes and macromolecules: Measurement by fluorescence correlation spectroscopy. J. Mol. Recognit. 2004, 17, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, D.E.; Levinger, N.E.; Spry, D.B.; Fayer, M.D. Confinement or the Nature of the Interface? Dynamics of Nanoscopic Water. J. Am. Chem. Soc. 2007, 129, 14311–14318. [Google Scholar] [CrossRef] [PubMed]
- Gardeniers, H.J.G.E. Chemistry in nanochannel confinement. Anal. Bioanal. Chem. 2009, 394, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Fayer, M.D.; Levinger, N.E. Analysis of water in confined geometries and at interfaces. Annu. Rev. Anal. Chem. 2010, 3, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.; Rubinovich, L. Remarkable nanoconfinement effects on chemical equilibrium manifested in nucleotide dimerization and H–D exchange reactions. Phys. Chem. Chem. Phys. 2011, 13, 16728–16734. [Google Scholar] [CrossRef] [PubMed]
- Norton, C.D.; Thompson, W.H. On the Diffusion of Acetonitrile in Nanoscale Amorphous Silica Pores. Understanding Anisotropy and the Effects of Hydrogen Bonding. J. Phys. Chem. C 2013, 117, 19107–19114. [Google Scholar] [CrossRef]
- Muñoz-Santiburcio, D.; Wittekindt, C.; Marx, D. Nanoconfinement effects on hydrated excess protons in layered materials. Nat. Commun. 2013, 4, 2349. [Google Scholar] [CrossRef] [PubMed]
- Binder, K.; Müller, M.; Kremer, M.; Schnurpfeil, A.; John von Neumann-Institut für Computing (Eds.) NIC Symposium 2016: 11.-12. Februar 2016, Jülich, Germany: Proceedings; NIC Series; Forschungszentrum Jülich: Jülich, Germany, 2016; ISBN 978-3-95806-109-5. [Google Scholar]
- Muñoz-Santiburcio, D.; Marx, D. Chemistry in nanoconfined water. Chem. Sci. 2017, 8, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Brasier, M.D.; Matthewman, R.; McMahon, S.; Wacey, D. Pumice as a Remarkable Substrate for the Origin of Life. Astrobiology 2011, 11, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Hazen, R.M. Life’s rocky start. Sci. Am. 2001, 284, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, C.M.; Jonsson, C.L.; Sverjensky, D.A.; Cleaves, H.J.; Hazen, R.M. Attachment of L-Glutamate to Rutile (α-TiO2): A Potentiometric, Adsorption, and Surface Complexation Study. Langmuir 2009, 25, 12127–12135. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, C.M.; Jonsson, C.L.; Estrada, C.; Sverjensky, D.A.; Cleaves, H.J.; Hazen, R.M. Adsorption of L-aspartate to rutile (α-TiO2): Experimental and theoretical surface complexation studies. Geochim. Cosmochim. Acta 2010, 74, 2356–2367. [Google Scholar] [CrossRef]
- Westall, F.; Hickman-Lewis, K.; Hinman, N.; Gautret, P.; Campbell, K.; Bréhéret, J.; Foucher, F.; Hubert, A.; Sorieul, S.; Dass, A.; et al. A hydrothermal-sedimentary context for the origin of life. Astrobiology 2017. [Google Scholar] [CrossRef] [PubMed]
- Hansma, H.G. Possible origin of life between mica sheets. J. Theor. Biol. 2010, 266, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Hansma, H.G. Possible origin of life between mica sheets: Does life imitate mica? J. Biomol. Struct. Dyn. 2013, 31, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Hansma, H.G. The Power of Crowding for the Origins of Life. Orig. Life Evol. Biosph. 2014, 44, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Hansma, H. Better than Membranes at the Origin of Life? Life 2017, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Costanzo, G.; Negri, R.; Di Mauro, E. A possible prebiotic synthesis of purine, adenine, cytosine, and 4 (3H)-pyrimidinone from formamide: Implications for the origin of life. Bioorg. Med. Chem. 2001, 9, 1249–1253. [Google Scholar] [CrossRef]
- Zaia, D.A.M. Adsorption of amino acids and nucleic acid bases onto minerals: A few suggestions for prebiotic chemistry experiments. Int. J. Astrobiol. 2012, 11, 229–234. [Google Scholar] [CrossRef]
- Banerjee, S.; Gnanamani, E.; Yan, X.; Zare, R.N. Can all bulk-phase reactions be accelerated in microdroplets? Analyst 2017, 142, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Bain, R.M.; Cooks, R.G. Organic Reactions in Microdroplets: Reaction Acceleration Revealed by Mass Spectrometry. Angew. Chem. Int. Ed. 2016, 55, 12960–12972. [Google Scholar] [CrossRef] [PubMed]
- Nam, I.; Lee, J.K.; Nam, H.G.; Zare, R.N. Abiotic production of sugar phosphates and uridine ribonucleoside in aqueous microdroplets. Proc. Natl. Acad. Sci. USA 2017, 114, 12396–12400. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.A.; Kee, T.P. On the Origin of Phosphorylated Biomolecules. In Origins of Life: The Primal Self-Organization; Egel, R., Lankenau, D.-H., Mulkidjanian, A.Y., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2011; pp. 57–84. ISBN 978-3-642-21624-4. [Google Scholar]
- Baltscheffsky, H.; Schultz, A.; Baltscheffsky, M. Energy for the Origin of Life. In Exobiology: Matter, Energy, and Information in the Origin and Evolution of Life in the Universe; Springer: Dordrecht, The Netherlands, 1998; pp. 95–102. ISBN 978-94-010-6124-7. [Google Scholar]
- Waehneldt, T.V.; Fox, S.W. Phosphorylation of nucleosides with polyphosphoric acid. Biochim. Biophys. Acta—Nucleic Acids Protein Synth. 1967, 134, 1–8. [Google Scholar] [CrossRef]
- Akouche, M.; Jaber, M.; Maurel, M.-C.; Lambert, J.-F.; Georgelin, T. Phosphoribosyl Pyrophosphate: A Molecular Vestige of the Origin of Life on Minerals. Angew. Chem. 2017, 129, 8028–8031. [Google Scholar] [CrossRef]
- Westheimer, F.H. Why nature chose phosphates. Science 1987, 235, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Miller, S.L. Are polyphosphates or phosphate esters prebiotic reagents? J. Mol. Evol. 1995, 41, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Gull, M.; Mojica, M.A.; Fernández, F.M.; Gaul, D.A.; Orlando, T.M.; Liotta, C.L.; Pasek, M.A. Nucleoside phosphorylation by the mineral schreibersite. Sci. Rep. 2015, 5, 17198. [Google Scholar] [CrossRef] [PubMed]
- Cleaves, H.J.; Miller, S.L. Oceanic protection of prebiotic organic compounds from UV radiation. Proc. Natl. Acad. Sci. USA 1998, 95, 7260–7263. [Google Scholar] [CrossRef] [PubMed]
- Alberty, R.A.; Goldberg, R.N. Standard thermodynamic formation properties for the adenosine 5′-triphosphate series. Biochemistry (Mosc.) 1992, 31, 10610–10615. [Google Scholar] [CrossRef]
- Bernal, J.D. The Physical Basis of Life; Routledge and Paul: London, UK, 1951. [Google Scholar]
- Orgel, L.E. Polymerization on the Rocks: Theoretical Introduction. Orig. Life Evol. Biosph. 1998, 28, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Gull, M. Prebiotic Phosphorylation Reactions on the Early Earth. Challenges 2014, 5, 193–212. [Google Scholar] [CrossRef]
- Georgelin, T.; Jaber, M.; Onfroy, T.; Hargrove, A.-A.; Costa-Torro, F.; Lambert, J.-F. Inorganic Phosphate and Nucleotides on Silica Surface: Condensation, Dismutation, and Phosphorylation. J. Phys. Chem. C 2013, 117, 12579–12590. [Google Scholar] [CrossRef]
- Faeder, J.; Ladanyi, B.M. Molecular Dynamics Simulations of the Interior of Aqueous Reverse Micelles. J. Phys. Chem. B 2000, 104, 1033–1046. [Google Scholar] [CrossRef]
- Levinger, N.E. Water in Confinement. Science 2002, 298, 1722–1723. [Google Scholar] [CrossRef] [PubMed]
- Harpham, M.R.; Ladanyi, B.M.; Levinger, N.E. The Effect of the Counterion on Water Mobility in Reverse Micelles Studied by Molecular Dynamics Simulations. J. Phys. Chem. B 2005, 109, 16891–16900. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Santiburcio, D.; Marx, D. On the complex structural diffusion of proton holes in nanoconfined alkaline solutions within slit pores. Nat. Commun. 2016, 7, 12625. [Google Scholar] [CrossRef] [PubMed]
- Pollack, G. Cells, Gels and the Engines of Life; Ebner and Sons Publishers: Seattle, WA, USA, 2001. [Google Scholar]
- Plazanet, M.; Torre, R.; Sacchetti, F. Confinement, entropic effects and hydrogen bond network fluctuations of water in Nafion membrane. J. Mol. Liq. 2016, 219, 1161–1164. [Google Scholar] [CrossRef]
- Fukatsu, Y.; Morikawa, K.; Ikeda, Y.; Tsukahara, T. Temperature and Size Effects on Structural and Dynamical Properties of Water Confined in 1–10 nm-scale Pores Using Proton NMR Spectroscopy. Anal. Sci. 2017, 33, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Why are cells powered by proton gradients. Nat. Educ. 2010, 3, 2. [Google Scholar]
- Kolesnikov, A.I.; Reiter, G.F.; Choudhury, N.; Prisk, T.R.; Mamontov, E.; Podlesnyak, A.; Ehlers, G.; Seel, A.G.; Wesolowski, D.J.; Anovitz, L.M. Quantum Tunneling of Water in Beryl: A New State of the Water Molecule. Phys. Rev. Lett. 2016, 116, 167802. [Google Scholar] [CrossRef] [PubMed]
- Laporte, S. The Electric Field at an Oxide Surface-Impact on Reactivity of Prebiotic Molecules; Université Pierre et Marie Curie-Paris VI: Paris, France, 2016. [Google Scholar]
- Kreuzer, H.J. Chemical Reactions in High Electric Fields. In Surface Science of Catalysis; Dwyer, D.J., Hoffmann, F.M., Eds.; American Chemical Society: Washington, DC, USA, 1992; Volume 482, pp. 268–286. ISBN 978-0-8412-2189-5. [Google Scholar]
- Morowitz, H.J. Beginnings of Cellular Life; Yale University Press: New Haven, CT, USA, 1992; ISBN 978-0-300-05483-5. [Google Scholar]
- Bachmann, P.A.; Luisi, P.L.; Lang, J. Autocatalytic self-replicating micelles as models for prebiotic structures. Nature 1992, 357, 57–59. [Google Scholar] [CrossRef]
- Sleep, N.H.; Zahnle, K.; Neuhoff, P.S. Initiation of clement surface conditions on the earliest Earth. Proc. Natl. Acad. Sci. USA 2001, 98, 3666–3672. [Google Scholar] [CrossRef] [PubMed]
- Sleep, N.H. The Hadean-Archaean Environment. Cold Spring Harb. Perspect. Biol. 2010, 2, a002527. [Google Scholar] [CrossRef] [PubMed]
- Westall, M.F.; Anbar, P.A.; Fischer, W.; Kump, L. The Great Oxidation Event: An Expert Discussion on the Causes, the Processes, and the Still Unknowns. Astrobiology 2012, 12, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Knauth, L.P.; Lowe, D.R. High Archean climatic temperature inferred from oxygen isotope geochemistry of cherts in the 3.5 Ga Swaziland Supergroup, South Africa. GSA Bull. 2003, 115, 566–580. [Google Scholar] [CrossRef]
- Knauth, L.P. Temperature and salinity history of the Precambrian ocean: Implications for the course of microbial evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 53–69. [Google Scholar] [CrossRef]
- Kranendonk, M.J.; van Bennett, V.; Smithies, H.R.H. Earth’s Oldest Rocks; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-08-055247-7. [Google Scholar]
- Dass, A.V.; Hickman-Lewis, K.; Brack, A.; Kee, T.P.; Westall, F. Stochastic Prebiotic Chemistry within Realistic Geological Systems. ChemistrySelect 2016, 1, 4906–4926. [Google Scholar] [CrossRef]
- Barge, L.M.; Cardoso, S.S.S.; Cartwright, J.H.E.; Cooper, G.J.T.; Cronin, L.; De Wit, A.; Doloboff, I.J.; Escribano, B.; Goldstein, R.E.; Haudin, F.; et al. From Chemical Gardens to Chemobrionics. Chem. Rev. 2015, 115, 8652–8703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negendank, W. Studies of ions and water in human lymphocytes. Biochim. Biophys. Acta—Rev. Biomembr. 1982, 694, 123–161. [Google Scholar] [CrossRef]
- Ling, G. In Search of the Physical Basis of Life; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-1-4613-2667-0. [Google Scholar]
- Zheng, J.; Pollack, G.H. Long-range forces extending from polymer-gel surfaces. Phys. Rev. E 2003, 68, 031408. [Google Scholar] [CrossRef] [PubMed]
- Barge, L.M.; Abedian, Y.; Doloboff, I.J.; Nuñez, J.E.; Russell, M.J.; Kidd, R.D.; Kanik, I. Chemical Gardens as Flow-through Reactors Simulating Natural Hydrothermal Systems. J. Vis. Exp. 2015. [Google Scholar] [CrossRef] [PubMed]
- Dušek, K.; Patterson, D. Transition in swollen polymer networks induced by intramolecular condensation. J. Polym. Sci. Part 2 Polym. Phys. 1968, 6, 1209–1216. [Google Scholar] [CrossRef]
- Osada, Y.; Gong, J. Stimuli-responsive polymer gels and their application to chemomechanical systems. Prog. Polym. Sci. 1993, 18, 187–226. [Google Scholar] [CrossRef]
- Saygin, Ö. Non-enzymatic photophosphorylation by visible light. Naturwissenschaften 1981, 68, 616–617. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed]
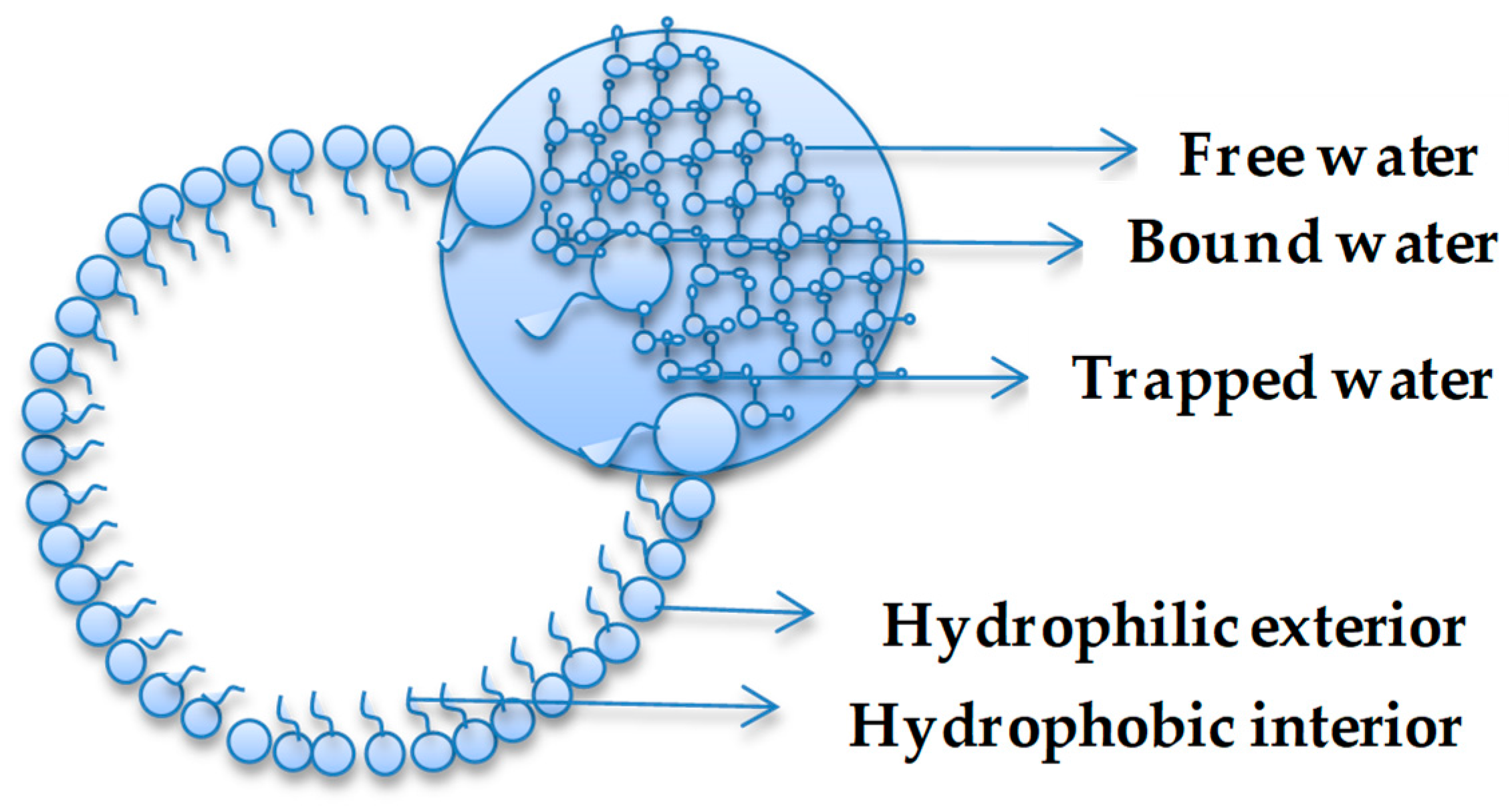
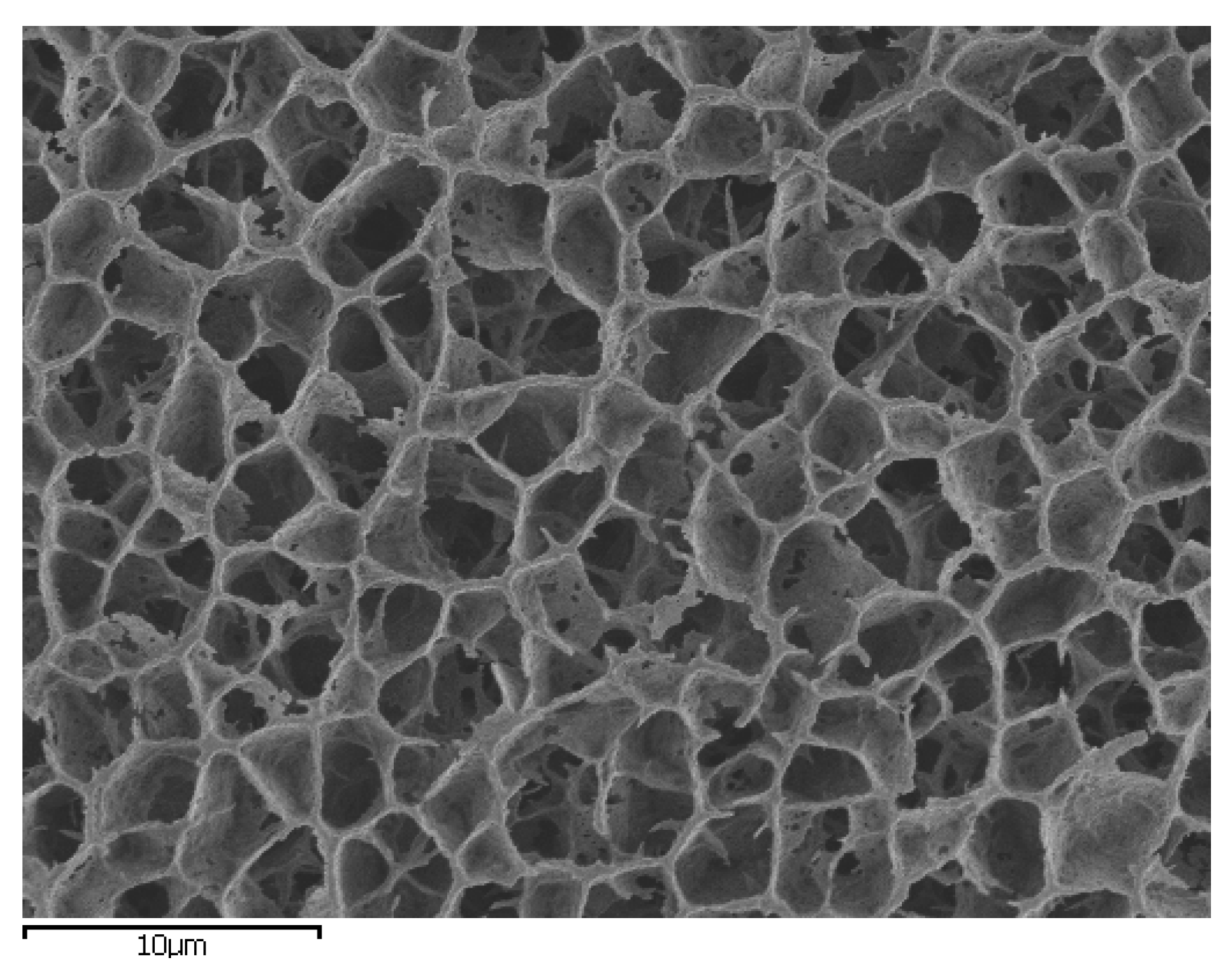
| Molecules | ∆rG° (kJ/mol) |
|---|---|
| Pyrophosphate | +42 |
| Adenosine-monophosphate | +183 |
| Adenosine-diphosphate | +279 |
| Adenosine-triphosphate | +550 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dass, A.V.; Jaber, M.; Brack, A.; Foucher, F.; Kee, T.P.; Georgelin, T.; Westall, F. Potential Role of Inorganic Confined Environments in Prebiotic Phosphorylation. Life 2018, 8, 7. https://doi.org/10.3390/life8010007
Dass AV, Jaber M, Brack A, Foucher F, Kee TP, Georgelin T, Westall F. Potential Role of Inorganic Confined Environments in Prebiotic Phosphorylation. Life. 2018; 8(1):7. https://doi.org/10.3390/life8010007
Chicago/Turabian StyleDass, Avinash Vicholous, Maguy Jaber, André Brack, Frédéric Foucher, Terence P. Kee, Thomas Georgelin, and Frances Westall. 2018. "Potential Role of Inorganic Confined Environments in Prebiotic Phosphorylation" Life 8, no. 1: 7. https://doi.org/10.3390/life8010007
APA StyleDass, A. V., Jaber, M., Brack, A., Foucher, F., Kee, T. P., Georgelin, T., & Westall, F. (2018). Potential Role of Inorganic Confined Environments in Prebiotic Phosphorylation. Life, 8(1), 7. https://doi.org/10.3390/life8010007







