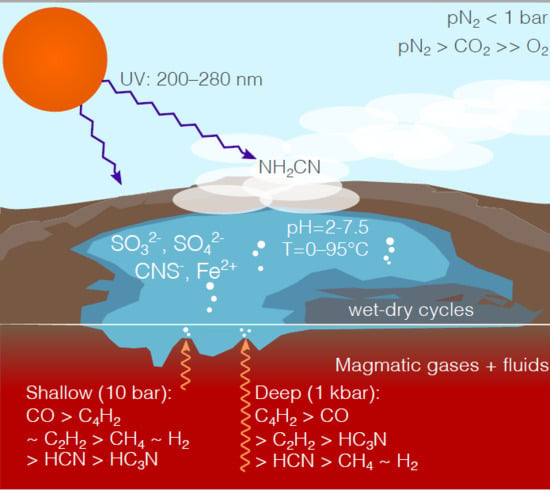
Origin of Life’s Building Blocks in Carbon- and Nitrogen-Rich Surface Hydrothermal Vents
Abstract
:1. Introduction
2. Nitrogen and Carbon on Early Earth
3. The Gas-Phase Chemistry of Early Earth Basalts
- Nitrogen-Poor () Carbon-Poor (, mean Oxidation State , NNO = )
- Nitrogen-Rich () Carbon-Poor (Section 3.2)
- Nitrogen-Poor, Carbon-Rich (, mean Oxidation State , NNO ≈; Section 3.1)
- Nitrogen-Rich and Carbon-Rich (Section 3.3)
3.1. Nitrogen-Rich Carbon-Poor Magma
3.2. Nitrogen-Poor and Carbon-Rich Magma
3.3. Carbon-Rich Nitrogen-Rich Magma
4. Surface Hydrothermal Vents with Carbon- Nitrogen-Rich Magma Degassing
5. Discussion
5.1. Challenges of Making Life on Land
5.2. Making the Surface Hydrothermal Vent Origin of Life Scenario Testable
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Partial Pressure of Nitrogen in Early Earth Atmosphere

Appendix B. Oxygen Fugacity in the Carbon-Rich Magma



References
- Powner, M.W.; Gerland, B.; Sutherland, J.D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef]
- Ritson, D.; Sutherland, J.D. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat. Chem. 2012, 4, 895–899. [Google Scholar] [CrossRef] [Green Version]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Ritson, D.J.; Ranjan, S.; Todd, Z.R.; Sasselov, D.D.; Sutherland, J.D. Photochemical reductive homologation of hydrogen cyanide using sulfite and ferrocyanide. Chem. Commun. 2018, 54, 5566–5569. [Google Scholar] [CrossRef]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef]
- Russell, M.J.; Martin, W. The rocky roots of the acetyl-CoA pathway. Trends Biochem. Sci. 2004, 29, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Russell, M.; Hall, A.; Martin, W. Serpentinization as a source of energy at the origin of life. Geobiology 2010, 8, 355–371. [Google Scholar] [CrossRef]
- Martin, W.; Russell, M.J. On the origin of biochemistry at an alkaline hydrothermal vent. Philos. Trans. R. Soc. Biol. Sci. 2007, 362, 1887–1926. [Google Scholar] [CrossRef] [Green Version]
- Preiner, M.; Xavier, J.; Sousa, F.; Zimorski, V.; Neubeck, A.; Lang, S.; Greenwell, H.; Kleinermanns, K.; Tüysüz, H.; McCollom, T.; et al. Serpentinization: Connecting Geochemistry, Ancient Metabolism and Industrial Hydrogenation. Life 2018, 8, 41. [Google Scholar] [CrossRef]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805. [Google Scholar] [CrossRef]
- Harrison, S.A.; Lane, N. Life as a guide to prebiotic nucleotide synthesis. Nat. Commun. 2018, 9, 5176. [Google Scholar] [CrossRef]
- Russell, M. Green Rust: The Simple Organizing ‘Seed’of All Life? Life 2018, 8, 35. [Google Scholar] [CrossRef]
- Ménez, B.; Pisapia, C.; Andreani, M.; Jamme, F.; Vanbellingen, Q.P.; Brunelle, A.; Richard, L.; Dumas, P.; Réfrégiers, M. Abiotic synthesis of amino acids in the recesses of the oceanic lithosphere. Nature 2018, 564, 59. [Google Scholar] [CrossRef]
- Jackson, J.B. Natural pH gradients in hydrothermal alkali vents were unlikely to have played a role in the origin of life. J. Mol. Evolut. 2016, 83, 1–11. [Google Scholar] [CrossRef]
- Jackson, J.B. Ancient Living Organisms Escaping from, or Imprisoned in, the Vents? Life 2017, 7, 36. [Google Scholar] [CrossRef]
- Jackson, J.B. The “Origin-of-Life Reactor” and reduction of CO2 by H2 in inorganic precipitates. J. Mol. Evolut. 2017, 85, 1–7. [Google Scholar] [CrossRef]
- Cleaves, H.; Aubrey, A.; Bada, J. An evaluation of the critical parameters for abiotic peptide synthesis in submarine hydrothermal systems. Orig. Life Evolut. Biosph. 2009, 39, 109–126. [Google Scholar] [CrossRef]
- Danger, G.; Plasson, R.; Pascal, R. Pathways for the formation and evolution of peptides in prebiotic environments. Chem. Soc. Rev. 2012, 41, 5416–5429. [Google Scholar] [CrossRef]
- Camprubi, E.; Jordan, S.F.; Vasiliadou, R.; Lane, N. Iron catalysis at the origin of life. IUBMB Life 2017, 69, 373–381. [Google Scholar] [CrossRef]
- Nisbet, E.G.; Sleep, N.H. The habitat and nature of early life. Nature 2001, 409, 1083–1091. [Google Scholar] [CrossRef]
- Mojzsis, S.J.; Harrison, T.M.; Pidgeon, R.T. Oxygen-isotope evidence from ancient zircons for liquid water at Earth’s surface 4,300 Myr ago. Nature 2001, 409, 178–181. [Google Scholar] [CrossRef]
- Kamber, B.S. Archean mafic-ultramafic volcanic landmasses and their effect on ocean-atmosphere chemistry. Chem. Geol. 2010, 274, 19–28. [Google Scholar] [CrossRef]
- Cousins, C.R.; Crawford, I.A.; Carrivick, J.L.; Gunn, M.; Harris, J.; Kee, T.P.; Karlsson, M.; Carmody, L.; Cockell, C.; Herschy, B.; et al. Glaciovolcanic hydrothermal environments in Iceland and implications for their detection on Mars. J. Volcanol. Geotherm. Res. 2013, 256, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef]
- Westall, F.; Hickman-Lewis, K.; Hinman, N.; Gautret, P.; Campbell, K.A.; Bréhéret, J.G.; Foucher, F.; Hubert, A.; Sorieul, S.; Dass, A.V.; et al. A Hydrothermal-Sedimentary Context for the Origin of Life. Astrobiology 2018, 18, 259–293. [Google Scholar] [CrossRef] [Green Version]
- Deamer, D.; Singaram, S.; Rajamani, S.; Kompanichenko, V.; Guggenheim, S. Self-assembly processes in the prebiotic environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1809–1818. [Google Scholar] [CrossRef] [Green Version]
- Djokic, T.; van Kranendonk, M.J.; Campbell, K.A.; Walter, M.R.; Ward, C.R. Earliest signs of life on land preserved in ca. 3.5 Ga hot spring deposits. Nat. Commun. 2017, 8, 15263. [Google Scholar] [CrossRef] [Green Version]
- Rimmer, P.B.; Rugheimer, S. Hydrogen Cyanide in Nitrogen-Rich Atmospheres of Rocky Exoplanets. Icarus 2018. submitted. [Google Scholar]
- Fischer, T.P. Fluxes of volatiles (H2O, CO2, N2, Cl, F) from arc volcanoes. Geochem. J. 2008, 42, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, C.; Nakazawa, K.; Mizuno, H. Earth’s melting due to the blanketing effect of the primordial dense atmosphere. Earth Planet. Sci. Lett. 1979, 43, 22–28. [Google Scholar] [CrossRef]
- Zahnle, K.; Schaefer, L.; Fegley, B. Earth’s earliest atmospheres. Cold Spring Harb. Perspect. Biol. 2010, 2, a004895. [Google Scholar] [CrossRef]
- Kasting, J.F. Earth’s early atmosphere. Science 1993, 259, 920–926. [Google Scholar] [CrossRef]
- Marty, B.; Zimmermann, L.; Pujol, M.; Burgess, R.; Philippot, P. Nitrogen Isotopic Composition and Density of the Archean Atmosphere. Science 2013, 342, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Avice, G.; Marty, B.; Burgess, R.; Hofmann, A.; Philippot, P.; Zahnle, K.; Zakharov, D. Evolution of atmospheric xenon and other noble gases inferred from Archean to Paleoproterozoic rocks. Geochim. Cosmochim. Acta 2018, 232, 82–100. [Google Scholar] [CrossRef]
- Lammer, H.; Stroßl, L.; Grenfell, J.L.; Scherf, M.; Fossatil, L.; Lendl, M.; Cubillos, P.E. The Role of N2 as a Geo-Biosignature for the Characterization and Detection of Earth-like Habitats. Astrobiology 2018. submitted. [Google Scholar]
- Mikhail, S.; Sverjensky, D.A. Nitrogen speciation in upper mantle fluids and the origin of Earth’s nitrogen-rich atmosphere. Nat. Geosci. 2014, 7, 816–819. [Google Scholar] [CrossRef]
- Mason, E.; Edmonds, M.; Turchyn, A.V. Remobilization of crustal carbon may dominate volcanic arc emissions. Science 2017, 357, 290–294. [Google Scholar] [CrossRef]
- Trail, D.; Watson, E.B.; Tailby, N.D. The oxidation state of Hadean magmas and implications for early Earth’s atmosphere. Nature 2011, 480, 79–82. [Google Scholar] [CrossRef]
- Shaw, G. Earth’s atmosphere—Hadean to early Proterozoic. Chemie der Erde Geochemistry 2008, 68, 235–264. [Google Scholar] [CrossRef]
- Yang, X.; Gaillard, F.; Scaillet, B. A relatively reduced Hadean continental crust and implications for the early atmosphere and crustal rheology. Earth Planet. Sci. Lett. 2014, 393, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Burnham, A.D.; Berry, A.J. Formation of Hadean granites by melting of igneous crust. Nat. Geosci. 2017, 10, 457–461. [Google Scholar] [CrossRef]
- Sharp, Z.D. Nebular ingassing as a source of volatiles to the Terrestrial planets. Chem. Geol. 2017, 448, 137–150. [Google Scholar] [CrossRef]
- Bali, E.; Audétat, A.; Keppler, H. Water and hydrogen are immiscible in Earth’s mantle. Nature 2013, 495, 220. [Google Scholar] [CrossRef] [PubMed]
- Burgisser, A.; Alletti, M.; Scaillet, B. Simulating the behavior of volatiles belonging to the C-O-H-S system in silicate melts under magmatic conditions with the software D-Compress. Comput. Geosci. 2015, 79, 1–14. [Google Scholar] [CrossRef]
- Rimmer, P.B.; Helling, C. A Chemical Kinetics Network for Lightning and Life in Planetary Atmospheres. ApJS 2016, 224, 9. [Google Scholar] [CrossRef]
- Burcat, A.; Ruscic, B. Third Millenium Ideal Gas and Condensed Phase Thermochemical Database for Combustion (With Update From Active Thermochemical Tables); Technical Report; Argonne National Laboratory (ANL): Lemont, IL, USA, 2005.
- Tsai, S.M.; Lyons, J.R.; Grosheintz, L.; Rimmer, P.B.; Kitzmann, D.; Heng, K. VULCAN: An Open-source, Validated Chemical Kinetics Python Code for Exoplanetary Atmospheres. ApJS 2017, 228, 20. [Google Scholar] [CrossRef]
- Stolper, E. The speciation of water in silicate melts. Geochim. Cosmochim. Acta 1982, 46, 2609–2620. [Google Scholar] [CrossRef]
- Dixon, J.E.; Stolper, E.M.; Delaney, J.R. Infrared spectroscopic measurements of CO2 and H2O in Jan de Fuca Ridge basaltic glasses. Earth Planet. Sci. Lett. 1988, 90, 87–104. [Google Scholar] [CrossRef]
- Gaillard, F.; Scaillet, B. A theoretical framework for volcanic degassing chemistry in a comparative planetology perspective and implications for planetary atmospheres. Earth Planet. Sci. Lett. 2014, 403, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Nisbet, E.G.; Cheadle, M.J.; Arndt, N.T.; Bickle, M.J. Constraining the potential temperature of the Archaean mantle: A review of the evidence from komatiites. Lithos 1993, 30, 291–307. [Google Scholar] [CrossRef]
- Coogan, L.A.; Saunders, A.D.; Wilson, R.N. Aluminum-in-olivine thermometry of primitive basalts: Evidence of an anomalously hot mantle source for large igneous provinces. Chem. Geol. 2014, 368, 1–10. [Google Scholar] [CrossRef]
- Turner, S.; Evans, P.; Hawkesworth, C. Ultrafast Source-to-Surface Movement of Melt at Island Arcs from 226Ra-230Th Systematics. Science 2001, 292, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Mutch, E.; Maclennan, J.; Shorttle, O. Rapid trans-crustal magma movement under Icelandic volcanoes. Nat. Geosci. 2018. under review. [Google Scholar]
- Hu, R.; Seager, S. Photochemistry in Terrestrial Exoplanet Atmospheres. III. Photochemistry and Thermochemistry in Thick Atmospheres on Super Earths and Mini Neptunes. ApJ 2014, 784, 63. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Ranjan, S.; Todd, Z.R.; Sutherland, J.D.; Sasselov, D.D. Sulfidic Anion Concentrations on Early Earth for Surficial Origins-of-Life Chemistry. arXiv, 2018; arXiv:astro-ph.EP/1801.07725. [Google Scholar] [CrossRef] [PubMed]
- Kamyshny, A.; Druschel, G.; Mansaray, Z.F.; Farquhar, J. Multiple sulfur isotopes fractionations associated with abiotic sulfur transformations in Yellowstone National Park geothermal springs. Geochem. Trans. 2014, 15, 7. [Google Scholar] [CrossRef] [Green Version]
- Ritson, D.; Battilocchio, C.; Ley, S.V.; Sutherland, J.D. Mimicking the surface and chemistry of early Earth; the first steps to life? Nat. Commun. 2018, 9, 1821. [Google Scholar] [CrossRef]
- Mariani, A.; Russell, D.A.; Javelle, T.; Sutherland, J.D. A light-releasable potentially prebiotic nucleotide activating agent. J. Am. Chem. Soc. 2018, 140, 8657–8661. [Google Scholar] [CrossRef]
- Matsunaga, T.; Hieda, K.; Nikaido, O. Wavelength dependent formation of thymine dimers and (6-4) photoproducts in DNA by monochromatic ultraviolet light ranging from 150 to 365 nm. Photochem. Photobiol. 1991, 54, 403–410. [Google Scholar] [CrossRef]
- Karentz, D.; Cleaver, J.E.; Mitchell, D.L. Cell Survival Characteristics and Molecular Responses of Antarctic Phytoplankton to Ultraviolet-B Radiation. J. Phycol. 1991, 27, 326–341. [Google Scholar] [CrossRef]
- Quickenden, T.I.; Irvin, J.A. The ultraviolet absorption spectrum of liquid water. J. Chem. Phys. 1980, 72, 4416–4428. [Google Scholar] [CrossRef]
- Laurion, I.; Ventura, M.; Catalan, J.; Psenner, R.; Sommaruga, R. Attenuation of ultraviolet radiation in mountain lakes: Factors controlling the among- and within-lake variability. Limnol. Oceanogr. 2000, 45, 1274–1288. [Google Scholar] [CrossRef] [Green Version]
- Abramov, O.; Mojzsis, S.J. Microbial habitability of the Hadean Earth during the late heavy bombardment. Nature 2009, 459, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Abramov, O.; Kring, D.A.; Mojzsis, S.J. The impact environment of the Hadean Earth. Chemie der Erde Geochemistry 2013, 73, 227–248. [Google Scholar] [CrossRef]
- Chapman, C.R.; Cohen, B.A.; Grinspoon, D.H. What are the real constraints on the existence and magnitude of the late heavy bombardment? Icarus 2007, 189, 233–245. [Google Scholar] [CrossRef]
- Boehnke, P.; Harrison, T.M. Illusory Late Heavy Bombardments. Proc. Natl. Acad. Sci. USA 2016, 113, 10802–10806. [Google Scholar] [CrossRef] [Green Version]


| Parameter | Value | Units |
|---|---|---|
| Pressure | 2 | kbar |
| Temperature | 1473 | K |
| Background NNO | ||
| Fe/Fe | 13.3 | |
| Porosity Volume | 1.5398 | % |
| Gas Weight | 0.5 | % |
| Gas Molar Weight | 52.52 | gmol |
| FeO Weight | 10.24 | % |
| 2.67 | g cm | |
| 0.86 | g cm | |
| H2O melt weight | 0.098 | % |
| H2 melt weight | 6.91 | ppb |
| SO2 melt weight | 0.60 | % |
| H2S melt weight | 732 | ppm |
| CO2 melt weight | 0.11 | % |
| S melt weight | 0.36828 | % |
| Species | p | c | ||
|---|---|---|---|---|
| Hydrogen Sulfide (H2S) | 0.2 | bar | 20 | mM |
| Sulfur Dioxide (SO2) | 3 | bar | 3 | M |
| Carbon Monoxide (CO) | 3.8 | bar | 3.8 | mM |
| Methane (CH4) | 63 | mbar | 88 | M |
| Acetylene (C2H2) | 1.8 | bar | 46 | mM |
| Diacetylene (C4H2) | 4.2 | bar | 0.19 | M |
| Hydrogen Cyanide (HCN) | 7.5 | mbar | 90 | mM |
| Cyanoacetylene (HC3N) | 5.6 | mbar | 0.23 | M |
| Acrylonitrile (C3H3N) | 0.28 | mbar | 3.2 | mM |
| Formaldehyde (HCHO) | 0.29 | mbar | 1.1 | mM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimmer, P.B.; Shorttle, O. Origin of Life’s Building Blocks in Carbon- and Nitrogen-Rich Surface Hydrothermal Vents. Life 2019, 9, 12. https://doi.org/10.3390/life9010012
Rimmer PB, Shorttle O. Origin of Life’s Building Blocks in Carbon- and Nitrogen-Rich Surface Hydrothermal Vents. Life. 2019; 9(1):12. https://doi.org/10.3390/life9010012
Chicago/Turabian StyleRimmer, Paul B., and Oliver Shorttle. 2019. "Origin of Life’s Building Blocks in Carbon- and Nitrogen-Rich Surface Hydrothermal Vents" Life 9, no. 1: 12. https://doi.org/10.3390/life9010012
APA StyleRimmer, P. B., & Shorttle, O. (2019). Origin of Life’s Building Blocks in Carbon- and Nitrogen-Rich Surface Hydrothermal Vents. Life, 9(1), 12. https://doi.org/10.3390/life9010012