How Prebiotic Chemistry and Early Life Chose Phosphate
Abstract
:1. Introduction
2. Phosphoryl Transfer Pathways
2.1. Phosphate Esters
2.2. Phosphate Anhydrides
2.3. Phosphate Mixed Anhydrides
2.4. Phosphoramidates
3. Which Phosphate Derivatives Could Play A Role as Early Energy Currencies?
4. The Question of Prebiotic Phosphorylation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lipmann, F. Metabolic generation and utilization of phosphate bond energy. Adv. Enzymol. Relat. Areas Mol. Biol. 1941, 1, 99–162. [Google Scholar]
- Lipmann, F. Phosphorus Metabolism; McElroy, W.D., Glass, H.B., Eds.; Johns Hopkins Press: Baltimore, MD, USA, 1951; Volume 1, p. 521. [Google Scholar]
- Westheimer, F.H. Why nature chose phosphate. Science 1987, 235, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Kumler, W.D.; Eiler, J.J. The Acid Strength of Mono and Diesters of Phosphoric Acid. The n-Alkyl Esters from Methyl to Butyl, the Esters of Biological Importance, and the Natural Guanidine Phosphoric Acids. J. Am. Chem. Soc. 1943, 65, 2355–2361. [Google Scholar] [CrossRef]
- Wolfenden, R.; Ridgway, C.; Young, G. Spontaneous hydrolysis of ionized phosphate monoesters and diesters and the proficiencies of phosphatases and phosphodiesterases as catalysts. J. Am. Chem. Soc. 1998, 120, 833–834. [Google Scholar] [CrossRef]
- Bowler, M.W.; Cliff, M.J.; Waltho, J.P.; Blackburn, G.M. Why did Nature select phosphate for its dominant roles in biology? New J. Chem. 2010, 34, 784–794. [Google Scholar] [CrossRef]
- Goldford, J.E.; Hartman, H.; Smith, T.F.; Segrè, D. Remnants of an Ancient Metabolism without Phosphate. Cell 2017, 168, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.F.; Thauer, R.K. Energy in Ancient Metabolism. Cell 2017, 168, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.A.; Kee, T.P.; Bryant, D.E.; Pavlov, A.A.; Lunine, J.I. Production of Potentially Prebiotic Condensed Phosphates by Phosphorus Redox Chemistry. Angew. Chem. Int. Ed. 2008, 47, 7918–7920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, D.E.; Greenfield, D.; Walshaw, R.D.; Evans, S.M.; Nimmo, A.E.; Smith, C.L.; Wang, L.; Pasek, M.A.; Kee, T.P. Electrochemical studies of iron meteorites: Phosphorus redox chemistry on the early Earth. Int. J. Astrobiol. 2009, 8, 27–36. [Google Scholar] [CrossRef]
- Bryant, D.E.; Marriott, K.E.R.; Macgregor, S.A.; Kilner, C.; Pasek, M.A.; Kee, T.P. On the prebiotic potential of reduced oxidation state phosphorus: The H-phosphinate–pyruvate system. Chem. Commun. 2010, 46, 3726–3728. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.; Bryant, D.; Marriott, K.; Ohara, S.; Fishwick, C.; Kee, T. Selective Phosphonylation of 5′-Adenosine Monophosphate (5′-AMP) via Pyrophosphite [PPi(III)]. Orig. Life Evol. Biosph. 2016, 46, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, G.K.; Lad, C.; Wyman, P.; Williams, N.H.; Wolfenden, R. The time required for water attack at the phosphorus atom of simple phosphodiesters and of DNA. Proc. Natl. Acad. Sci. USA 2006, 103, 4052–4055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfenden, R. Benchmark reaction rates, the stability of biological molecules in water, and the evolution of catalytic power in enzymes. Annu. Rev. Biochem. 2011, 80, 645–667. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.J. Effective molarities for intramolecular reactions. Adv. Phys. Org. Chem. 1980, 17, 183–278. [Google Scholar]
- Pascal, R. Catalysis through Induced Intramolecularity: What Can Be Learned by Mimicking Enzymes with Carbonyl Compounds that Covalently Bind Substrates? Eur. J. Org. Chem. 2003, 2003, 1813–1824. [Google Scholar] [CrossRef]
- Pascal, R. Kinetic Barriers and the Self-organization of Life. Isr. J. Chem. 2015, 55, 865–874. [Google Scholar] [CrossRef]
- Li, B.-J.; El-Nachef, C.; Beauchemin, A. Organocatalysis Using Aldehydes: The Development and Improvement of Catalytic Hydroaminations, Hydrations and Hydrolyses. Chem. Commun. 2017, 53, 13192–13204. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.J.; Varvoglis, A.G. The Reactivity of Phosphate Esters. Monoester Hydrolysis. J. Am. Chem. Soc. 1967, 89, 415–423. [Google Scholar] [CrossRef]
- Westheimer, F.H. Monomeric metaphosphate ion. Chem. Rev. 1981, 81, 313–326. [Google Scholar] [CrossRef]
- Kamerlin, S.C.; Sharma, P.K.; Prasad, R.B.; Warshel, A. Why nature really chose phosphate. Q. Rev. Biophys. 2013, 46, 1–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipmann, F. Projecting backward from the present stage of evolution of biosynthesis. In The Origins of Prebiological Systems and of Their Molecular Matrices; Fox, S.W., Ed.; Academic Press: New York, NY, USA, 1965; pp. 259–280. [Google Scholar]
- Miller, S.L.; Parris, M. Synthesis of pyrophosphate under primitive Earth conditions. Nature 1964, 204, 1248–1250. [Google Scholar] [CrossRef]
- Vieyra, A.; Meyer-Fernandes, J.R.; Gama, O.B. Phosphorolysis of acetyl phosphate by orthophosphate with energy conservation in the phosphoanhydride linkage of pyrophosphate. Arch. Biochem. Biophys. 1985, 238, 574–583. [Google Scholar] [CrossRef]
- Vieyra, A.; Gueiros-Filho, F.; Meyer-Fernandes, J.R.; Costa-Sarmento, G.; De Souza-Barros, F. Reactions involving carbamyl phosphate in the presence of precipitated calcium phosphate with formation of pyrophosphate: A model for primitive energy-conservation pathways. Orig. Life Evol. Biosph. 1995, 25, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Miller, S.L. Are polyphosphate or phosphate esters prebiotic reagents? J. Mol. Evol. 1995, 41, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Miller, S.L. Potentially prebiotic synthesis of condensed phosphates. Orig. Life Evol. Biosph. 1996, 26, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W. The First Living Systems: A Bioenergetic Perspective. Microbiol. Mol. Biol. Rev. 1997, 61, 239–261. [Google Scholar] [PubMed]
- Russell, M.J. The Alkaline Solution to the Emergence of Life: Energy, Entropy and Early Evolution. Acta Biotheor. 2007, 55, 133–179. [Google Scholar] [CrossRef] [PubMed]
- Hagan, W.J., Jr.; Parker, A.; Steuerwald, A.; Hathaway, M. Phosphate Solubility and the Cyanate-Mediated Synthesis of Pyrophosphate. Orig. Life Evol. Biosph. 2007, 37, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J. The Origin of Life on Earth and the Design of Alternative Life Forms. Mol. Front. J. 2017, 1, 121–131. [Google Scholar] [CrossRef]
- Lipmann, F. Acetyl Phosphate. Adv. Enzymol. Relat. Areas Mol. Biol. 1946, 6, 231–267. [Google Scholar]
- Jencks, W.P. Free energies of hydrolysis and decarboxylation. In Handbook of Biochemistry and Molecular Biology, 3rd ed.; Fasman, G.D., Ed.; CRC Press: Cleveland, OH, USA, 1976; Volume 1, pp. 296–304. [Google Scholar]
- Di Sabato, G.; Jencks, W.P. Mechanism and catalysis of reactions of acyl phosphates II. Hydrolysis. J. Am. Chem. Soc. 1961, 83, 4400–4405. [Google Scholar] [CrossRef]
- Biron, J.-P.; Pascal, R. Amino acid N-carboxyanhydrides: Activated peptide monomers behaving as phosphate-activating agents in aqueous solution. J. Am. Chem. Soc. 2004, 126, 9198–9199. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.J.; Wallace, R.G. A phosphate-catalysed acyl transfer reaction. Hydrolysis of 4-nitrophenyl acetate in phosphate buffers. Aust. J. Chem. 1984, 37, 2559–2569. [Google Scholar] [CrossRef]
- Andrés, G.O.; Granados, A.M.; de Rossi, R.H. Kinetic Study of the Hydrolysis of Phthalic Anhydride and Aryl Hydrogen Phthalates. J. Org. Chem. 2001, 66, 7653–7657. [Google Scholar] [CrossRef] [PubMed]
- El Seoud, O.A.; Ruasse, M.-F.; Rodrigues, W.A. Kinetics and mechanism of phosphate-catalyzed hydrolysis of benzoate esters: Comparison with nucleophilic catalysis by imidazole and o-iodosobenzoate. J. Chem. Soc. Perkin Trans. 2 2002, 1053–1058. [Google Scholar] [CrossRef]
- Gill, M.S.; Neverov, A.A.; Brown, R.S. Dissection of nucleophilic and general base roles for the reaction of phosphate with p-nitrophenylthiolacetate, p-nitrophenylthiolformate, phenylthiolacetate. J. Org. Chem. 1997, 62, 7351–7357. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.L.; Payne, R.J. Phosphate-assisted peptide ligation. Chem. Commun. 2009, 4260–4262. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Flynn, G.L.; Shah, A.C. Reversible Formation and Hydrolysis of Phthaloyl and Succinyl Monophosphates in Aqueous Solution. J. Am. Chem. Soc. 1967, 89, 616–622. [Google Scholar] [CrossRef]
- Hagan, W.J., Jr. Uracil-Catalyzed Synthesis of Acetyl Phosphate: A Photochemical Driver for Protometabolism. ChemBioChem 2010, 11, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Whicher, A.; Camprubi, E.; Pinna, S.; Herschy, B.; Lane, N. Acetyl Phosphate as a Primordial Energy Currency at the Origin of Life. Orig. Life Evol. Biosph. 2018, 48, 159–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, T.N.C.; Ho, C.K.; Fersht, A.R. Free Energy of Hydrolysis of Tyrosyl Adenylate and Its Binding to Wild-Type and Engineered Mutant Tyrosyl-tRNA Synthetases. Biochemistry 1986, 25, 6603–6608. [Google Scholar] [CrossRef] [PubMed]
- Ribas de Pouplana, L.; Schimmel, P. Aminoacyl-tRNA synthetases: Potential markers of genetic code development. Trends Biochem. Sci. 2001, 26, 591–596. [Google Scholar] [CrossRef]
- Pascal, R.; Boiteau, L.; Commeyras, A. From the Prebiotic Synthesis of α-Amino Acids Towards a Primitive Translation Apparatus for the Synthesis of Peptides. Top. Curr. Chem. 2005, 259, 69–122. [Google Scholar]
- Pascal, R.; Boiteau, L. Energetic constraints on prebiotic pathways: Application to the emergence of translation. In Origin and Evolution of Life: An Astrobiology Perspective; Gargaud, M., Lopez-Garcia, P., Martin, H., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 247–258. [Google Scholar]
- Liu, Z.; Beaufils, D.; Rossi, J.-C.; Pascal, R. Evolutionary importance of the intramolecular pathways of hydrolysis of phosphate ester mixed anhydrides with amino acids and peptides. Sci. Rep. 2014, 4, 7440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Rigger, L.; Rossi, J.-C.; Sutherland, J.D.; Pascal, R. Mixed Anhydride Intermediates in the Reaction of 5(4H)-Oxazolones with Phosphate Esters and Nucleotides. Chem. Eur. J. 2016, 22, 14940–14949. [Google Scholar] [CrossRef] [PubMed]
- Biron, J.-P.; Parkes, A.L.; Pascal, R.; Sutherland, J.D. Expeditious, potentially primordial, aminoacylation of nucleotides. Angew. Chem. Int. Ed. Engl. 2005, 44, 6731–6734. [Google Scholar] [CrossRef] [PubMed]
- Leman, L.; Orgel, L.; Ghadiri, M.R. Amino Acid Dependent Formation of Phosphate Anhydrides in Water Mediated by Carbonyl Sulfide. J. Am. Chem. Soc. 2006, 128, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Brack, A. Selective emergence and survival of early polypeptides in water. Orig. Life 1987, 17, 367–379. [Google Scholar] [CrossRef]
- Commeyras, A.; Taillades, J.; Collet, H.; Boiteau, L.; Vandenabeele-Trambouze, O.; Pascal, R.; Rousset, A.; Garrel, L.; Rossi, J.-C.; Biron, J.-P.; et al. Dynamic co-evolution of peptides and chemical energetics, a gateway to the emergence of homochirality and the catalytic activity of peptides. Orig. Life Evol. Biosph. 2004, 34, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Leman, L.; Orgel, L.; Ghadiri, M.R. Carbonyl Sulfide–Mediated Prebiotic Formation of Peptides. Science 2004, 306, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Danger, G.; Boiteau, L.; Cottet, H.; Pascal, R. The peptide formation mediated by cyanate revisited. N-carboxyanhydrides as accessible intermediates in the decomposition of N-carbamoylamino acids. J. Am. Chem. Soc. 2006, 128, 7412–7413. [Google Scholar] [PubMed]
- Wickramasinghe, N.S.M.D.; Staves, M.P.; Lacey, J.C. Stereoselective, nonenzymatic, intramolecular transfer of amino acids. Biochemistry 1991, 30, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Paecht-Horowitz, M.; Berger, J.; Katchalsky, A. Prebiotic synthesis of polypeptides by heterogeneous polycondensation of aminoacid adenylates. Nature 1970, 228, 636–639. [Google Scholar] [CrossRef]
- Liu, Z.; Hanson, C.; Ajram, G.; Boiteau, L.; Rossi, J.-C.; Danger, G.; Pascal, R. 5(4H)-Oxazolones as Effective Aminoacylation Reagents for the 3′-Terminus of RNA. Synlett 2017, 28, 73–77. [Google Scholar]
- Bowler, F.R.; Chan, C.K.; Duffy, C.D.; Gerland, B.; Islam, S.; Powner, M.W.; Sutherland, J.D.; Xu, J. Prebiotically plausible oligoribonucleotide ligation facilitated by chemoselective acetylation. Nat. Chem. 2013, 5, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Ajram, G.; Rossi, J.-C.; Pascal, R. The chemical likelihood of ribonucleotide-α-amino acid copolymers as players for early stages of evolution. J. Mol. Evol. 2019, in press. [Google Scholar] [CrossRef]
- Page, M.I.; Jencks, W.P. Entropic contribution to rate accelerations in enzymic and intramolecular reactions and the chelate effect. Proc. Natl. Acad. Sci. USA 1971, 68, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C. A research proposal on the origin of life. Orig. Life Evol. Biosph. 2003, 33, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Adelfinskaya, O.; Herdewijn, P. Amino Acid Phosphoramidate Nucleotides as Alternative Substrates for HIV-1 Reverse Transcriptase. Angew. Chem. Int. Ed. 2007, 46, 4356–4358. [Google Scholar] [CrossRef] [PubMed]
- Adelfinskaya, O.; Terrazas, M.; Froeyen, M.; Marlière, P.; Nauwelaerts, K.; Herdewijn, P. Polymerase-catalyzed synthesis of DNA from phosphoramidate conjugates of deoxynucleotides and amino acids. Nucleic Acids Res. 2007, 35, 5060–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraut, A.; Herdewijn, P. Influence of the Linkage between Leaving Group and Nucleoside on Substrate Efficiency for Incorporation in DNA Catalyzed by Reverse Transcriptase. ChemBioChem 2010, 11, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-P.; Bouillon, C.; Lescrinier, E.; Herdewijn, P. Iminodipropionic acid as the leaving group for DNA polymerization by HIV-1 reverse transcriptase. ChemBioChem 2011, 12, 1868–1880. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-P.; Bouillon, C.; Lescrinier, E.; Herdewijn, P. Dipeptides as Leaving Group in the Enzyme-Catalyzed DNA Synthesis. Chem. Biodivers. 2012, 9, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Giraut, A.; Abu El-Asrar, R.; Marlière, P.; Delarue, M.; Herdewijn, P. 2′-Deoxyribonucleoside phosphoramidate triphosphate analogues as alternative substrates for E. coli polymerase III. ChemBioChem 2012, 13, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Liu, X.H.; Li, Y.M.; Ma, Y.; Tan, B.; Wan, R.; Zhao, Y.F. N-Phosphoryl amino acids and biomolecular origins. Orig. Life Evol. Biosph. 2004, 34, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Deng, H.; Tang, G.; Liu, Y.; Xu, P.; Zhao, Y. Intermolecular Phosphoryl Transfer of N-Phosphoryl Amino Acids. Eur. J. Org. Chem. 2011, 2011, 3220–3228. [Google Scholar] [CrossRef]
- Ying, J.; Fu, S.; Li, X.; Fen, L.; Pengxiang, X.; Liu, Y.; Gao, X.; Zhao, Y. A plausible model correlates prebiotic peptide synthesis with the primordial genetic code. Chem. Commun. 2018, 54, 8598–8601. [Google Scholar] [CrossRef] [PubMed]
- Griesser, H.; Tremmel, P.; Kervio, E.; Pfeffer, C.; Steiner, U.E.; Richert, C. Ribonucleotides and RNA Promote Peptide Chain Growth. Angew. Chem. Int. Ed. 2017, 56, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Jauker, M.; Griesser, H.; Richert, C. Spontaneous Formation of RNA Strands, Peptidyl RNA, and Cofactors. Angew. Chem. Int. Ed. 2015, 54, 14564–14569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamurthy, R.; Arrhenius, G.; Eschenmoser, A. Formation of glycolaldehyde phosphate from glycolaldehyde in aqueous solution. Orig. Life Evol. Biosph. 1999, 29, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.; Guntha, S.; Eschenmoser, A. Regioselective α-phosphorylation of aldoses in aqueous solution. Angew. Chem. Int. Ed. 2000, 39, 2281–2285. [Google Scholar] [CrossRef]
- Karki, M.; Gibard, C.; Bhowmik, S.; Krishnamurthy, R. Nitrogenous Derivatives of Phosphorus and the Origins of Life: Plausible Prebiotic Phosphorylating Agents in Water. Life 2017, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Gibard, C.; Bhowmik, S.; Karki, M.; Kim, E.-K.; Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 2017, 10, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, R.; Orgel, L.E. Template-directed synthesis of high molecular weight polynucleotide analogues. Nature 1976, 261, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, W.S.; Orgel, L.E. Oligomerization of activated derivatives of 3′-amino-3′-deoxyguanosine on poly(C) and poly(dC) templates. Nucleic Acids Res. 1985, 13, 2469–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielinski, W.S.; Orgel, L.E. Autocatalytic synthesis of a tetranucleotide analogue. Nature 1987, 327, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, W.S.; Orgel, L.E. Oligoaminonucleoside phosphoramidates. Oligomerization of dimers of 3′-amino-3′-deoxynudeotides (GC and CG) in aqueous solution. Nucleic Acids Res. 1987, 15, 1699–1715. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, W.S.; Orgel, L.E. Polymerization of a Monomeric Guanosine Derivative in a Hydrogen-Bonded Aggregate. J. Mol. Evol. 1989, 29, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Sievers, D.; von Kiedrowski, G. Self-replication of complementary nucleotide-based oligomers. Nature 1994, 369, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Mansy, S.S.; Schrum, J.P.; Krishnamurthy, M.; Tobé, S.; Treco, D.A.; Szostak, J.W. Template-directed synthesis of a genetic polymer in a model protocell. Nature 2008, 454, 122–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Blain, C.; Zielinska, D.; Gryaznov, S.; Szostak, J. Fast and accurate nonenzymatic copying of an RNA-like synthetic genetic polymer. Proc. Natl. Acad. Sci. USA 2013, 110, 17732–17737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.L.; Lohrmann, R.; Orgel, L.E. Poly(U)-Directed Transamidation between Adenosine 5′-Phosphorimidazolide and 5′ Phosphoadenosine 2′(3′)-Glycine Ester. J. Am. Chem. Soc. 1974, 96, 5283–5284. [Google Scholar] [CrossRef] [PubMed]
- Nicolis, G.; Prigogine, I. Self-Organization in Nonequilibrium Systems: From Dissipative Structures to Order through Fluctuations; Wiley: New York, NY, USA, 1977. [Google Scholar]
- Eschenmoser, A. Chemistry of potentially prebiological natural products. Orig. Life Evol. Biosph. 1994, 24, 389–423. [Google Scholar] [CrossRef]
- Eschenmoser, A. Question 1: Commentary Referring to the Statement “The Origin of Life can be Traced Back to the Origin of Kinetic Control” and the Question “Do You Agree with this Statement; and How Would You Envisage the Prebiotic Evolutionary Bridge Between Thermodynamic and Kinetic Control?” Stated in Section 1.1. Orig. Life Evol. Biosph. 2007, 37, 309–314. [Google Scholar] [PubMed]
- Danger, G.; Plasson, R.; Pascal, R. Pathways for the formation and evolution of peptides in prebiotic environments. Chem. Soc. Rev. 2012, 41, 5416–5429. [Google Scholar] [CrossRef] [PubMed]
- Boiteau, L.; Pascal, R. Energy sources, self-organization, and the origin of life. Orig. Life Evol. Biosph. 2011, 41, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.W. Phosphorus in prebiotic chemistry. Philos. Trans. R. Soc. Lond. Ser. B 2006, 361, 1743–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-García, C.; Coggins, A.J.; Powner, M.W. A Chemist’s Perspective on the Role of Phosphorus at the Origins of Life. Life 2017, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Tordini, F.; Bencini, A.; Bruschi, M.; De Gioia, L.; Zampella, G.; Fantucci, P. Theoretical Study of Hydration of Cyanamide and Carbodiimide. J. Phys. Chem. A 2003, 107, 1188–1196. [Google Scholar] [CrossRef]
- Steinman, G.; Lemmon, R.M.; Calvin, M. Cyanamide: A possible key compound in chemical evolution. Proc. Natl. Acad. Sci. USA 1964, 52, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Spector, L.; Lipmann, F. Carbamyl phosphate, the carbamyl donor in enzymatic citrulline synthesis. J. Am. Chem. Soc. 1955, 77, 819–820. [Google Scholar] [CrossRef]
- Jones, M.E.; Lipmann, F. Chemical and enzymatic synthesis of carbamyl phosphate. Proc. Natl. Acad. Sci. USA 1960, 46, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Halmann, M.; Lapidot, A.; Samuel, D. Kinetic and tracer studies of the reaction of carbamoyl phosphate in aqueous solution. J. Chem. Soc. 1962, 1944–1957. [Google Scholar] [CrossRef]
- Allen, C.M., Jr.; Jones, M.E. Decomposition of Carbamylphosphate in Aqueous Solutions. Biochemistry 1964, 3, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Vogels, G.D.; Uffink, L.; Van der Drift, C. Cyanate decomposition catalyzed by certain divalent anions. Recl. Trav. Chim. Pays-Bas 1970, 89, 500–508. [Google Scholar] [CrossRef]
- Danger, G.; Charlot, S.; Boiteau, L.; Pascal, R. Activation of carboxyl group with cyanate: Peptide bond formation from dicarboxylic acids. Amino Acids 2012, 42, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Saygin, Ö. Non-enzymatic photophosphorylation with visible-light—A possible mode of prebiotic ATP. Naturwissenschaften 1981, 68, 617–619. [Google Scholar] [CrossRef]
- Saygin, Ö. Non-enzymatic phosphorylation of acetate by carbamyl-phosphate—A model reaction for prebiotic activation of carboxyl groups. Orig. Life 1983, 13, 43–48. [Google Scholar] [CrossRef]
- Saygin, Ö. Photochemical carbamylphosphate formation and metal ion-catalysed transphosphorylations between carbamylphosphate and adenine nucleotides or carboxyl groups. Orig. Life 1984, 14, 131–137. [Google Scholar] [CrossRef]
- Hosseini, M.W.; Lehn, J.M. Supramolecular catalysis of phosphoryl transfer: Pyrophosphate synthesis from acetyl phosphate mediated by macrocyclic polyamines. J. Am. Chem. Soc. 1987, 109, 7047–7058. [Google Scholar] [CrossRef]
- Hosseini, M.W.; Lehn, J.M. Supramolecular catalysis of adenosine triphosphate synthesis in aqueous solution mediated by a macrocyclic polyamine and divalent metal cations. J. Chem. Soc. Chem. Commun. 1991, 451–453. [Google Scholar] [CrossRef]
- Costanzo, G.; Saladino, R.; Crestini, C.; Ciciriello, F.; Di Mauro, E. Nucleoside Phosphorylation by Phosphate Minerals. J. Biol. Chem. 2007, 282, 16729–16735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohrmann, R.; Orgel, L.E. Urea-Inorganic Phosphate Mixtures as Prebiotic Phosphorylating Agents. Science 1971, 171, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Reimann, R.; Zubay, G. Nucleoside phosphorylation: A feasible step in the prebiotic pathway to RNA. Orig. Life Evol. Biosph. 1999, 29, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, A.; Duffy, C.; Sutherland, J.; Monnard, P. Self-Assembly of Phosphate Amphiphiles in Mixtures of Prebiotically Plausible Surfactants. Astrobiology 2014, 14, 462–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orgel, L.E.; Lohrmann, R. Prebiotic Chemistry and Nucleic Acid replication. Acc. Chem. Res. 1974, 7, 368–377. [Google Scholar] [CrossRef]
- Osterberg, R.; Orgel, L.E. Polyphosphate and Trimetaphosphate Formation under Potentially Prebiotic Conditions. J. Mol. Evol. 1972, 1, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Madanamoothoo, W.; Berlioz-Barbier, A.; Maniti, O.; Girard-Egrot, A.; Buchet, R.; Strazewski, P. Giant vesicles from rehydrated crude mixtures containing unexpected mixtures of amphiphiles formed under plausibly prebiotic conditions. Org. Biomol. Chem. 2017, 15, 4231–4240. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.H.R.; Bordeaux, J.J. The Decomposition of Urea in Aqueous Media. J. Am. Chem. Soc. 1955, 77, 4729–4733. [Google Scholar] [CrossRef]
- Orgel, L.E. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123. [Google Scholar] [PubMed]
- Fahrenbach, A.C.; Giurgiu, C.; Tam, C.P.; Li, L.; Hongo, Y.; Aono, M.; Szostak, J.W. Common and Potentially Prebiotic Origin for Precursors of Nucleotide Synthesis and Activation. J. Am. Chem. Soc. 2017, 139, 8780–8783. [Google Scholar] [CrossRef] [PubMed]


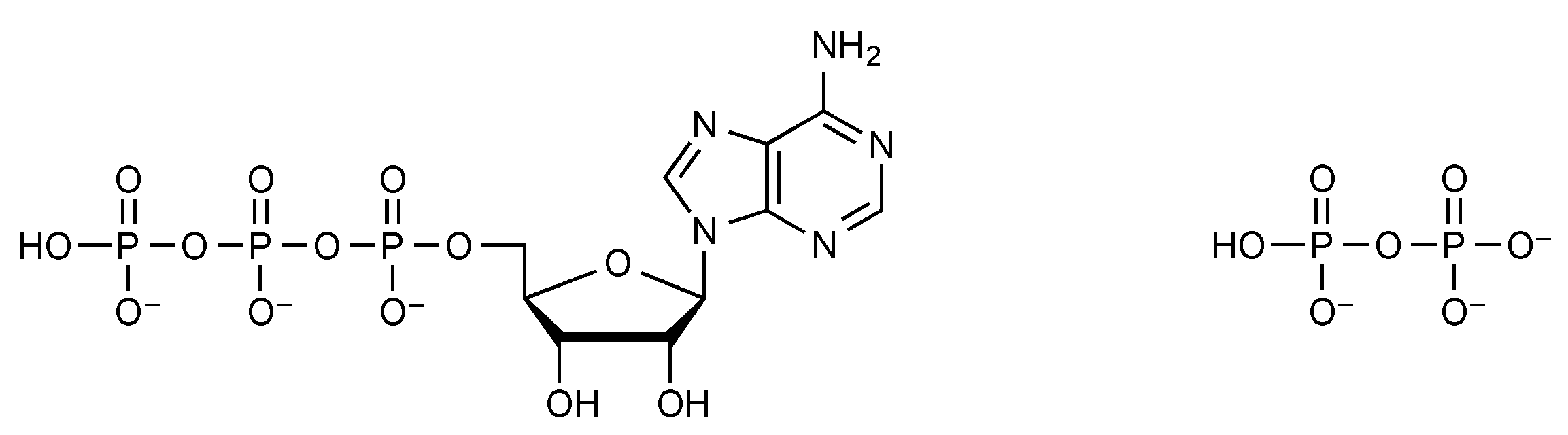


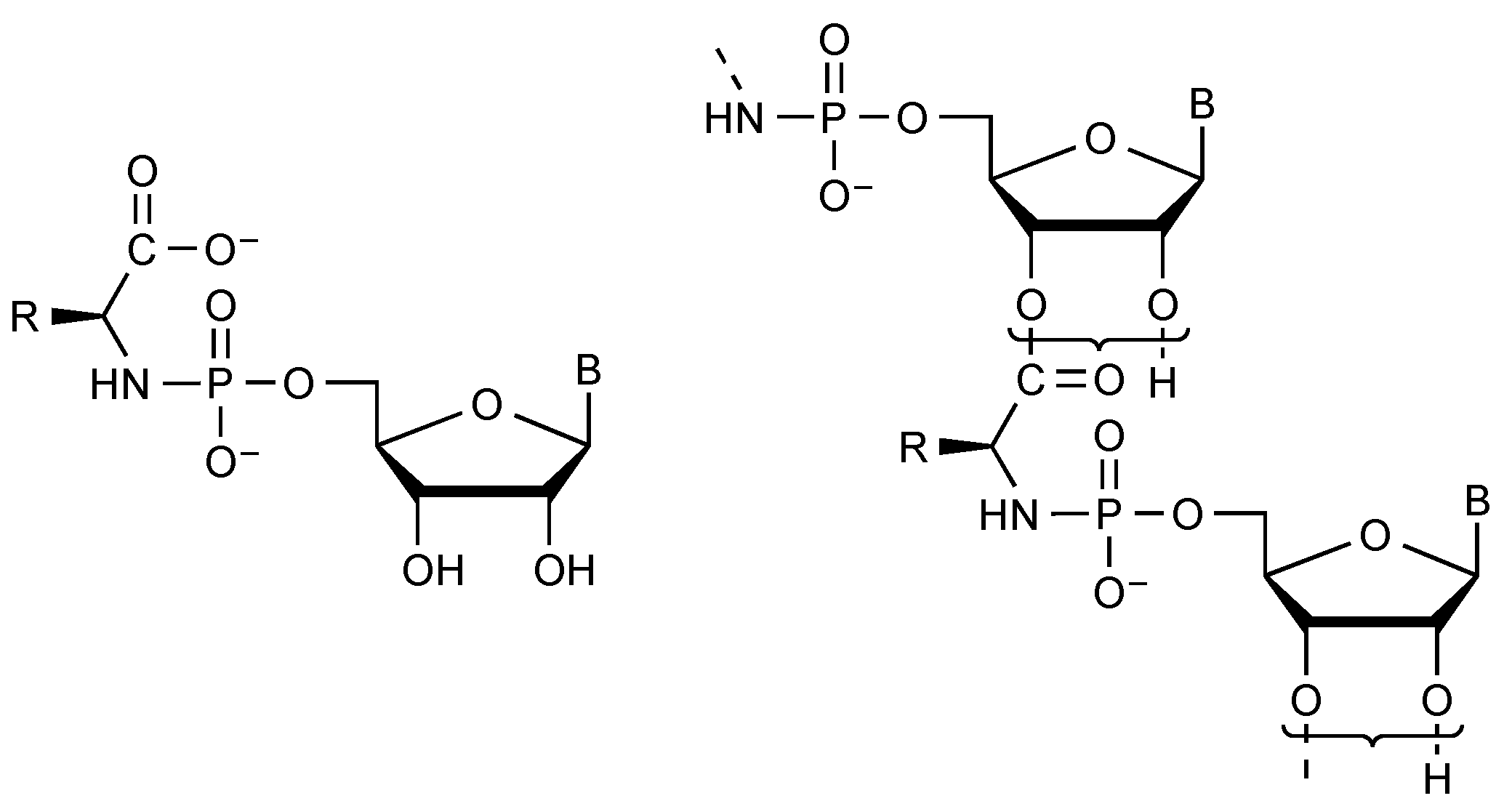



| Reagent | Product(s) | ΔG°’ kJ mol−1 | Reference |
|---|---|---|---|
| PPi | 2 Pi | −19 | [33] |
| ATP | AMP + PPi | −32.2 | [33] |
| ATP | ADP + Pi | −30.5 | [33] |
| Acetyl phosphate | AcOH + Pi | −43.1 | [33] |
| Carbamyl phosphate | CO2 + NH3 + Pi | ca. −51 1 | [33] |
| Aminoacyl phosphate | Amino acid + Pi | ca. −50 | [91] |
| Aminoacyl adenylate | Amino acid + AMP | −70 | [44] |
| Phosphoenol pyruvate | Pyruvate + Pi | −62 | [33] |
| Reagent | Product(s) | ΔG°’ kJ mol−1 | Reference |
|---|---|---|---|
| HNCO | CO2 + NH3 | −54 | [91] |
| Urea | CO2 + NH3 | −28 | [91] |
| Cyanamide | Isourea | −83 | [94] |
| Carbodiimide | Isourea | −97 | [94] |
| Acetic anhydride | Acetic acid | −91 | [33] |
| NCA | Amino acid + CO2 | −60 | [46] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Rossi, J.-C.; Pascal, R. How Prebiotic Chemistry and Early Life Chose Phosphate. Life 2019, 9, 26. https://doi.org/10.3390/life9010026
Liu Z, Rossi J-C, Pascal R. How Prebiotic Chemistry and Early Life Chose Phosphate. Life. 2019; 9(1):26. https://doi.org/10.3390/life9010026
Chicago/Turabian StyleLiu, Ziwei, Jean-Christophe Rossi, and Robert Pascal. 2019. "How Prebiotic Chemistry and Early Life Chose Phosphate" Life 9, no. 1: 26. https://doi.org/10.3390/life9010026
APA StyleLiu, Z., Rossi, J.-C., & Pascal, R. (2019). How Prebiotic Chemistry and Early Life Chose Phosphate. Life, 9(1), 26. https://doi.org/10.3390/life9010026





